Introduction
Ameloblastoma is an odontogenic epithelial neoplasm
that may originate from the enamel organ, remnants of the dental
lamina, epithelium of dentigerous cysts, or possibly from the basal
cells of the oral mucosa epithelium (1). Although the lesion is histologically
benign, this neoplasm behaves as a slow-growing invasive tumor.
Usually the tumor remains asymptomatic until it reaches a large
enough size to provoke expansion and perforation of the adjacent
soft tissue, at which point the patient may perceive its existence
(2).
Ameloblastomas account for ∼1% of all jaw tumors and
cysts (3). They appear between
30–40 years of age, except in the unicystic variety which usually
appear prior to the age of 30 (4).
In >80% of cases ameloblastomas present as an intraosseous
neoformation in the mandible, particularly in the molar area or the
ascending ramus (5). Since 1992
the World Health Organization has accepted three subtypes of benign
ameloblastomas: solid/multicystic, unicystic and
extraosseous/peripheral (6). The
most common subtype is multicystic, representing >80% of cases
including the follicular, plexiform, acanthomatous and granular
types. Since 2005, two new subtypes have been added to the
classification: desmoplastic and mixed (with areas of desmoplastic
and solid pattern). The unicystic type also comprises mural,
luminal and intraluminal ameloblastoma arising in dentigerous
cysts. Diagnosis is based on imaging examinations (panoramic
radiography, CT and MRI) and histopathological studies by means of
a biopsy. Radiographically, multicystic ameloblastomas usually
present as multilocular radiolucent images in ‘soap bubbles’
(7). In more than half of cases
associated with impacted teeth, unicystic ameloblastomas appear as
well-defined radiolucent images, with a scalloped or lobed edge.
Therefore the tumors are visualized around the tooth crown, similar
to that observed in dentigerous cysts (6).
Treatment of these neoplasms remains a matter of
debate due to their locally aggressive behavior and high rate of
recurrence following treatment (8). The therapeutic challenge is to
achieve a complete lesion excision with the least possible
morbidity. For this purpose the surgeon is required to assess the
location, size and subtype of the ameloblastoma, as well as age of
the patient. A number of different treatment strategies have been
previously reported including local techniques (curettage,
enucleation or marsupialization) or radical treatments (marginal or
en-bloc segmental resection with safety margins and reconstruction
of bone defect) (2,4,8,9–11).
Solid/multicystic ameloblastomas have been identified as the most
aggressive subtype, with a high recurrence rate following local
excision. By contrast, unicystic ameloblastomas are described to
have a lower rate of recurrence and enucleation, with curettage
potentially being sufficient for their management. Local treatment
has an increased risk of recurrence, therefore it may be
complemented with further application of Carnoy's solution,
cryotherapy or diathermy in order to reduce the recurrence rate
(12). Peripheral ameloblastomas
occur in the gingiva or alveolar mucosa and usually respond well to
local treatment. The purpose of this study was to analyze the
therapeutic results obtained from a series of patients with
mandibular ameloblastomas and to specifically focus attention on
evaluating the surgical management of recurrent ameloblastoma.
Materials and methods
A retrospective study was performed on 31 patients
with mandibular ameloblastomas, treated at the Department of Oral
and Maxillofacial Surgery, ‘Virgen del Rocío’ University Hospital
of Seville, Spain, between 2000 and 2010. The patients included 17
men and 14 women, aged between 13–82 years at the time of the
initial diagnosis (mean age 43.1 years). Patients with at least one
positive ameloblastoma biopsy and who were undergoing surgery were
included in the present study. Therapeutic modalities were divided
into enucleation, curettage, marginal mandibulectomy, segmental
mandibulectomy and mandibulectomy with reconstruction. Enucleation
(with or without curettage) involves tumoral removal and avoidance
of neoplasm spillage. Marginal mandibulectomy consists of an ‘en
bloc’ resection of the tumor with a safety margin of 1 cm of the
adjacent bone, and sometimes the adjacent periosteum may be invaded
by the tumor. Segmental resection suggests a discontinuity defect
of the mandible. Reconstructive surgery of the mandible may be
performed with a bone graft or a free flap with or without
subsequent placement of dental implants. Initial surgical treatment
consisted of enucleation and curettage (26 patients), marginal
mandibulectomy (4 patients) and segmental mandibulectomy (1
patient).
The data collected included age, gender, tumor
location, histological findings, initial treatment, number of
recurrences, year of recurrence onset, surgical treatment option,
reconstruction and follow-up. Follow-up was performed by routine
annual clinical and radiographic examination by panoramic
radiograph and CT. Recurrent ameloblastoma was defined as a relapse
after a minimum disease free period of 1 year following initial
surgery. Due to the small number of patients enrolled, no
statistical analysis was performed.
Results
In the 10-year period studied, 31 patients underwent
surgery for mandibular ameloblastomas. This included 17 men and 14
women, aged 13–82 years at the time of the initial diagnosis (mean
age 43.1 years). Sixteen of the 31 patients were under 40 years of
age at the time of diagnosis. Multicystic pattern predominated in
26 patients (83.9%), and 5 patients (16.1%) exhibited unicystic
pattern. Tumors were located in the molar region of the mandibular
body in 16 cases (51.6%), 11 cases affected the ramus and angle
(35.5%), and in 4 cases, the anterior and premolar areas were
involved (12.9%).
Initial surgical treatment involved enucleation and
curettage (26 patients), marginal mandibulectomy (4 patients) and
segmental mandibulectomy (1 patient). Nine patients (29%) presented
tumor recurrence subsequent to the first surgery. In the 31
patients, a total of 40 surgical procedures were performed,
including 26 enucleations and curettages (65%), 9 marginal
mandibulectomies (22.5%) and 5 segmental mandibulectomies (12.5%).
Primary bone reconstruction was performed in 10 cases: five
cancellous bone grafts obtained from proximal tibia, three cortical
non-vascularized iliac bone grafts, one iliac crest bone graft and
one vascularized fibula free flap were harvested. In the oldest
patient (82 years of age), the mandibular continuity defect was
reconstructed with a single titanium reconstruction plate. In cases
where a segmental resection was performed patients showed permanent
anesthesia of the lower lip.
The group of patients with recurrent ameloblastomas
comprised 2 men and 7 women (mean age of 36.1 years at the time of
recurrence). Initial diagnosis was multicystic ameloblastoma in 6
cases and unicystic ameloblastoma in 3 cases. Initial procedures
consisted of curettage in 5 cases, and enucleation and curettage in
the remaining 4 cases. Recurrences were detected at 32 months on
average following initial treatment and were surgically treated by
means of marginal mandibulectomies (5 cases) and segmental
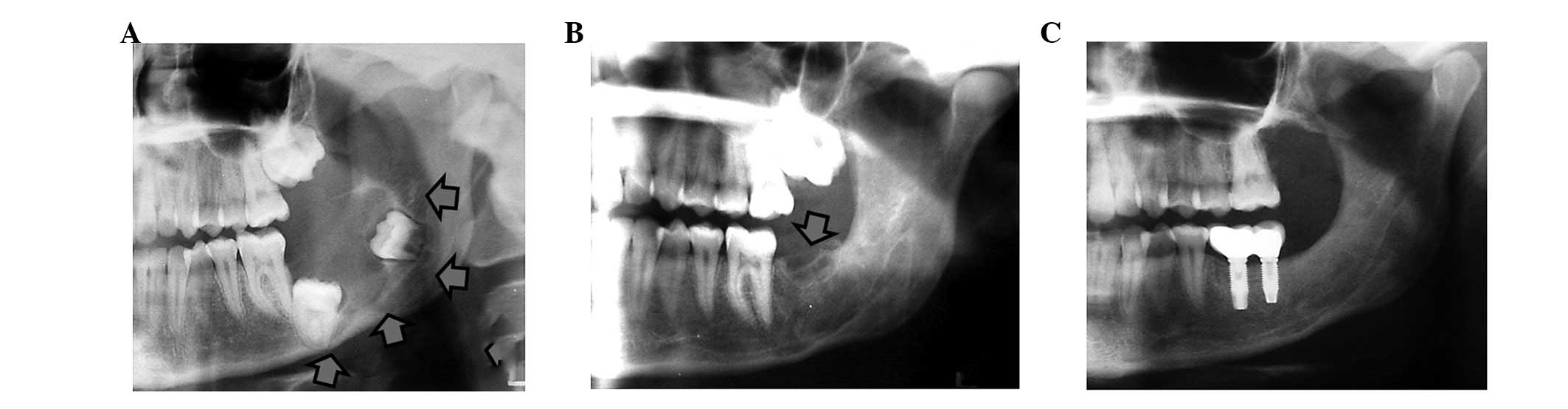
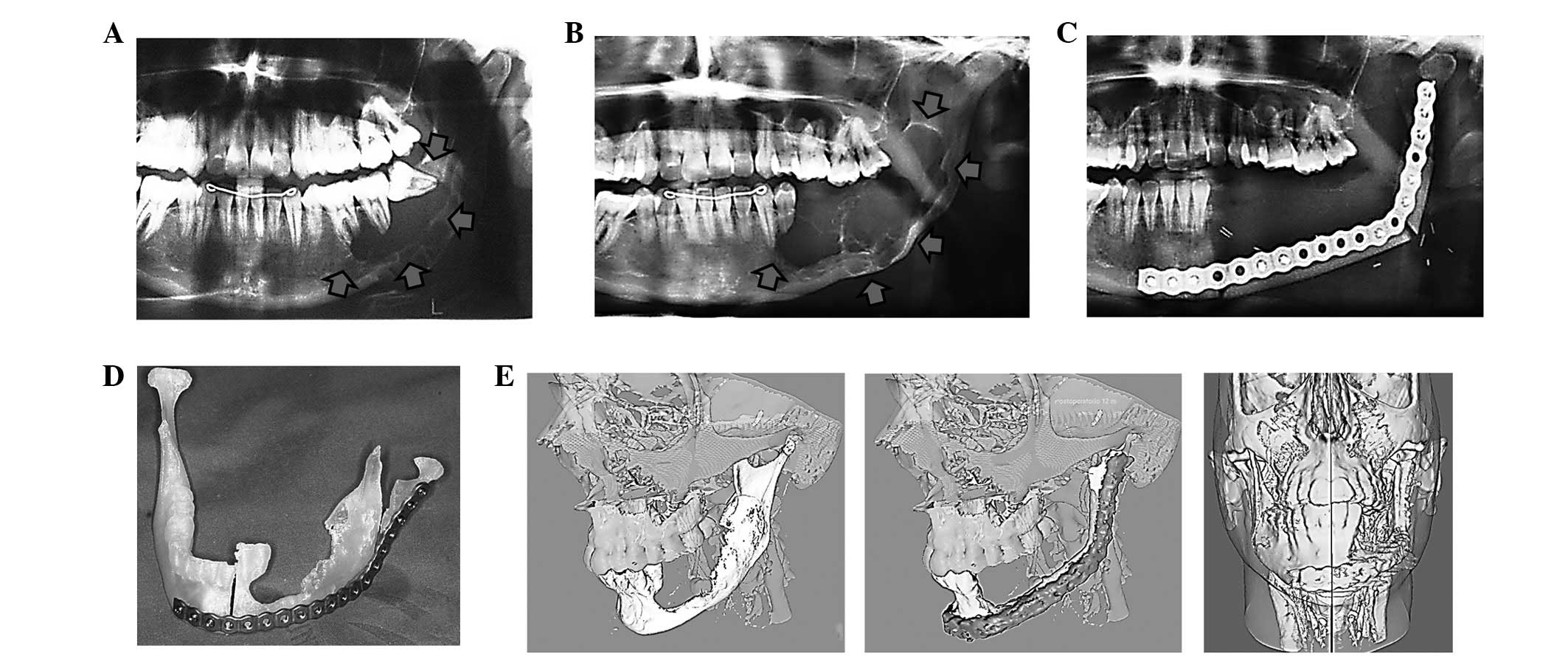
mandibulectomies (4 cases) (Figs.
1–3). In 6 cases, bone
reconstruction was performed primarily (3 patients with cortical
iliac crest grafts, 1 patient with cancellous iliac crest bone
graft, 1 patient with proximal tibia bone graft and 1 patient with
fibula free flap). No further recurrences were observed after the
second operation. In 3 patients dental implants were placed
posteriorly and implant-supported fixed prosthesis were
constructed. Three-dimensional reconstruction of preoperative
planning and outcome following surgical treatment was performed
using AYRA software (formerly VirSSPA, Andalusian Health Service,
Seville, Spain) (Fig. 3E).
Discussion
Mandibular ameloblastoma management remains a
subject of debate. The preferred treatment is surgical excision.
However, there is no unanimous consensus with regards to the extent
and type of surgery. While the main objective remains to achieve a
complete resection, in order to prevent tumor recurrence, the focus
of various investigations has been on how to achieve this without
performing a disproportionate surgery, for which it is necessary to
assess the location, size and type of ameloblastoma, as well as the
age of the patient. Currently, conservative local treatment appears
to be acceptable in young, growing patients, in order to minimize
the psychological impact of an aggressive resection and future
functional or growth problems, and in elderly patients to avoid
major surgical complications. It is also acceptable in unicystic
luminal ameloblastomas, if the tumor has not spread beyond the
basement membrane of the cyst, and in those lesions treated without
a previously accurate diagnosis (6). Extensive surgical treatment is
recommended in large or aggressive ameloblastomas (multicystic)
with evidence of cortical bone infiltration or soft tissue
extension (13,14).
The sample of 31 ameloblastomas used in the present
study reproduce the relatively uniform documented data previously
reported with regards to epidemiology, clinical features, treatment
and outcomes (4,15–17).
The mandibular molar region was the most common location and, in
terms of patient age, the group fell within the age ranges reported
in the literature. Surgical treatment consisted of conservative
treatment in 65% of cases and an en-bloc bone resection (marginal,
22.5% and segmental, 12.5%) in the remaining cases. In accordance
with the literature, a more conservative approach to unicyst
lesions, which could be treated with simple enucleation and/or
curettage, was preferred in young patients (18). In solid and multicystic
ameloblastomas we followed the procedure recommended most in the
literature, i.e., radical resection including a healthy bone margin
of at least 1 cm (19–21).
Recurrence after initial surgical treatment is the
result of the infiltrative growth of the ameloblastoma through the
adjacent bone, responsible for the local bone cancellous invasion
beyond the radiographically visible margins. To a large extent,
recurrence is the result of performing an inadequate initial
procedure. The recurrence rate should be assessed in a large
sample, during a prolonged postoperative period, and varies with
regard to location, tumor histology and radicality of the surgical
resection. Kim and Jang (22) and
Escande et al (2) reported
an overall recurrence rate of 21.1% and 45%, respectively. In the
present study, the overall rate of recurrence was 29%. Hong et
al (20), in a retrospective
analysis of 239 patients with ameloblastomas, reported a recurrence
rate of 4.5% after treatment by segmental resection or
maxillectomy, 11.6% after marginal resection and 29.3% after
conservative treatment (enucleation, curettage and
marsupialization), obtaining a statistically significant
correlation between method of treatment and recurrence. Attempts
have been made to use various markers to differentiate the types of
ameloblastoma and prevent recurrences, although this has not yet
yielded encouraging results (23).
At present, the prognosis of recurrence appears to be associated
with the surgical planning prior to evaluation of the histological
subtype (1,17,21).
In our study, all ameloblastomas that recurred had initially
undergone local procedures regardless of type of ameloblastoma.
Radical and aggressive surgery is the preferred
option for recurrent ameloblastoma management (4,7,21).
This method supports that the mandibular resection should be at
least 1–2 cm beyond the radiological limit to ensure that all
microlesions are removed (7). In 4
cases a radical treatment option, by means of a segmental
mandibulectomy, was selected due to aggressive features (cortical
bone perforation, tumor extension, infiltration of the dental
nerve, and multicystic type). In all other cases a marginal
mandibulectomy was selected, taking into account the preservation
of surrounding anatomical structures including dental nerve and
basal mandibular cortex.
Mandibular reconstruction is necessary following
tumor resection resulting in severe defects of mandibular arch
continuity and sacrifice of teeth. Basic reconstruction involves
the use of non-vascularized bone grafts together with restoration
of lost teeth by means of dental implants and implant-supported
prostheses (13,24,25).
In 3 cases presenting with <5 cm mandibular segmental defect,
reconstruction was achieved using a non-vascularized iliac crest
graft. By contrast, in cases where bone resection results in a
severe defect of continuity, reconstruction using a
micro-vascularized free flap is required (26,27).
In the present study, an 8 cm defect involving the body, angle and
ramus of the mandible was reconstructed using a fibula free flap.
Surgical planning in this particular case was performed using the
software VirSSPA AYRA based on the images from CT angiography,
which allowed creation of a preoperative stereolithographic model
to shape the titanium plate and perform the virtual surgical
simulation of bone resection. A template, made using rapid
prototyping technology, was used to carry out the fibula
osteotomies (28). In marginal
mandibulectomies bone regeneration can be expected following
preservation of the mandibular basal cortex, particularly in young
patients. In 2 patients a cancellous graft, obtained from the
proximal tibia and iliac crest respectively, was used to restore
the bone defect following a marginal mandibulectomy. In 3 cases,
restoration of missing teeth was achieved by secondary dental
implant placement in the bone grafts and by using implant-supported
prostheses. Previous studies have demonstrated the advantages of
implant placement and subsequent restoration with prostheses for
the rehabilitation of oral competence, mastication, speech and
facial contour in patients with mandibular defects (25,29,30).
In conclusion, ameloblastoma is a benign, locally
invasive odontogenic tumor with a high rate of long-term recurrence
associated with an inappropriate initial therapeutic approach. When
detecting tumor recurrence, the ideal treatment method is bone
resection with a safety margin of at least 1 cm beyond the
radiographically visible margins. This may be performed by either
marginal or segmental mandibulectomy depending on the location and
extent of recurrence. Immediate reconstruction of the bone defect
with free grafts or flaps, placement of dental implants and
rehabilitation with implant-supported prostheses in a second stage
can improve jaw function and facial harmony of the patient.
Acknowledgements
The authors would like to acknowledge
CIBER-BBN, an initiative funded by the VI National R&D&i
Plan 2008–2011, Iniciativa Ingenio 2010, Consolider Program, CIBER
Actions and financed by the Instituto de Salud Carlos III with
assistance from the European Regional Development Fund.
References
|
1.
|
Ghandhi D, Ayoub AF, Pogrel MA, MacDonald
G, Brocklebank LM and Moos KF: Ameloblastoma: a surgeon's dilemma.
J Oral Maxillofac Surg. 64:1010–1014. 2006.
|
|
2.
|
Escande C, Chaine A, Menard P, et al: A
treatment algorithm for adult ameloblastomas according to the
Pitié-Salpêtrière Hospital experience. J Craniomaxillofac Surg.
37:363–369. 2009.PubMed/NCBI
|
|
3.
|
Torres-Lagares D, Infante-Cossio P,
Hernandez-Guisado JM and Gutiérrez-Pérez JL: Mandibular
ameloblastoma. A review of the literature and presentation of six
cases. Med Oral Patol Oral Cir Bucal. 10:231–238. 2005.PubMed/NCBI
|
|
4.
|
Fregnani ER, da Cruz Perez DE, de Almeida
OP, Kowalski LP, Soares FA and de Abreu Alves F:
Clinicopathological study and treatment outcomes of 121 cases of
ameloblastomas. Int J Oral Maxillofac Surg. 39:145–149. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Varkhede A, Tupkari JV and Sardar M:
Odontogenic tumors: a study of 120 cases in an Indian teaching
hospital. Med Oral Patol Oral Cir Bucal. 16:e895–e899. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Black CC, Addante RR and Mohila CA:
Intraosseous ameloblastoma. Oral Surg Oral Med Oral Pathol Oral
Radiol Endod. 110:585–592. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Becelli R, Morello R, Renzi G, Matarazzo G
and Dominici C: Treatment of recurrent mandibular ameloblastoma
with segmental resection and revascularized fibula free flap. J
Craniofac Surg. 22:1163–1165. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Junquera L, Ascani G, Vicente JC,
Garcia-Consuegra L and Roig P: Ameloblastoma revisited. Ann Otol
Rhinol Laryngol. 112:1034–1039. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Chaine A, Pitak-Arnnop P, Dhanuthai K,
Ruhin-Poncet B, Bertrand JC and Bertolus C: A treatment algorithm
for managing giant mandibular ameloblastoma: 5-year experiences in
a Paris university hospital. Eur J Surg Oncol. 35:999–1005.
2009.
|
|
10.
|
Pogrel MA and Montes DM: Is there a role
for enucleation in the management of ameloblastoma? Int J Oral
Maxillofac Surg. 38:807–812. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Sammartino G, Zarrelli C, Urciuolo V, et
al: Effectiveness of a new decisional algorithm in managing
mandibular ameloblastomas: a 10-years experience. Br J Oral
Maxillofac Surg. 45:306–310. 2007.PubMed/NCBI
|
|
12.
|
Mendenhall WM, Werning JW, Fernandes R,
Malyapa RS and Mendenhall NP: Ameloblastoma. Am J Clin Oncol.
30:645–348. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Mareque Bueno J, Mareque Bueno S, Pamias
Romero J, Bescos Atin MS, Huguet Redecilla P and Raspall Martin G:
Mandibular ameloblastoma. Reconstruction with iliac crest graft and
implants. Med Oral Patol Oral Cir Bucal. 12:E73–E75.
2007.PubMed/NCBI
|
|
14.
|
Belli E, Rendine G and Mazzone N:
Ameloblastoma relapse after 50 years from resection treatment. J
Craniofac Surg. 20:1146–1149. 2009.PubMed/NCBI
|
|
15.
|
Eckardt AM, Kokemüller H, Flemming P and
Schultze A: Recurrent ameloblastoma following osseous
reconstruction - a review of twenty years. J Craniomaxillofac Surg.
37:36–41. 2009.PubMed/NCBI
|
|
16.
|
Tamme T, Soots M, Kulla A, et al:
Odontogenic tumors, a collaborative retrospective study of 75 cases
covering more than 25 years from Estonia. J Craniomaxillofac Surg.
32:161–165. 2004.PubMed/NCBI
|
|
17.
|
Hertog D, Bloemena E, Aartman IH and van
der Waal I: Histopathology of ameloblastoma of the jaws; some
critical observations based on a 40 years single institution
experience. Med Oral Patol Oral Cir Bucal. 17:e76–e82.
2012.PubMed/NCBI
|
|
18.
|
Carlson ER and Marx RE: The ameloblastoma:
primary, curative surgical management. J Oral Maxillofac Surg.
64:484–494. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Nakamura N, Mitsuyasu T, Higuchi Y, Sandra
F and Ohishi M: Growth characteristics of ameloblastoma involving
the inferior alveolar nerve. A clinical and histopathologic study.
Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 91:557–562. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Hong J, Yun PY, Chung IH, et al: Long-term
follow up on recurrence of 305 ameloblastoma cases. Int J Oral
Maxillofac Surg. 36:283–288. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Hertog D, Schulten EA, Leemans CR, Winters
HA and Van der Waal I: Management of recurrent ameloblastoma of the
jaws; a 40-year single institution experience. Oral Oncol.
47:145–146. 2011.PubMed/NCBI
|
|
22.
|
Kim SG and Jang HS: Ameloblastoma: a
clinical, radiographic, and histopathologic analysis of 71 cases.
Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 91:649–653. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Coleman H, Altini M, Ali H, Doglioni C,
Favia G and Maiorano E: Use of calretinin in the differential
diagnosis of unicystic ameloblastomas. Histopathology. 38:312–317.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Nakamura N, Higuchi Y, Mitsuyasu T, Sandra
F and Ohishi M: Comparison of long-term results between different
approaches to ameloblastoma. Oral Surg Oral Med Oral Pathol Oral
Radiol Endod. 93:13–20. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Chiapasco M, Colletti G, Romeo E, Zaniboni
M and Brusati R: Long-term results of mandibular reconstruction
with autogenous bone grafts and oral implants after tumor
resection. Clin Oral Implants Res. 19:1074–1080. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Chana JS, Chang YM, Wei FC, et al:
Segmental mandibulectomy and immediate free fibula
osteoseptocutaneous flap reconstruction with endosteal implants: an
ideal treatment method for mandibular ameloblastoma. Plast Reconstr
Surg. 113:80–87. 2004. View Article : Google Scholar
|
|
27.
|
Zemann W, Feichtinger M, Kowatsch E and
Kärcher H: Extensive ameloblastoma of the jaws: surgical management
and immediate reconstruction using microvascular flaps. Oral Surg
Oral Med Oral Pathol Oral Radiol Endod. 103:190–196. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Infante-Cossio P, Gacto-Sanchez P,
Gomez-Cia T and Gomez-Ciriza G: Stereolithographic cutting guide
for fibula osteotomy. Oral Surg Oral Med Oral Pathol Oral Radiol.
113:712–713. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Chiapasco M, Abati S, Ramundo G, Rossi A,
Romeo E and Vogel G: Behaviour of implants in bone grafts or free
flaps after tumor resection. Clin Oral Implants Res. 11:66–75.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Tözüm TF, Sönmez E, Askin SB, Tulunoglu I
and Safak T: Implant stability and peri-implant parameters in free
vascularized iliac graft transplantation patients: report of three
ameloblastoma cases. J Periodontol. 82:329–335. 2011.PubMed/NCBI
|