Introduction
Acute pain occurs as a result of tissue damage,
often accidentally due to an injury or surgery. Acute postoperative
pain is a manifestation of inflammation due to tissue injury. The
management of postoperative pain and inflammation is a critical
component of patient care (1).
Non-steroidal anti-inflammatory drugs (NSAIDs) are
commonly used in the management of post-operative pain. NSAIDs
inhibit cyclooxygenase (COX) enzymes, which are involved in the
synthesis of prostaglandins and thereby reduce pain and
inflammation. The inhibition of COX is the principal mechanism for
the efficacy and the toxicity of NSAIDs (2) and it has been demonstrated that COX
exists as at least two isoenzymes, COX-1 and COX-2 (3). Traditional NSAIDs non-specifically
inhibit COX-1 and COX-2, whereas specific COX-2 inhibitors only
affect the activity of COX-2. The major reason for development of
specific COX-2 inhibitors was the maintenance of the
anti-inflammatory and analgesic effects without altering the
homeostatic functions of COX-1 (4). To represent an attractive alternative
for patients requiring NSAIDs perioperatively, the selective COX-2
inhibitors, besides their improved side-effect profile, should have
an equipotent analgesic efficacy relative to traditional
NSAIDs.
Furthermore, oral NSAIDs are used post-operatively;
however, when patients are unable to tolerate oral medications or
require a faster onset of analgesia, parenteral administration may
be preferred. Parecoxib is a COX-2 selective inhibitor, which may
be administered as an intravenous or intramuscular injection for
the short-term management of postoperative pain. It is a prodrug
(the parent drug is inactive) that is rapidly hydrolysed in
vivo to its active form, valdecoxib (5). Clinical trials have indicated that
parecoxib is effective in treating postoperative pain resulting
from oral surgery, orthopedic surgery and abdominal hysterectomy
pain. Other studies have demonstrated no significant effects on
platelet function or upper gastrointestinal mucosa (6–9). As
a result, parecoxib sodium has been approved in European countries
for the treatment of postoperative pain.
The combination of PCA and the selective COX-2
inhibitor parecoxib has reportedly been used for acute
postoperative pain for years in European countries; however, the
efficacy and safety of the combination has not yet been
investigated. Therefore, to investigate the efficacy and safety
profile of the combination of PCA and parecoxib for postoperative
analgesic effects, we conducted a meta-analysis of randomized
controlled trials (RCTs).
Materials and methods
Search sources and strategy
The search strategy was produced according to
working handbook version 4.2.7 from the Cochrane collaboration
(10). Studies were identified by
extensively searching PubMed, Cochrane Central Register of
Controlled Trials, EBSCO, Springer, Ovid and Chinese National
Knowledge Infrastructure (CNKI) databases from January 1999 to
January 2013. In addition, a manual search of abstracts from
selected conferences was conducted, as well as a search by hand of
the bibliographies of all relevant trials. The following search
criteria were used: ‘parecoxib sodium’, ‘cyclooxygenase-2
inhibitor’ and ‘RCTs’. The language of the studies was not
restricted to English.
Study selection
Two reviewers independently conducted the literature
search and extraction of relevant articles. The title and abstract
of potentially relevant studies were screened for appropriateness
before retrieval of the full articles. Any disagreement concerning
study selection or data extraction was resolved by consensus with
the third reviewer. For meta-analysis, all studies had to meet the
following inclusion criteria: i) a study described as an RCT; ii)
patients with no statistically significant differences in baseline
characteristics; iii) intervention: a) treatment group, PCA
combined with parecoxib sodium (successively injected for <3
days) intravenously at 40+20/40 mg bid; b) control group, same
volume of saline; and iv) outcome variables: a) according to
patients’ global evaluation of study medication (PGESM), pain
relief 24, 48 and 72 h after the initial intravenous dose of 40 mg
parecoxib was assessed on a four-point scale (0, none; 1, a little
or some; 2, a lot; and 3, complete; scores 1 and 2 were defined as
‘ineffective’ and 3 and 4 were defined as ‘effective’); b) adverse
reactions of opioids, including respiratory depression, pruritus,
fever, headache, nausea and vomiting.
The exclusion criteria were as follows: i) a single
injection of parecoxib sodium before PCA; and ii) PCA not combined
with parecoxib sodium following surgery.
Data extraction and assessment of study
quality
Two of the authors independently extracted data from
the trials that met the inclusion criteria. Authors were contacted
for missing data when necessary. From each study, the following
information was extracted: author, year of publication, sample size
and intervention measures.
Quality assessment of the RCTs included in the
meta-analysis was independently performed by the same reviewers
according to the Cochrane Handbook 5.0.1 and Juni et al
(11,12). Jadad grade was evaluated using the
following items: i) was the study a randomized trial; ii) was the
randomization scheme described and appropriate; iii) was the study
described as double-blinded; iv) was the method of double-blinding
appropriate; v) was there a description of allocation concealment;
vi) was there a description of dropouts and withdrawals; and (vii)
did the patients have statistically significant differences in
baseline characteristics. Each author rated the quality of the
trials using Jadad grade (maximum grade, A; minimum grade, C; grade
≥B, good quality).
Statistical analysis
Data were analyzed using Review Manager 5.1
(provided by The Nordic Cochrane Centre, The Cochrane
Collaboration). Included articles were pooled and weighted
(13). Relative risk (RR) and 95%
confidence interval (CI) were calculated in a random-effects model
or in a fixed-effects model. Heterogeneity was assessed by
χ2 test and the quantity of heterogeneity was measured
with I2 statistic. If heterogeneity (P<0.01 or
I2>50%) was identified among the trials, a
random-effects model was selected, otherwise a fixed-effects model
was selected. If heterogeneity was evident (I2>70%),
the inferior quality study was eliminated for analysis.
Results
Study characteristics
There were 121 articles relevant to the search terms
and seven articles (14–20) involving 1,939 patients (treatment
group, 1,207 patients; control group, 732 patients) were included
in this meta-analysis. The flow chart for the selection of RCTs is
shown in Fig. 1. The
characteristics of the included trials are shown in Table I.
 | Table I.Study characteristics and quality
assessment of included RCTs. |
Table I.
Study characteristics and quality
assessment of included RCTs.
| Study (ref.) | Sample size (n)
| Intervention measures
| Methodological
quality evaluation
|
|---|
| T | C | T | C | Randomization | Blinding | Allocation
concealed | Quality grade |
|---|
| Apfelbaum 2008
(13) |
T1:151 | 151 | T1: 40+20
mg qd i.v. prior before the end of surgery | NS | Computer
stochastic | Double blinding | Unclear | B |
|
T2:152 | | T2: 40+20
mg bid i.v. before the end of surgery | | | | | |
| Michael 2007
(14) | 211 | 203 | 40+20 mg bid i.v.
before the end of surgery | NS | Computer
stochastic | Double blinding | Unclear | B |
| Viscusi 2008
(15) |
T1:166 | 167 | T1:40+20
mg qd i.v. before the end of surgery | NS | Computer
stochastic | Double blinding | Unclear | B |
|
T2:167 | | T2: 40+20
mg bid i.v. before the end of surgery | | | | | |
| Tang 2002 (16) | T1:19 | 18 | T1: 20 mg
bid i.v. before the end of surgery | NS | Computer
stochastic | Double blinding | Unclear | B |
| T2:18 | | T2: 40 mg
bid i.v. before the end of surgery | | | | | |
| Malan 2003 (17) | T1:67 | 70 | T1: 20 mg
bid i.v. before the end of surgery | NS | Computer
stochastic | Double blinding | Unclear | B |
| T2:64 | | T2: 40 mg
bid i.v. before the end of surgery | | | | | |
| Jirarattanaphochai
2008 (18) | 60 | 60 | 40+40 mg bid i.v.
before the end of surgery | NS | Computer
stochastic | Double blinding | Unclear | B |
| Hubbard 2003
(19) | T1:65 | 63 | T1: 20 mg bid i.v.
before the end of surgery | NS | Computer
stochastic | Double
blinding | Unclear | B |
|
T2:67 | | T2: 40
mg bid i.v. before the end of surgery | | | | | |
Methodological quality assessment
The quality assessment of included RCTs is presented
in Table I. All the trials
included in this meta-analysis clarified adequate randomization
procedures, used double-blinding and reported numbers of
dropouts/withdrawals during the treatment; however, no study
reported allocation concealment clearly. According to the Jadad
score, all studies were eventually assessed to be good in terms of
methodology with Jadad score B.
Comparisons of effectiveness
Patient global evaluation 24 h after
the initial dose of parecoxib
Two studies (17,20)
(n=166) provided specific data for analysis of PGESM at 24 h after
surgery. We selected the fixed-effect model to perform the
meta-analysis since there were no significant heterogeneities
(effective, χ2=0.46, P=0.50, I2= 0%; ineffective,
χ2= 0.15, P= 0.70, I2= 0%). The incidence of
‘effective’ and ‘ineffective’ results in the analgesic effect
evaluation was significantly different between the two groups
[RR=1.41, 95% CI (1.13–1.75), P=0.002; and RR=0.43, 95% CI
(0.26–0.72), P=001, respectively; Fig.
2].
Patient global evaluation 48 h after
the initial dose of parecoxib
Three studies (15,16,19)
(n=868) provided specific data for analysis of PGESM at 48 h after
surgery. We selected the fixed-effect model to perform the
meta-analysis since there were no significant heterogeneities
(effective, χ2=1.73, P=0.42, I2= 0%; ineffective
χ2= 0.47, P= 0.79, I2= 0%). The incidence of
‘effective’ and ‘ineffective’ results in the analgesic effect
evaluation was significantly different between the two groups
[RR=1.25, 95% CI (1.15–1.35), P<0.00001; and RR=0.44, 95% CI
(0.34–0.57), P<0.00001, respectively; Fig. 3].
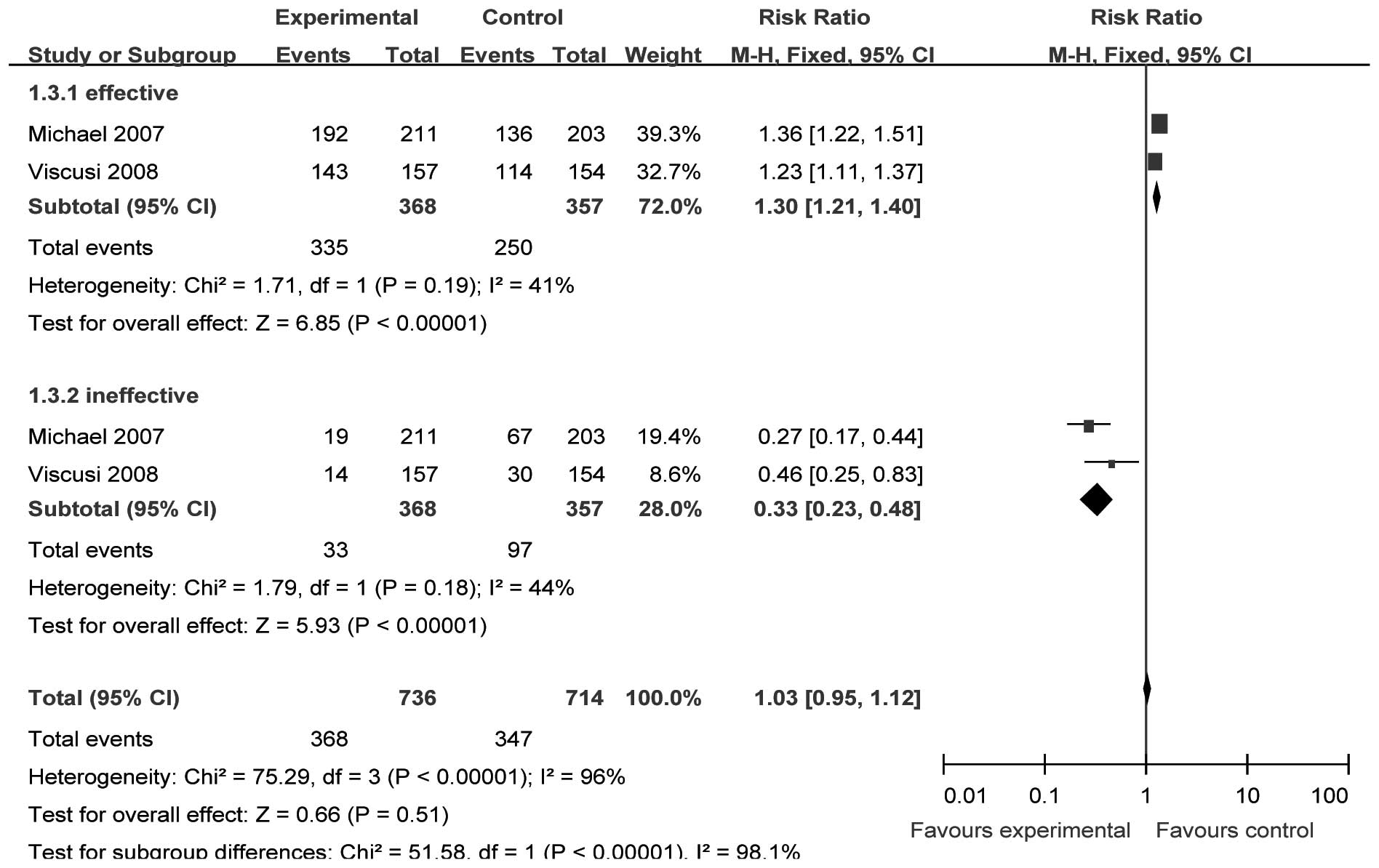
Patient global evaluation 72 h after
the initial dose of parecoxib
Two studies (15,16)
(n=748) provided specific data for analysis of PGESM at 72 h after
surgery. We selected the fixed-effect model to perform the
meta-analysis since there were no significant heterogeneities
(effective, χ2=1.71, P= 0.19, I2= 41.4%;
ineffective χ2=1.79, P=0.18, I 2= 44.2%). The
incidence of ‘effective’ and ‘ineffective’ results in the analgesic
effect evaluation was significantly different between the two
groups [RR=1.30, 95% CI (1.21–1.40), P<0.00001; and RR=0.33, 95%
CI (0.23–0.48), P<0.00001, respectively; Fig. 4].
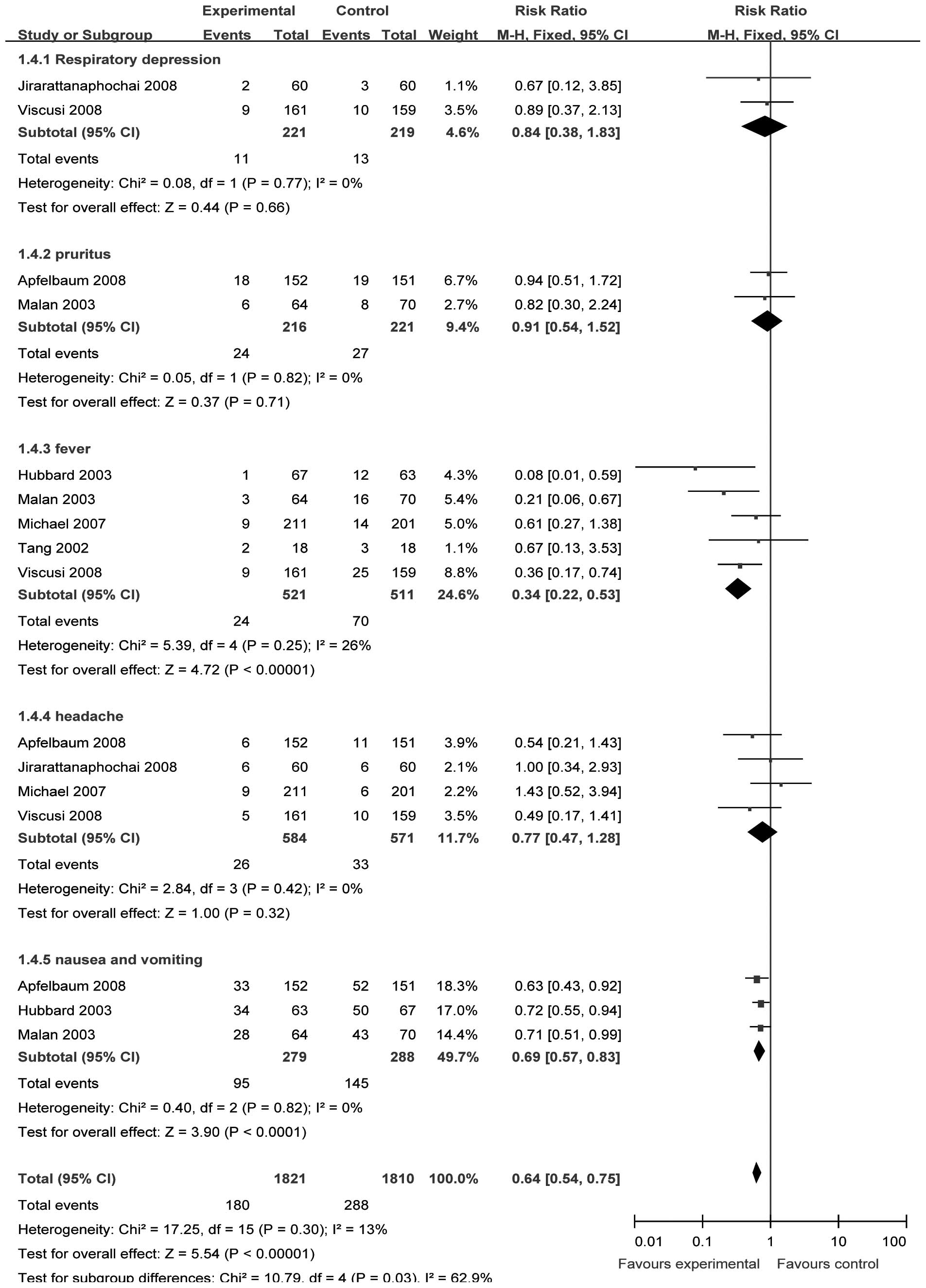
Comparisons of safety
We selected the fixed-effect model to perform the
meta-analysis since there were no significant heterogeneities
(respiratory depression, χ2= 0.08, P= 0.77, I2= 0%;
pruritus, χ2= 0.05, P= 0.82, I2= 0%; fever,
χ2=5.39, P= 0.25, I2=25.8%; headache,
χ2=2.84, P=0.42, I2=0%; nausea and vomiting,
χ2= 0.40, P= 0.82, I2=0%). Two (15,18)
(n=454), two (14,18) (n=437) and four studies (14–16,19)
(n=1171) provided data of respiratory depression, pruritus and
headache, respectively. The incidence of respiratory depression,
pruritus and headache between the treatment and control groups was
not significantly different [RR= 0.84, 95% CI (0.38–1.83), P=0.66;
RR=0.91, 95% CI (0.54–1.52), P=0.71; and RR=0.77, 95% CI
(0.47–1.28), P=0.32, respectively; Fig. 5].
Five (15–18,20)
(n=1048) and three studies (14,18,20)
(n=567) provided data on fever, and nausea and vomiting,
respectively. The incidences of fever, and nausea and vomiting
between the treatment and control groups were significantly
different [RR= 0.34, 95% CI (0.22–0.53), P<0.00001; and RR=0.69,
95% CI (0.57–0.83), P<0.00001, respectively; Fig. 5].
Discussion
NSAIDs are known to induce analgesia mainly via
inhibition of COX. Parecoxib exhibits anti-inflammatory, analgesic
and antipyretic properties in animal models and humans due to
inhibition of prostanoid synthesis primarily by affecting COX-2.
Although the inhibition of COX in the periphery is commonly
accepted as the primary mechanism, experimental and clinical data
suggest a potential role for central COX inhibition to produce
antinociception and reduce hypersensitivity. Additionally, it has
double analgesic actions (21).
Multimodal analgesia, where opioids, including morphine, are
administered with a non-opioid, is often used to reduce
opioid-related adverse effects, including postoperative nausea and
vomiting, drowsiness, respiratory depression and gastrointestinal
and bladder dysfunction (22). The
underlying principle is that the different modes of action of
morphine and the non-opioid drug allow optimum analgesia to be
maintained with a lower dose of morphine and consequently a lower
incidence of morphine-related adverse effects (23).
We conducted the current meta-analysis to compare
the efficacy and safety of parecoxib sodium plus PCA with PCA alone
for acute postoperative pain. The results of the meta-analysis
indicated that the efficacy of PCA combined with parecoxib sodium
(successively injected for <3 days intravenously) was superior
to that of PCA alone with a statistically significant difference.
After 24, 48 and 72 h of the initial dose of 40 mg parecoxib i.v.,
the percentage of ‘effective’ treatment as described by PGESM was
higher compared with that of the control group; the percentage of
‘ineffective’ treatment was lower compared with that of the control
group. Moreover, PCA plus parecoxib sodium reduced the incidence of
postoperative fever, nausea and vomiting; however, it did not
significantly reduce the incidence of respiratory depression,
pruritus and headache. The incidence of postoperative bleeding,
urinary retention, digestive tract ulcer, pulmonary embolism,
massive hemorrhage and cardiovascular events in all the included
studies were extremely low, which demonstrated an improved security
of parecoxib sodium. However, a previous study indicated that there
was a reduction in 24-h morphine consumption, leading to a
reduction in morphine-related adverse effects when COX-2 inhibitors
were administered in addition to PCA morphine following surgery,
with no clear difference between them (23). Therefore, our findings indicate
that parecoxib sodium may be beneficial in pain relief following
surgery; however, further studies are required to confirm this.
Certain limitations affecting the results of the
current meta-analysis should be taken into account. Firstly, our
findings may be affected by the quality of trials included in the
meta-analysis. A well-designed randomized controlled trial requires
a thorough understanding of randomization so that better results
are achieved. However, none of the included trials clarified
allocation concealment clearly. All studies were only assessed to
be a Jadad score B in terms of methodology. Secondly, this
meta-analysis is based on a relatively small number of RCTs and we
acknowledge that using a limited number of studies raises the
possibility of a second-order sampling error (24). Thirdly, the distinct differences in
administration times, dose, treatment course, different surgery and
initial pain level of patients used exist (Table I), which may affect the consistency
of effects across those included studies.
In conclusion, although certain limitations exist in
this meta-analysis, based on the results of our meta-analysis, we
identified that parecoxib is an effective and relatively safe
option for acute postoperative pain. However, further high quality
RCTs are required to determine the long-term effects of parecoxib
for postoperative pain.
References
|
1.
|
Barden J, Derry S, McQuay HJ and Moore RA:
Single dose oral ketoprofen and dexketoprofen for acute
postoperative pain in adults. Cochrane Database Syst Rev.
4:CD0073552009.PubMed/NCBI
|
|
2.
|
Vane JR: Inhibition of prostaglandin
synthesis as a mechanism of action for the aspirin-like drugs. Nat
New Biol. 231:232–235. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Needleman P and Isakson PC: The discovery
and function of COX-2. J Rheumatol Suppl. 49:6–8. 1997.
|
|
4.
|
Rømsing J and Møiniche S: A systematic
review of COX-2 inhibitors compared with traditional NSAIDs, or
different COX-2 inhibitors for post-operative pain. Acta
Anaesthesiol Scand. 48:525–546. 2004.PubMed/NCBI
|
|
5.
|
Lloyd R, Derry S, Moore RA and McQuay HJ:
Intravenous or intramuscular parecoxib for acute postoperative pain
in adults. Cochrane Database Syst Rev. 2:CD0047712009.PubMed/NCBI
|
|
6.
|
Graff J, Arabmotlagh M, Cheung R,
Geisslinger G and Harder S: Effects of parecoxib and dipyrone on
platelet aggregation in patients undergoing meniscectomy: a
double-blind, randomized, parallel-group study. Clin Ther.
29:438–447. 2007. View Article : Google Scholar
|
|
7.
|
Harris SI, Stoltz RR, LeComte D and
Hubbard RC: Parecoxib sodium demonstrates gastrointestinal safety
comparable to placebo in healthy subjects. J Clin Gastroenterol.
38:575–580. 2004. View Article : Google Scholar
|
|
8.
|
Noveck RJ, Laurent A, Kuss M, Talwalker S
and Hubbard RC: Parecoxib sodium does not impair platelet function
in healthy elderly and non-elderly individuals: two randomised,
controlled trials. Clin Drug Investig. 21:465–476. 2001. View Article : Google Scholar
|
|
9.
|
Stoltz RR, Harris SI, Kuss ME, LeComte D,
Talwalker S, Dhadda S and Hubbard RC: Upper GI mucosal effects of
parecoxib sodium in healthy elderly subjects. Am J Gastroenterol.
97:65–71. 2002. View Article : Google Scholar
|
|
10.
|
Sackett DL, Clarke M and Oxman AD:
Cochrane Reviewers, Handbook 4.2. Renew Manager. Versions 4.2
Oxford, England: The cochrane collaboration; pp. 13–36. 2002
|
|
11.
|
Jüni P, Altman DG and Egger M: Systematic
reviews in health care: Assessing the quality of controlled
clinical trials. BMJ. 323:42–46. 2001.
|
|
12.
|
Jadad AR, Moore RA, Carroll D, Jenkinson
C, Reynolds DJ, Gavaghan DJ and McQuay HJ: Assessing the quality of
reports of randomized clinical trials: is blinding necessary?
Control Clin Trials. 17:1–12. 1996. View Article : Google Scholar
|
|
13.
|
Jüni P and Egger M: PRISMAtic reporting of
systematic reviews and meta-analyses. Lancet. 374:1221–1223.
2009.
|
|
14.
|
Apfelbaum JL, Desjardins PJ, Brown MT and
Verburg KM: Multiple-day efficacy of parecoxib sodium treatment in
postoperative bunionectomy pain. Clin J Pain. 24:784–792. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Michael C, Artur J, Kotarski J, Katz TK,
Brown MT and Verburg KM: Parecoxib sodium administered over several
days reduces pain after gynecologic surgery via laparotomy. J Clin
Anesth. 19:448–455. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Viscusi ER, Gimbel JS, Halder AM, Snabes
M, Brown MT and Verburg KM: A multiple-day regimen of parecoxib
sodium 20 mg twice daily provides pain relief after total hip
arthroplasty. Anesth Analg. 107:652–660. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Tang J, Li S, White PF, Chen X, Wender RH,
Quon R, Sloninsky A, Naruse R, Kariger R, Webb T and Norel E:
Effect of parecoxib, a novel intravenous cyclooxygenase type-2
inhibitor, on the postoperative opioid requirement and quality of
pain control. Anesthesiology. 96:1305–1309. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Malan TP Jr, Marsh G, Hakki SI, Grossman
E, Traylor L and Hubbard RC: Parecoxib sodium, a parenteral
cyclooxygenase-selective inhibitor, improves morphine analgesia and
is opioid-sparing following total hip arthroplasty. Anesthesiology.
98:950–957. 2003. View Article : Google Scholar
|
|
19.
|
Jirarattanaphochai K, Thienthong S, Sriraj
W, Jung S, Pulnitiporn A, Lertsinudom S and Foocharoen T: Effect of
parecoxib on postoperative pain after lumbar spine surgery. Spine.
33:132–139. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Hubbard RC, Naumann TM, Traylor L and
Dhadda S: Parecoxib sodium has opioid-sparing effects in patients
undergoing total knee arthroplasty under spinal anaesthesia. Br J
Anaesth. 90:166–172. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Koppert W, Wehrfritz A, Körber N, Sittl R,
Albrecht S, Schüttler J and Schmelz M: The cyclooxygenase isozyme
inhibitors parecoxib and paracetamol reduce central hyperalgesia in
humans. Pain. 108:148–153. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Beaulieu P: Non-opioid strategies for
acute pain management. Can J Anaesth. 54:481–485. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Maund E, McDaid C, Rice S, Wright K,
Jenkins B and Woolacott N: Paracetamol and selective and
non-selective non-steroidal anti-inflammatory drugs for the
reduction in morphine-related side-effects after major surgery: a
systematic review. Br J Anaesth. 106:292–297. 2011. View Article : Google Scholar
|
|
24.
|
Higgins J, Thompson S, Deeks J and Altman
D: Statistical heterogeneity in systematic reviews of clinical
trials: a critical appraisal of guidelines and practice. J Health
Serv Res Policy. 7:51–61. 2002. View Article : Google Scholar : PubMed/NCBI
|