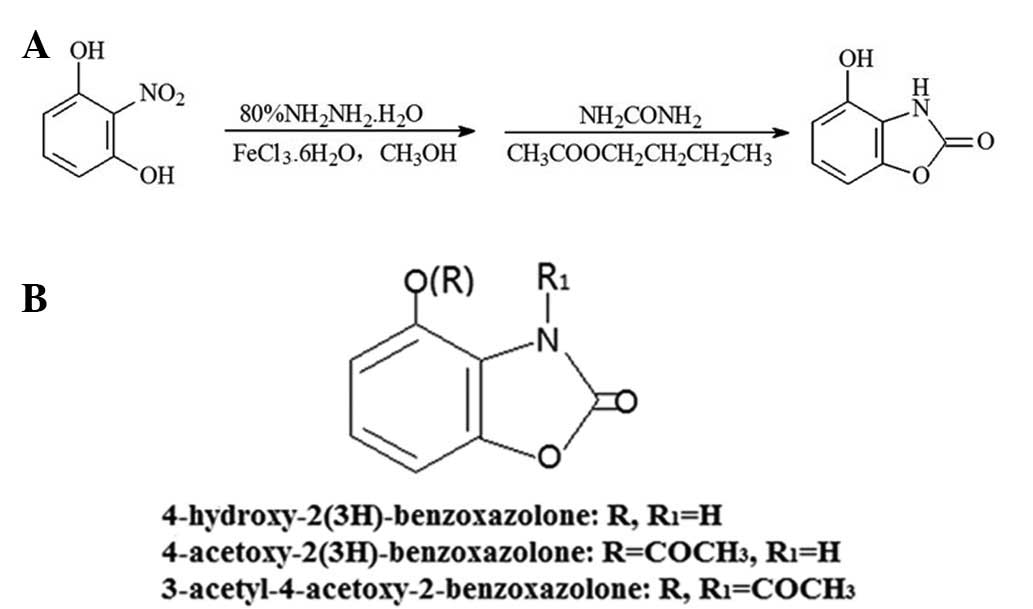
Introduction
The liver is a vital organ in mammals, including
rodents. Hepatotoxic agents may cause severe damage to hepatocytes;
not only do they potentially catabolize radical-induced lipid
peroxidation, damage the membranes of liver cells and organelles
and cause swelling and necrosis of hepatocytes, but they may also
induce an inflammatory response and release mediators of
inflammation (1). CCl4
is used as a model agent for inducing acute liver injury in mice.
CCl4-induced liver injury is associated with the
aggravation of lipid peroxidation and the attenuation of
antioxidant enzyme function (2).
These changes result from the formation of reactive intermediates
such as trichloromethyl (•CCl3) free radicals
and reactive oxygen species (ROS) (3). Furthermore, CCl4-induced
liver injury has been reported to cause significant increases in
the levels of alanine aminotransferase (ALT), aspartate
aminotransferase (AST), lactic dehydrogenase (LDH) and
malondialdehyde (MDA) and reductions in the levels of superoxide
dismutase (SOD), catalase (CAT), glutathione (GSH) and glutathione
peroxidase (Gpx) (4). Therefore,
reducing lipid peroxidation or oxidative stress may be an effective
therapeutic strategy for preventing and treating
CCl4-induced liver injury.
Acanthus ilicifolius L. (Acanthaceae)
has spinous edges to its evergreen leaves and stipular spines at
the stem nodes. It is a plant found in marshy habitats throughout
the mangroves of China, including those of the Guangxi region and
Guangdong and Fujian provinces, and in various Asian countries such
as India, Burma and Thailand. In traditional Chinese medicine,
A. ilicifolius is used to treat inflammation, hepatitis,
swollen spleens, asthma, gastralgia and malignant tumors. Previous
studies have reported that the crude alcoholic extract of A.
ilicifolius exhibits antioxidant, hepatoprotective (5), antitumor (6) and antimicrobial activities (7).
Acanthus ilicifolius is protected in China.
Acanthus ilicifolius alkaloid A
(4-hydroxy-2(3H)-benzoxazolone, HBOA) has been separated from the
Acanthus ilicifolius plant in the pharmaceutical chemistry
laboratory of Guangxi Medical University. Moreover, in order to
avoid the exploitation of the mangroves, we attempted to synthesize
HBOA and successfully synthesized the core heterocyclic nucleus,
2(3H)-benzoxazolone (BOA) of HBOA. Benzoxazolone heterocycles are
considered to be ‘privileged scaffolds’ in the design of
pharmacological probes. Medicinal chemists have paid considerable
attention to these units due to their capacity for mimicking phenol
or catechol moieties in a metabolically stable template (8). Furthermore, 3-substituted BOA
derivatives with more potent anti-inflammatory activity than
aspirin have been reported (9).
Therefore, HBOA, as a lead compound, may be modified to synthesize
novel drugs with pharmacological activities.
There are a number of methods that may be used for
the synthesis of BOA, including reactions between 2-aminophenol and
urea phosgene or carbon monoxide; the reaction with urea is
normally used in industry (10). A
method of synthesizing HBOA from 2-aminoresorcinol hydrochloride
and carbonyldiimidazole (CDI) by cyclization in dichloromethane,
and a method for synthesizing 4-acetoxy-2(3H)-benzoxazolone
(AcO-BOA) have been reported (11). The reaction scheme for HBOA is
presented in Fig. 1A.
Pharmacological studies of HBOA and its derivatives have been
reported, and describe its use as an anti-inflammatory agent
(12), analgesic (13,14),
antioxidant, anti-convulsant and as a Type 2 diabetes agent
(15–17). Our previous in vitro study
demonstrated that HBOA and its derivatives possess hepatoprotective
activity against the induction of apoptosis in hepatic stellate
cells (HSC-T6), and the two acyl derivatives were shown to be more
potent than HBOA (18). These
findings prompted us to conduct the current study to investigate
the hepatoprotective activity of HBOA and its acetylated
derivatives in mice.
Materials and methods
Materials
Melting points (M.p.) were determined using an
electrothermal melting point apparatus (Beijing Tech Instrument
Co., Ltd., Beijing, China) and are uncorrected. The IR spectra of
the compounds were recorded using a Spectrum One FT-IR spectrometer
(Perkin-Elmer, Waltham, MA, USA). NMR (1H and
13C) spectra were recorded on a Bruker AV-500 MHz NMR
spectrometer (Bruker BioSpin, Shanghai, China), and chemical shifts
are expressed in parts per million (ppm, for δ) relative to
tetramethylsilane (TMS) as an internal standard with
CDCl3 as the solvent. Spin multiplets are provided as s
(singlet), d (doublet), t (triplet), q (quartet) and m (multiplet).
All compounds reported in the present study had IR, 1H
and 13C-NMR data consistent with their structures. TLC
analyses were performed on TLC plates (silica gel,
GF254; Qingdao Haiyang Chemical Co., Ltd., Qingdao,
China).
Bifendate (Bif) was purchased from Guangzhou Xingqun
Pharmaceutical Co., Ltd. (Guangzhou, China); ALT, AST, LDH, MDA,
SOD, CAT, GSH and Gpx testing kits were obtained from Nanjing
Jiancheng Bioengineering Research Institute (Nanjing, China). The
tumor necrosis factor-α (TNF-α) kit was obtained from Wuhan Boster
Bio-engineering Co., Ltd (Wuhan, China).
Methods
Synthesis of 2-nitroresorcinol
The intermediate product, 2-nitroresorcinol was
synthesized by the reaction of resorcinol with a mixed acid
consisting of concentrated sulfuric acid and nitric acid according
to a method reported previously (19) (yield, 30.12%; M.p., 83–84°C).
Synthesis of HBOA
Activated carbon (18 g) was added to a solution of 8
g hexahydrated ferric chloride dissolved in 480 ml methanol and
refluxed for ~30 min. To this mixture, 62 g 2-nitroresorcinol was
added and the mixture was refluxed with stirring. Subsequently, 100
ml (75%) hydrazine hydrate was added dropwise to the mixture which
was then refluxed for 4–5 h. The mixture was filtered to remove the
activated carbon, and the solvent was evaporated under reduced
pressure. Upon obtaining a homogenous paste, 620 ml ethyl acetate
was added and the mixture was heated to 90°C. To the stirred
mixture, 144 g urea was added and refluxing was conducted for 4–5
h. Following the removal of solvent under reduced pressure, 500 ml
water was added and the mixture was refluxed twice for 30 min each.
A crude product was obtained through filtration and subsequent
drying. The product was extracted with n-butyl alcohol (3 × 800
ml). The extract was evaporated to dryness under reduced pressure
and the resulting light brown crystals were filtered and
recrystallized from ethanol
(C7H5NO3; yield, 63.08%; M.p.,
293-29°C). IR (KBr, cm−1), 3,289 (-OH, phenolic
hydroxy), 1,741 (C=O, lactam) and 1,659 (C=O, amide);
1H-NMR (500 MHz; CDCl3) δ (ppm), 6.76 (1H,
dd, H5), 6.74 (1H, dd, H7) and 6.94 (1H, m, H6).
Synthesis of AcO-BOA (TC-2)
To a stirred and cooled solution of 10.7 g HBOA in
750 ml acetone, at <5°C, was added 11.9 g triethylamine.
Subsequently, a solution of 6.9 g acetyl chloride in 20 ml of
acetone was added dropwise and the mixture was stirred for 5 h; the
temperature did not rise above 5°C. Stirring proceeded for a
further 5 h at room temperature and the mixture was left for a
further 24 h. Following evaporation to dryness, the white
precipitate of AcO-BOA was collected by filtration, washed with
water, dried and recrystallized from water
(C9H7NO4; yield, 68.22%; M.p.,
191–193°C). IR (KBr, cm−1), 3,127 (-OH, phenolic
hydroxy), 1,761 (C=O, lactam) and 1,639 (C=O, amide);
1H-NMR (500 MHz; CDCl3) δ (ppm), 7.15 (1H,
dd, H5), 6.99 (1H, dd, H7), 7.17 (1H, m, H6) and 2.07 (3H, s,
-CH3).
Synthesis of TC-3
To a solution of 11.0 g AcO-BOA in 750 ml acetone,
which was stirred and cooled at <5°C, was added 11.9 g
triethylamine. Then, 11.36 g acetyl chloride in 20 ml acetone was
added dropwise. The reaction mixture was stirred for 2 h at
<5°C. After stirring at room temperature for another day, the
solvent was removed under reduced pressure and the residue was
obtained in water and extracted with methenyl trichloride.
Trichloromethane was recovered and white crystals of TC-3 were
collected by filtration and recrystallized from ethanol
(C11H9NO5; yield, 64.62%; M.p.,
91–93°C). IR (KBr, cm−1), 1,787 (C=O, lactam) and 1,602
(C=O, amide); 1H-NMR (500 MHz; CDCl3) δ
(ppm), 7.12 (1H, dd, H5), 6.96 (1H, dd, H7), 7.25 (1H, m, H6), 2.71
(3H, s, phenyl-OCOCH3) and 2.32 (3H, s,
-NCOCH3).
Determination of compound purity
A Shimadzu HPLC system (Shimadzu, Tokyo, Japan) was
utilized for the experiments and consisted of the following
components: a 3500 pump, AS 3000 auto sampler, and PDAD 1000
detector. A welchrom-C18, 250×4.6 mm, 5 μm analytical column (Welch
Materials, Inc., Potomac, MD, USA) was used. The instrumental
settings were: flow rate, 1 ml/min; column oven temperature, 30°C;
detector wavelength, 215 nm, 215 nm and 210 nm, respectively; and
injection volume, 10 μl. Data acquisition was made using LCsolution
version 1.2 (Shimadzu). Peak purities were checked using a
photodiode array detector. The mobile phase consisted of methanol
and water (50:50, v/v). The mobile phases were filtered through a
0.45-μm nylon filter and degassed. The three compounds were
dissolved in the mobile phase.
Animals and treatment
Local Kunming mice of both genders, ranging from
18–22 g in weight, were provided by the Experimental Animal Center
of Guangxi Medical University (Guangxi, China). This study was
conducted in accordance with protocols approved by the
Institutional Ethics Committee of Guangxi Medical University.
The mice were divided randomly into various groups
containing ten mice in each group: the normal control group, the
sodium carboxymethyl cellulose (CMC-Na) group, the CCl4
model group, the Bif group and the HBOA, AcO-BOA and TC-3 (high
dose, middle dose and low dose) groups. The acute liver injury
model was established through the intraperitoneal injection of 0.1
ml/10 g body weight of 0.15% (v/v) CCl4 solution in
peanut oil to the mice, with the exception of the control and
CMC-Na groups. The synthesized compounds were administered orally
(at 200, 100 and 50 mg/kg) in 6% CMC solution and the Bif group
mice were treated with Bif (150 mg/kg) 8 h later. The mice in the
control group received the same volume of normal saline solution,
and the CMC-Na and model groups received the same volume of 6% CMC.
An additional dose of the treatment was administered after a
further 16 h. At 8 h after the last treatment the mice were
sacrificed by cervical dislocation. Serum samples were obtained
from the eyeballs of the mice and stored at −20°C. The livers were
removed from the mice and weighed. Liver tissue (0.2 mg) was
accurately weighed, homogenized and centrifuged (1,575 × g for 10
min). The supernatant was separated and stored at −20°C. The
remaining liver samples were fixed in 10% formaldehyde for
histological analysis.
Liver function and biochemical
assays
The liver index was calculated for each group using
the following formula: Liver index = [mouse liver weight (g)/mouse
weight (g)] × 100. The serum levels of ALT and AST were determined
using commercially available kits according to the manufacturer's
instructions. Levels of LDH in the serum and levels of MDA, SOD,
CAT, GSH and Gpx in the liver tissue were measured in accordance
with the kit manufacturer's instructions. The inhibition rate of
MDA was calculated as follows: (level in the model group - level in
the treatment group)/level in the model group × 100. The induction
rates of SOD and GSH were calculated as follows: (level in the
treatment group - level in the model group)/level in the model
group × 100.
Histopathology and
immunohistochemistry
Liver tissue sections fixed in 10% formaldehyde were
embedded in paraffin. Paraffin sections were stained with
hematoxylin and eosin (H&E). The TNF-α levels in the liver
tissues were measured using an immunohistochemical assay. The
degree of liver damage was examined blindly using an Olympus CX41
biological microscope (Tokyo, Japan).
Statistical analysis
Statistical analysis was performed using SPSS 13.0
for Windows (SPSS, Inc., Chicago, IL, USA). One way analysis of
variance (ANOVA) was used to compare the means among the groups.
Data are presented as the means ± SE. P<0.05 was considered to
indicate a statistically significant difference.
Results
Chemistry
HBOA and its derivatives were synthesized and the
purities of the products were determined. The chemical structures
of the products are presented in Fig.
1B. The purities of HBOA, AcO-BOA and TC-3 were 99.26, 94.42
and 93.73%, respectively.
Pharmacology
Liver index
The liver index in the model group was significantly
higher compared with that of the normal control group. However, the
liver indices in the high-dose HBOA and AcO-BOA and mid- and
high-dose TC-3 groups were markedly reduced compared with that of
the model group (Table I).
 | Table IEffect of HBOA, AcO-BOA and TC-3 on
the liver index in mice (%). |
Table I
Effect of HBOA, AcO-BOA and TC-3 on
the liver index in mice (%).
| Group | HBOA | AcO-BOA | TC-3 |
|---|
| Control | 4.56±0.51 | 4.93±0.41 | 5.59±0.34 |
| CMC-Na | 4.60±0.36 | 5.17±0.59 | 5.35±0.49 |
|
CCl4 | 5.28±0.45a | 5.66±0.75b | 6.06±0.46a |
| Bif | 4.61±0.35c | 4.83±0.30c | 5.29±0.18d |
| High-dose | 4.73±0.44d | 4.88±0.49d | 5.43±0.38d |
| Med-dose | 5.38±0.79 | 5.23±0.59 | 5.44±0.20d |
| Low-dose | 5.77±0.86 | 5.84±0.98 | 5.52±0.47c |
Effect of HBOA
The levels of serological indicators (AST, ALT, MDA)
were significantly higher in the model group compared with the
normal control group. Compared with the values in the model group,
high-dose HBOA induced significant reductions in the activities of
ALT and AST and increases in the activities of SOD, Gpx and CAT,
and mid- and high-dose HBOA induced significant reductions in the
levels of MDA and LDH and increases in the activity of GSH
(Fig. 2Aa; Table II). The expression of TNF-α was
significantly reduced by high-dose HBOA (Fig. 3).
 | Figure 2(A) Effects of (a) HBOA, (b) AcO-BOA
and (c) TC-3 on serum ALT and AST levels. Data are presented as the
means ± SE (n=10). **P<0.01 compared with the normal
control group; #P<0.05 and ##P<0.01
compared with the CCl4 group. (B) Comparison of (a) the
MDA inhibition rate and the induction rates of (b) SOD and (c) GSH
following treatment with HBOA, AcO-BOA or TC-3 in mice with acute
liver injury. Results are presented as: (level in the model group -
level in the treatment group)/level in the model group × 100 and
(level in the treatment group - level in the model group)/level in
the model group × 100, respectively. HBOA,
4-hydroxy-2(3H)-benzoxazolone; AcO-BOA,
4-acetoxy-2(3H)-benzoxazolone; TC-3,
3-acetyl-4-acetoxy-2-benzoxazolone; ALT; alanine aminotransferase;
AST, aspartate aminotransferase; MDA, malondialdehyde; SOD,
superoxide dismutase; GSH, glutathione. |
 | Table IIEffect of HBOA on SOD, MDA, GSH, Gpx,
CAT and LDH levels (n=10). |
Table II
Effect of HBOA on SOD, MDA, GSH, Gpx,
CAT and LDH levels (n=10).
| Group | SOD (U/mgprot) | MDA
(nmol/mgprot) | GSH
(nmol/mgprot) | Gpx (U/mgprot) | CAT (U/mgprot) | LDH (U/l) |
|---|
| Control | 200.84±41.95 | 2.20±0.60 | 2.20±0.83 | 368.74±101.27 | 12.74±3.29 |
5423.47±1054.40 |
| CMC-Na | 215.18±43.38 | 2.36±0.93 | 2.95±1.41 | 374.33±103.56 | 11.30±5.53 |
5942.01±1074.70 |
|
CCl4 |
139.49±30.52a | 3.79±1.74a | 1.02±0.36a |
269.06±61.43a | 8.18±2.82a |
7907.96±2420.00a |
| Bif |
191.61±36.57c | 1.88±0.78c | 1.63±0.89b |
344.46±72.22b | 11.41±1.81c |
5967.66±1305.49b |
| High-dose HBOA |
196.81±27.16c | 1.90±0.91c | 1.66±1.00b |
341.57±76.65b | 12.92±5.41b |
5288.04±1151.85c |
| Med-dose HBOA | 168.85±42.47 | 2.08±0.49c | 1.42±0.53b | 263.04±81.16 | 11.38±6.18 |
5764.20±865.38b |
| Low-dose HBOA | 160.99±44.77 | 2.81±1.89 | 1.31±0.91 | 278.41±68.83 | 7.25±3.25 | 7172.09±370.51 |
Effect of AcO-BOA
Acute liver injury induced by CCl4
provoked significant reductions in the activities of liver SOD and
GSH, and increased the liver MDA and serum ALT and AST levels. The
results demonstrated that the activities of liver SOD and GSH were
increased by treatment with high-dose AcO-BOA, whereas the liver
MDA and serum ALT and AST levels were markedly reduced (Fig. 2Ab; Table III).
 | Table IIIEffect of high-, mid- and low-dose
AcO-BOA on SOD, MDA and GSH levels (n=10). |
Table III
Effect of high-, mid- and low-dose
AcO-BOA on SOD, MDA and GSH levels (n=10).
| Group | SOD (U/mgprot) | MDA
(nmol/mgprot) | GSH
(nmol/mgprot) |
|---|
| Control | 291.51±92.07 | 1.59±0.48 | 4.42±2.32 |
| CMC-Na | 305.37±84.18 | 1.89±0.59 | 3.68±1.59 |
|
CCl4 |
203.72±49.49a | 2.58±0.85b | 2.16±0.78b |
| Bif |
269.34±82.90c | 1.32±0.47d | 3.27±0.86d |
| High-dose |
288.98±102.00c | 1.79±0.70c | 3.33±0.95d |
| Mid-dose | 217.58±74.44 | 2.65±0.67 | 1.11±0.56 |
| Low-dose | 227.66±56.68 | 2.80±1.04 | 2.77±0.15 |
Effect of TC-3
CCl4 intoxication reduced the activities
of SOD and GSH and raised the levels of ALT, AST and MDA.
Conversely, in mice treated with TC-3, the levels of AST and MDA
were reduced and SOD and GSH levels were elevated (Fig. 2Ac; Table IV).
 | Table IVEffect of high-, mid- and low-dose
TC-3 on SOD, MDA and GSH levels (n=10). |
Table IV
Effect of high-, mid- and low-dose
TC-3 on SOD, MDA and GSH levels (n=10).
| Group | SOD (U/mgprot) | MDA
(nmol/mgprot) | GSH
(nmol/mgprot) |
|---|
| Control | 246.81±86.41 | 2.81±0.62 | 4.57±2.30 |
| CMC-Na | 262.09±33.99 | 2.94±0.23 | 4.96±1.07 |
|
CCl4 |
193.37±22.36a | 3.62±0.92a | 2.57±0.84a |
| Bif |
290.39±63.25b | 2.74±0.90b | 4.81±1.43c |
| High-dose |
288.45±42.52c | 2.32±0.65c | 3.94±1.14c |
| Mid-dose |
216.15±20.75c | 2.33±0.45c | 2.32±1.05c |
| Low-dose | 208.76±54.45 | 4.30±1.35 | 2.19±1.15c |
Inhibition rate of MDA and induction
rate of SOD and GSH
A high-dose of each of the three compounds
demonstrated the highest MDA inhibition rates and the highest SOD
and GSH induction rates (Fig.
2B).
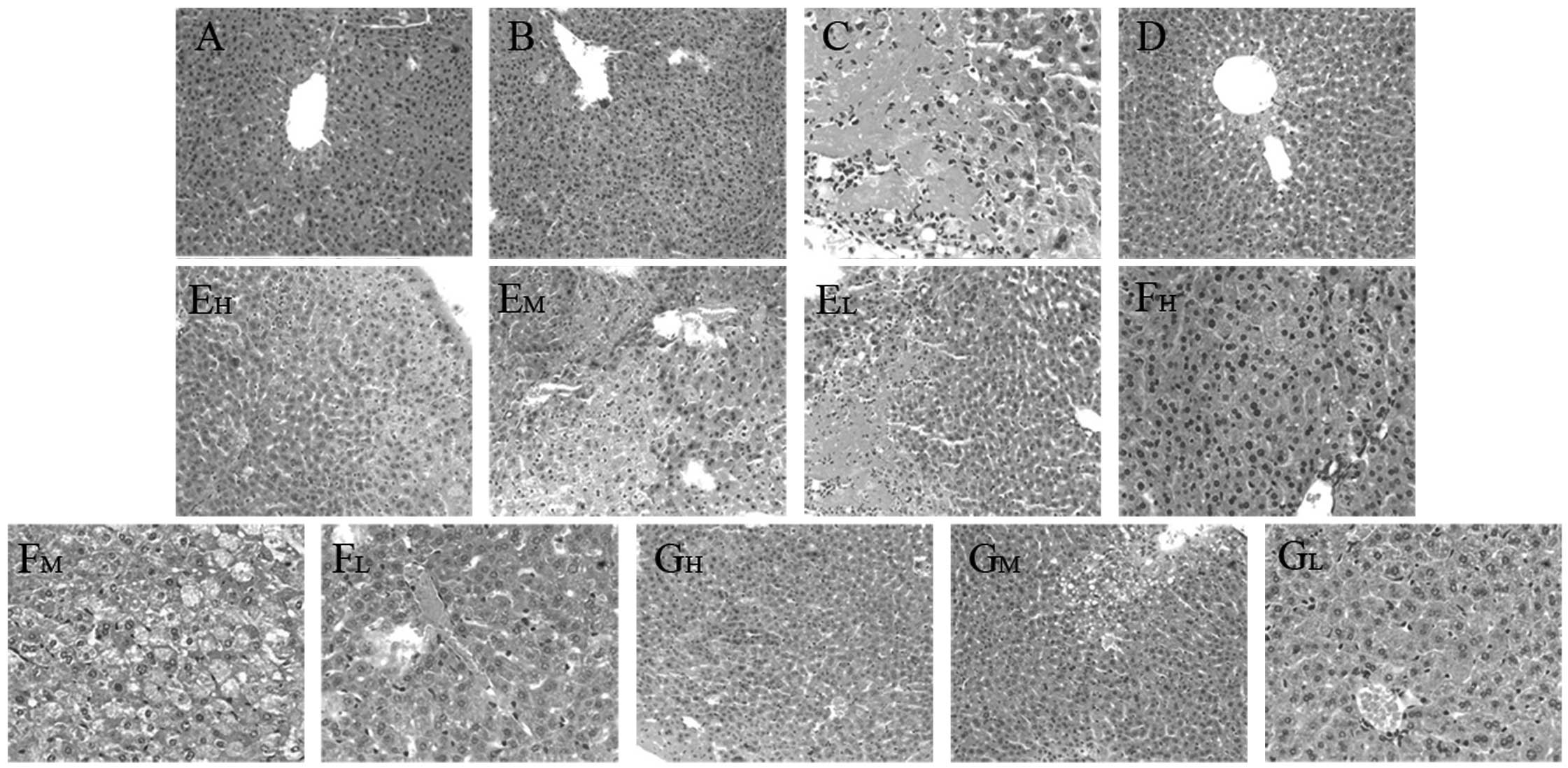
Histopathological observation
The degree of liver injury observed in mice was
evaluated by H&E staining, which demonstrated that the livers
from the normal control and CMC-Na control mice exhibited a normal
liver lobular architecture with a central vein and radiating
hepatic cords (Fig. 4A and B). In
contrast to normal mouse liver morphology, CCl4-induced
injury was evidenced by disruption of the liver lobular
architecture, liver cell edema, fatty degeneration of the liver,
inflammation and granuloma (Fig.
4C). These alterations were reduced in the liver sections of
mice that received the three compounds in high and medium doses
(Fig. 4E–G). Concurrent
administration of bifendate attenuated the CCl4-induced
changes in the liver architecture (Fig. 4D).
Discussion
Regarding the chemical structure of HBOA, it
contains hydroxyl and imine bases that may act as either hydrogen
bond donors or acceptors and play a role in the action of the drug
and in the process of receptor binding. Calış et al observed
that the substitution of the benzene ring of benzoxazolinone
derivatives by an acyl moiety resulted in enhanced
anti-inflammatory and analgesic activities (20). Therefore, we hypothesize that
3-substituted or 3,4-disubstituted derivatives of HBOA may possess
a greater protective effect on the liver than HBOA.
Products were carbonized by concentrated sulfuric
acid. In order to avoid carbonization and maintain the
concentration of sulfuric acid, we added distilled water or
filtrate when distilling. We obtained 2-nitroresorcinol by drip
type water vapor distillation in order to avoid carbonising the
product. HBOA was obtained from 2-nitroresorcinol by a ‘one-pot’
reduction reaction and subsequent cyclization with urea. In this
reaction, hexahydrated ferric chloride-activated carbon was used as
a catalytic agent and hydrazine hydrate was used as a reducing
agent. We have previously compared the applicability of reduction
with hydrazine hydrate with that of stannous chloride dissolved in
hydrochloric acid; reduction by hydrazine hydrate was observed to
be simpler and more convenient (21). However, hydrazine hydrate must be
evaporated to dryness to prevent it from reacting with urea.
AcO-BOA and TC-3 were subsequently obtained by the reaction of HBOA
with acetyl chloride, an acylating agent, in a basic triethylamine
solution.
HPLC was used to determine the purity of the
compounds. The method provides excellent separation of target
compounds from unknown impurities with a resolution of >1.5 and
a tailing factor range of 0.95–1.5. We observed that AcO-BOA and
TC-3 are readily hydrolyzed in aqueous solution. Therefore, we
employed a solution of CMC as required in order to avoid
hydrolysis.
CCl4-induced liver injury is dependent
upon reductive dehalogenation catalyzed by cytochrome p450 in the
endoplasmic reticulum of hepatic cells leading to the generation of
an unstable complex trichloromethyl radical
(•CCl3). The superoxide anion
O2−, H2O2 and the
hydroxyl radical (•OH) are reactive oxygen species (ROS)
produced in mitochondria (4).
Lipid peroxidation is an important consequence of the metabolism of
CCl4(22). These oxygen
radicals contribute to the process of lipid peroxidation. However,
cells have various mechanisms for protecting themselves from the
toxic effects of ROS, including free radical scavengers and chain
reaction terminators such as SOD, CAT, GSH and Gpx systems
(23). Liver injury via the ROS
pathway causes increases in the levels of ALT, AST, MDA and LDH,
the syntheses of which are increased due to the extensive damage of
the liver cells (4,24).
The current study demonstrates that HBOA and its
derivatives exhibit protective effects against acute liver injury,
confirmed by the serum ALT and AST levels and H&E staining. Our
investigation revealed that HBOA and its derivatives exhibit potent
hepatoprotective effects against CCl4-induced liver
damage in mice. This may be the result of increasing the activity
of the antioxidant-defense system and the inhibition of lipid
peroxidation.
Many hepatoprotective drugs have been of interest
due to their antioxidant activity (25). In the current study, the induction
of acute liver injury by CCl4 increased the levels of
MDA in liver tissue. MDA is a product of lipid peroxidation;
elevated levels of MDA may reflect the degree of lipid peroxidation
injury in liver cells (2). High
doses of HBOA and its derivatives are able to reduce the levels of
hepatic MDA. Compared with the CCl4 model group,
treatment with HBOA increased the activity or level of SOD, CAT,
Gpx and GSH, which scavenge free radicals and simultaneously reduce
lipid peroxidation, thus alleviating the oxidative damage caused by
CCl4. The results suggest that HBOA protects against the
damage caused by the oxidation of hepatic cellular membranes by
free radical scavenging. Treatment of the mice with the two
derivatives of HBOA attenuated the hepatic SOD and GSH depletion
induced by the intraperitoneal administration of CCl4.
Therefore, we hypothesize that the protective actions of the two
derivatives are related to their ability to scavenge free
radicals.
TNF-α activates various intracellular pathways which
regulate inflammation, cell death and proliferation. In the liver,
TNF-α not only mediates hepatotoxicity but also contributes to the
restoration of functional liver mass by promoting hepatocyte
proliferation and liver regeneration (26). TNF-α expression was observed to be
lower in the group treated with HBOA than in the model group, which
demonstrates that the hepatoprotective effect of HBOA involved an
anti-inflammatory mechanism and inhibiting the activity of secreted
TNF-α on Kupffer cells (KCs).
In conclusion, these preliminary studies indicate
that HBOA and its two acyl derivatives elicit a protective effect
on CCl4-induced liver injury in vivo. This study
supports the traditional use of Acanthus ilicifolius as an
herbal remedy for various liver diseases. However, further studies
are required in order to evaluate the mechanism of action these
compounds.
Acknowledgements
The authors acknowledge financial support from the
Science and Technology Research Development of Guangxi Province,
Guangxi Natural Science Foundation (2009AM4019) and the Department
of Education, Guangxi Province.
Abbreviations:
|
CCl4
|
carbon tetrachloride
|
|
ALT
|
alanine aminotransferase
|
|
AST
|
aspartate aminotransferase
|
|
LDH
|
lactic dehydrogenase
|
|
MDA
|
malondialdehyde
|
|
SOD
|
superoxide dismutase
|
|
CAT
|
catalase
|
|
GSH
|
glutathione
|
|
Gpx
|
glutathione peroxidase
|
References
|
1
|
Spiteller G: Are lipid peroxidation
processes induced by changes in the cell wall structure and how are
these processes connected with diseases? Med Hypotheses. 60:69–83.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Amin A and Mahmoud-Ghoneim D: Zizyphus
spina-christi protects against carbon tetrachloride-induced
liver fibrosis in rats. Food Chem Toxicol. 47:2111–2119. 2009.
View Article : Google Scholar
|
|
3
|
Lin HM, Tseng HC, Wang CJ, Lin JJ, Lo CW
and Chou FP: Hepatoprotective effects of Solanum nigrum Linn
extract against CCl4-induced oxidative damage in rats.
Chem Biol Interact. 171:283–293. 2008.
|
|
4
|
Wang CY, Ma FL, Liu JT, Tian JW and Fu FH:
Protective effect of salvianic acid A on acute liver injury induced
by carbon tetrachloride in rats. Biol Pharm Bull. 30:44–47. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Babu BH, Shylesh BS and Padikkala J:
Antioxidant and hepatoprotective effect of Acanthus
ilicifolius. Fitoterapia. 72:272–277. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Babu BH, Shylesh BS and Padikkala J:
Tumour reducing and anticarcinogenic activity of Acanthus
ilicifolius in mice. J Ethnopharmacol. 79:27–33. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ganesh S and Vennila JJ: Screening for
antimicrobial activity in Acanthus ilicifolius. Arch Appl
Sci Res. 2:311–315. 2010.
|
|
8
|
Poupaert J, Carato P, Colacino E and Yous
S: 2(3H)-Benzoxazolone and bioisosters as ‘privileged scaffold’ in
the design of pharmacological probes. Curr Med Chem. 12:877–885.
2005.PubMed/NCBI
|
|
9
|
Gulcan HO, Kupeli E, Unlu S, Yesilada E
and Sahin MF: 4-(5-chloro-2(3H)-benzoxazolon-3-yl) butanoic acid
derivatives: synthesis, antinociceptive and anti-inflammatory
properties. Arch Pharm (Weinheim). 336:477–482. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tang L, Ban SR, Feng XE, Lin WH and Li QS:
Synthesis and activities of new 4-hydroxy benzoxazolone
derivatives. Chin Chem Lett. 21:63–66. 2010. View Article : Google Scholar
|
|
11
|
Gülcan HO, Ünlü S, Banoğlu E, Şahin MF,
Küpeli E and Yeşilada E: Synthesis of new
4-(5-chloro-2-oxo-3Hbenzoxazol-3-yl) butanamide derivatives and
their analgesic and anti-inflammatory properties. Turk J Chem.
27:467–476. 2003.
|
|
12
|
Charlier C and Michaux C: Dual inhibition
of cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LOX) as a new
strategy to provide safer non-steroidal anti-inflammatory drugs.
Eur J Med Chem. 38:645–659. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Önkol T, Sahin MF, Yildirim E, Erol K and
Ito S: Synthesis and antinociceptive activity of
(5-chloro-2(3H)-benzoxazolon-3-yl) propanamide derivatives. Arch
Pharm Res. 27:1086–1092. 2004.PubMed/NCBI
|
|
14
|
Wang ZY, Chen YH, Zheng GJ, Yang CM and
Long SJ: Study on the anti-inflammatory and analgesic effects of
ilicifolius alkaloids A and its derivatives acetyl ilicifolius
alkaloids A. Chin Hosp Pharm J. 31:807–810. 2011.
|
|
15
|
Soyer Z, Bas M, Pabuccuoglu A and
Pabuccuoglu V: Synthesis of some 2(3H)-benzoxazolone derivatives
and their in-vitro effects on human leukocyte myeloperoxidase
activity. Arch Pharm (Weinheim). 338:405–410. 2005. View Article : Google Scholar
|
|
16
|
Ucar H, Van derpoorten K, Cacciaguerra S,
Spampinato S, Stables JP, Depovere P, Isa M, Masereel B, Delarge J
and Poupaert JH: Synthesis and anticonvulsant activity of 2(3
H)-benzoxazolone and 2(3H)-benzothiazolone derivatives. J Med Chem.
41:1138–1145. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bell M, Crowell T, Matthews D, Mcdonald J,
Neel D and Shuker A: Selective β3 adrenergic agonists. US Patent
5,786,356. Filed June 25, 1997; issued July 28, 1998.
|
|
18
|
Lin J and Fan H: Effect of
4-hydroxy-2-benzoxazolone on the proliferation of HSC-T6 cells.
Lishizhen Med Materia Medica Res. 10:2371–2372. 2011.
|
|
19
|
Wei CM: Optimizing the preparation process
for 2-nitroresorcinol. J Huaiyin Teach (Nat Sci). 3:135–138.
2004.
|
|
20
|
Calış Ü, Gökhan N and Erdoğan H: Synthesis
of some novel 3-methyl-6-(2-substituted
propanoyl/propyl)-2-benzoxazolinone derivatives and
anti-nociceptive activity. Farmaco. 56:719–724. 2001.PubMed/NCBI
|
|
21
|
Wei X, Chen YH and Long SJ: Synthesis of
2-aminoresorcinol hydrochloride. Northwest Pharmaceutical Journal.
3:168–169. 2008.
|
|
22
|
Recknagel RO, Glende EA Jr, Dolak JA and
Waller RL: Mechanisms of carbon tetrachloride toxicity. Pharmacol
Ther. 43:139–154. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu J, Wang Y, Qian H, Zhao Y, Liu B and Fu
C: Polyprenols from Taxus chinensis var. mairei prevent the
development of CCl4-induced liver fibrosis in rats. J
Ethnopharmacol. 142:151–160. 2012.
|
|
24
|
Al-Dbass AM, Al-Daihan SK and Bhat RS:
Agaricus blazei Murill as an efficient hepatoprotective and
antioxidant agent against CCl4-induced liver injury in
rats. Saudi J Biol Sci. 19:303–309. 2012. View Article : Google Scholar
|
|
25
|
Domitrović R, Jakovac H and Blagojević G:
Hepatoprotective activity of berberine is mediated by inhibition of
TNF-α, COX-2, and iNOS expression in CCl4-intoxicated
mice. Toxicology. 280:33–43. 2011.PubMed/NCBI
|
|
26
|
Schwabe RF and Brenner DA: Mechanisms of
liver injury. I TNF-alpha-induced liver injury: role of IKK, JNK
and ROS pathways. Am J Physiol Gastrointest Liver Physiol.
290:G583–G589. 2006. View Article : Google Scholar : PubMed/NCBI
|