Introduction
Gastric cancer is one of the most common malignant
tumors, with the fourth highest incidence rate and the second
highest mortality rate among the malignant types of cancer. It also
has the highest incidence rate among the digestive tract cancers
(1,2,3).
Surgery is considered to be the only radical treatment method for
gastric cancer. Due to an abundant gastric blood supply, complex
anatomical section and lymphatic metastasis pathway, the
anastomosis is difficult to operate on. The D2 radical operation
for gastric cancer during the progression period is even more
challenging. The surgery under laparoscope requires the physician
to have a great experience in open surgery and skilled in
laparoscopy techniques. The indications of laparoscopy-assisted D2
radical operation for gastric cancer in progressed stage remain the
topics of controversy. The most important question is whether the
gastric cancer surgery under laparoscope is able to achieve the
radical cure. The D2 radical resection of gastric cancer consists
of at least the three aspects: i) complete resection of the primary
foci and surrounding tissues and organs with a sufficiently wide
margin; ii) complete dissection of the gastric lymph nodes; iii)
complete elimination of shed cancer cells in the abdominal cavity.
The laparoscopy-assisted radical surgery for gastric cancer must
conform to these rules, therefore a long-term survival may be
assured and the advantage of minimally invasive surgery may be
maximized. The laparoscope has a favorable local amplifying effect
and is able to clearly visualize the blood vessels, nerves and
fascia. The laparoscope is able to guarantee a higher precision for
local operation and treatment of large vessels. The
laparoscopy-assisted surgery is superior. Goh et al were the
first to implement the laparoscopy-assisted D2 radical gastrectomy
in advanced cases of gastric cancer in 1997 (4). The minimal invasiveness of the
surgery is the predominant advantage when compared with traditional
laparotomy. However, the safety, feasibility and prognosis of
laparoscopy-assisted surgery have been the focus of debate. In this
study, we retrospectively analyzed the clinical data of 50 patients
receiving laparoscopic treatment (Group A) and 62 patients
receiving conventional laparotomy (Group B), at The Affiliated
Tumor Hospital of Xinjiang Medical University (Urumqi, China) from
August 2009 to January 2011. The surgical incision length, volume
of blood loss, postoperative recovery rate and complications, and
the cumulative survival rates were compared between the two groups
of patients, to investigate the advantages of laparoscopy in the
treatment of locally advanced gastric antral cancer.
Patients and methods
Patients
The present study involved 112 patients with locally
advanced gastric antral cancer, who received gastrointestinal
surgery at our hospital from August 2009 to December 2010. The 112
cases comprised 52 males and 60 females (age, 33–77 years), 98 of
whom were of Han ethnicity and 14 of whom were of an ethnic
minority. In accordance with the willingness of the patients to
undergo surgery, the patients were divided into two groups
according to the surgical approach. These were Group A (50 cases),
which comprised patients receiving laparoscopy, and Group B (62
cases), which comprised patients undergoing laparotomy. A general
comparison of the patients in the two groups revealed no
significant differences (P>0.05 for all parameters; Table I). The study protocol was approved
by the ethics committee of Xinjiang Medical University (Urumqi,
China), and written informed consent was obtained from each
participant prior to data collection.
 | Table IComparison of clinical data between
patients with distal gastric cancer in laparoscopy and laparotomy
groups. |
Table I
Comparison of clinical data between
patients with distal gastric cancer in laparoscopy and laparotomy
groups.
| Parameters | Group A | Group B | P-value |
|---|
| Gender |
| Male | 24 | 28 | 0.849 |
| Female | 26 | 34 | |
| Age (years) | 61.8±12.17 | 60.66±13.15 | 0.419 |
| Ethnicity |
| Han | 44 | 54 | 1.000 |
| Minority | 6 | 8 | |
| Tumor size (cm) | 3.83±1.05 | 3.98±1.17 | 0.457 |
| Preoperative
complications |
| Present | 24 | 26 | 0.569 |
| Absent | 26 | 36 | |
| Method |
| Billroth I | 32 | 36 | 0.564 |
| Billroth II | 18 | 26 | |
| Pathological
type |
| Mucinous
adenocarcinoma, signet ring cell carcinoma and undifferentiated
adenocarcinoma | 40 | 39 | 0.061 |
| Highly/moderately
differentiated adenocarcinoma | 10 | 23 | |
| Gross tumor type |
| Protruded | 30 | 30 | 0.256 |
| Ulcerated | 20 | 32 | |
Inclusion and exclusion criteria
The inclusion criteria for laparoscopy-assisted
surgery were as follows: pathologically confirmed adenocarcinoma;
preoperative clinical tumor stage T2–T3; the absence of extensive
peritoneal implantation metastasis and lung, liver and bone
metastasis according to preoperative clinical investigations,
including chest X-ray, thoracoabdominal cavity computed tomography
(CT) scan and a tumor marker test; and the absence at a general
physical checkup of other factors that indicated that the patient
was unsuitable for surgery. The exclusion criteria comprised:
multiple primary gastric tumors; distant metastasis discovered
during surgery; cases requiring emergency surgery for acute pyloric
obstruction; and cases unsuitable for laparoscopy for other
reasons.
Methods
The same group of surgeons performed the surgical
procedures in the two groups. The laparoscopy-assisted surgery
required five incisions to be made in the patient. The
pneumoperitoneum was established through an infraumbilical
puncture, with a pressure of 12–14 mmHg. A 10-mm trocar was
inserted through the infraumbilical route. The intraoperative
principles for laparoscopy-assisted surgery included: i)
application of D2 radical gastrectomy in all patients, ii) en bloc
resection of the cancerous tumor and the surrounding tissues; and
prevention of iatrogenic tumor spread. During the surgery, contact
between the forceps and the tumor, as well as squeezing of the
tumor, were prohibited. To prevent cancer implantation in the
peritoneal wall incisions, there was no direct clamping of the
tumor, and the tumor was removed through the protective ring. In
addition, following removal, fluorouracil implants were distributed
in the peritoneal cavity, after washing with saline. The protective
ring was subsequently removed, and the incisions were washed for a
second time. The pneumoperitoneum was relieved and the casing was
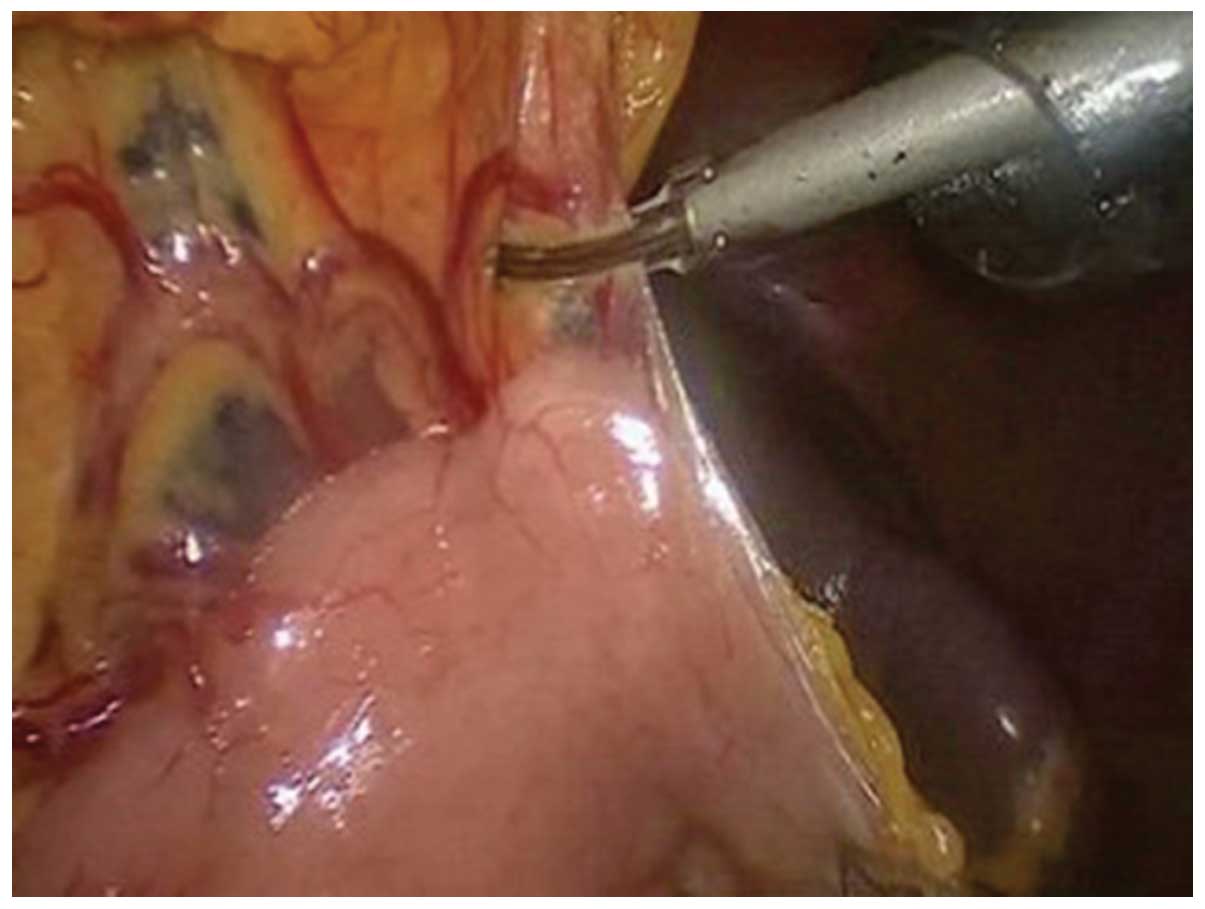
removed. An ultrasound knife (HARMONIC ACE36E, Johnson &
Johnson, New Brunswick, NJ, USA) was used to separate the anterior
lobe of the transverse mesocolon, with laparoscopic assistance
(Fig. 1). The perigastric blood
vessels were next treated as follows: the left gastric artery,
right gastric artery, right gastroomental vessels and right
gastroomental vessels were clipped by biological vascular clips and
other small blood vessels were closed by ultrasonic scalpel
(Fig. 2). The lymph nodes were
dissected as described previously (5), (Figs.
3 and 4). Of the patients
receiving laparoscopy, 32 were treated with the Billorth I method
and 18 were treated with the Billorth II method (6). For patients undergoing laparotomy,
the periumbilical incision was made on the middle upper abdomen for
traditional D2 radical gastrectomy.
Follow-up
The follow-up included re-examination during
hospitalization and as an outpatient, and regular telephone surveys
for discharged patients who had received radical gastrectomy. The
medical records of the patients were also evaluated for 14–30
months following the surgery (average duration, 18 months). A chest
X-ray, an abdominal B ultrasound and the detection of
carcinoembryonic antigen (CEA) were performed every month in the
first six months following the surgery, and a fibergastroscopy was
performed every 4–6 months. The follow-up period ended in April
2012. The patient follow-up rate was 93.75% (105/112). A case was
lost according to the last follow-up calculation. Lost cases and
fatalities that were not due to cancer were evaluated by
statistical analysis for censored data processing requirements.
Statistics
SPSS software, version 13.0 (SPSS, Inc., Chicago,
IL, USA) was used for statistical analysis of the data. The results
of Groups A and B were compared using the χ2 test for
enumeration data, a t-test for numerical data and a Wilcoxon rank
sum test for skewed data. In addition, the Kaplan-Meier method of
single factor analysis was utilized to compare the 1- and 3-year
cumulative survival rates, as well as the average survival time,
between the two groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
In Group A, 46 of the 50 patients underwent
successful surgery with complete tumor resection and the median
number of dissected lymph nodes per patient was 16.3. Four patients
(8%) in Group A were transferred to the laparotomy procedure; three
patients had severe adhesions due to obesity and one patient
demonstrated hematoma and blood oozing due to low levels of blood
coagulation. In Group B, the surgery was successful for all
patients, the tumors were completely resected and the median number
of dissected lymph nodes per patient was 17.2. No significant
difference was identified in the number of dissected lymph nodes
between Groups A and B (P>0.05).
The time taken for the gastrointestinal function to
recover and the incidence of postoperative complications are shown
in Table II. In Group A, the
complications observed were: fat liquefaction at the incision, lung
infection, anastomotic stoma stenosis, urinary tract infection,
subcutaneous emphysema and lymphatic leakage (one case of each). In
Group B, one case of each of small bowel obstruction,
intra-abdominal hemorrhage, peritoneal infection and lymphatic
leakage occurred; along with two cases of each of delayed gastric
emptying, lung infection and urinary tract infection, and eight
cases of fat liquefaction at the incision. Following the surgery,
the gastrointestinal function recovery time of Group A was shorter
than that of Group B (3.12±0.82 vs. 3.8±1.31 days; P<0.05), and
the postoperative hospital stay of Group A was significantly
shorter than that of Group B (18.94±7.81 vs. 23.61±9.02 days,
respectively; P<0.05; Table
II). The incidence of complications in Group A was
significantly lower than that of Group B (6/50 vs. 18/62,
respectively; P<0.05; Table
II).
 | Table IIPostoperative conditions of patients
with distal gastric cancer in laparoscopy and laparotomy
groups. |
Table II
Postoperative conditions of patients
with distal gastric cancer in laparoscopy and laparotomy
groups.
| Parameters | Group A | Group B | P-value |
|---|
| Surgery time
(min) | 251.10±87.38 | 218.41±60.62 | 0.046 |
| Intraoperative
bleeding volume (ml)a | 101 | 210 | 0.000 |
| Perioperative blood
transfusion (fraction of patients) | 1/49 | 9/53 | 0.023 |
| Distance of the
tumor mass from the cut distal end (cm) | 3.82±1.11 | 3.73±1.17 | 0.791 |
| Incision length
(cm) | 6.4±0.7 | 15.6±2.3 | 0.000 |
| Number of dissected
lymph nodesa | 16.3 | 17.2 | 0.435 |
| Time to
gastrointestinal function recovery (days) | 3.12±0.82 | 3.8±1.31 | 0.000 |
| Postoperative
complications |
| Present | 6 | 18 | |
| Absent | 44 | 44 |
0.037b |
| Postoperative
hospital stay (days) | 18.94±7.81 | 23.61±9.02 |
0.010b |
Kaplan-Meier univariate analysis showed that in
Groups A and B, the 1-year (86.0 and 88.6%, respectively) and
3-year cumulative survival rates (52.6 and 53.7%, respectively),
and average survival times (28.13 and 28.65 months, respectively),
were not statistically significantly different (P>0.05; Fig. 5).
Discussion
The rapid development of minimally invasive surgery
has facilitated a new approach to surgical treatment. Laparoscopic
cholecystectomy has become a gold standard treatment, while
laparoscopic radical resection is now recognized as an effective
technique for colorectal cancer. This novel technique has also been
applied to radical surgery for other types of tumors. However, the
application of laparoscopic gastrectomy is limited due to the
numerous gastric blood vessels, the levels of anatomical structure,
the complex lymph node metastasis pathway and the presence of
anastomosis. In 1994, Kitano et al reported the first use of
the radical gastrectomy technique (7). Subsequently, Kitano et al
described 116 cases of early-stage gastric cancer who received
laparoscopy-assisted radical gastrectomy (8). The cases were followed up for an
average of 45 months, and no cases of tumor recurrence or cancer
implantation in the incisions were identified. The present study
indicated that the number of dissected lymph nodes and the surgical
margin of the groups receiving either laparoscopy-assisted surgery
or laparotomy were similar (Table
II), which was concordant with the results of previous studies
(9–12). This is likely due to the fact that
the tips of the laparoscope assisted the radical gastrectomy for
gastric cancer, achieving a similar radical effect to that of
conventional open surgery.
The duration of the surgery is an important
indicator when evaluating a novel surgical technique. In the
present study, the duration of the laparoscopy-assisted surgery was
longer compared with that of the laparotomy. This may have been due
to the fact that laparoscopy-assisted surgery is a more complex
procedure than laparotomy, as it involves several abdominal regions
and the surgical area is difficult to access. The cooperation
between the surgeon and the assistants was suboptimal, which added
to the surgical difficulty; and the laparoscopic technique takes
time to master (13,14). Tokunaga et al determined
that following professional training in laparoscopic gastrectomy,
the duration of the surgery was decreased to marginally longer than
that of the laparotomy, which indicates that physicians who are
skilled at performing the laparotomy require additional experience
prior to becoming skilled at conducting laparoscopic surgery
(15). We propose that with an
increasing number of patients undergoing laparoscopy-assisted
surgery, the improvement in surgical skills may reduce the duration
of the surgery to that of laparotomy.
The introduction of an ultrasound knife may decrease
the volume of bleeding and the difficulty of tissue separation
experienced in laparoscopic surgery. It may also produce less
surgical smoke and carbon compared with an electric knife during
surgery (16,17). However, how the ultrasound knife is
used directly impacts the hemostatic effect. It will cause bleeding
if the blood vessels are severed when the blood has not completely
clotted; for areas with numerous blood vessels, if only part of the
blood vessels are clamped, then the blood vessel severing will also
cause bleeding. A frequently used method is to clean the field of
vision with an aspirator, while clamping the bleeding points with a
ultrasonic knife, set to a slow mode, to stop the bleeding
(18–22). Varela et al revealed that
the mean volume of intraoperative blood loss in patients receiving
laparoscopy was significantly lower than that of patients
undergoing laparotomy (138 vs. 57 ml, respectively) (23). The present study identified that
the length of the incision, intraoperative blood loss,
postoperative ventilation time, duration of hospitalization and the
incidence of complications in the laparoscopic group were decreased
compared with those in the laparotomy group. These advantages of
laparoscopic gastrectomy are in accordance with the results of
previous studies (24,25).
Implantation metastasis of the tumor in the
incisions and peritoneal cavity is a significant disadvantage of
laparoscopic surgery. However, whether the pressure difference in
the CO2 pneumoperitoneum results in the shedding and
implantation of tumor cells remains controversial. Certain scholars
consider that the pressure of the CO2 pneumoperitoneum
is not directly related to tumor metastasis (26,27).
The present study involved a follow-up of 50 patients who underwent
laparoscopic gastrectomy, in which one patient with implantation
metastasis in the peritoneal cavity was identified shortly
following the surgery. This may have been due to the following: i)
the direct implantation of the shedded tumor cells; ii) trocar
incision injury and leakage of CO2 along the trocar;
iii) atomization of the tumor cells; and iv) the effect of
artificial pneumoperitoneum on cellular immunity (28–31).
Furthermore, when the number of tumor cells reaches a certain
level, the cells escape from the collection pore due to the
pressure difference in the CO2 pneumoperitoneum. A
number of the cells adhere to the incisions or the incision
margins, resulting in cancer implantation in the incisions, which
is known as the ‘chimney effect’ (32). Therefore, tumor-free technology is
particularly important in laparoscopy, to reduce implantation
metastasis of the tumor in the incisions. In the present study,
relevant measures were taken, such as strictly complying with the
tumor-free principle (33,34), protecting the incisions when
removing the tumor specimens, soaking and rinsing prior to the
abdominal closure, and killing residual tumor cells by the
intraperitoneal application of fluorouracil implants. These
measures are important for reducing the risk of tumor
implantation.
All patients receiving the laparoscopy-assisted
surgery face the possibility of transferring to laparotomy, which
limits the application of laparoscopic surgery. The reduction of
the transferal rate is a key issue. Dulucq et al described
eight patients who received laparoscopic total gastrectomy and 11
patients who underwent laparoscopic subtotal gastrectomy, among
which there were no cases that were transferred to laparotomy
(35). Pugliese et al
investigated 48 cases who underwent laparoscopic total gastrectomy
and subtotal gastrectomy, where only one patient was transferred to
laparotomy due to a large tumor size (36). Shimizu et al revealed that
eight out of 100 cases (8%) were transferred to laparotomy
(37). In the present study, the
transferal rate in the laparoscopy group was 8%, and postoperative
complications occurred in three of the four patients who were
transferred. The reason may be that the surgeon did not fully
understand the indications of transferring to laparotomy, and
failed to transfer in a timely manner, which extended the surgery
time and affected the postoperative recovery. However, patients
with combined cardiopulmonary diseases may benefit from the
advantages of laparoscopic surgery, as it is minimally invasive. In
practice, we propose that transferal to laparotomy should be
considered when the following conditions are observed: i) a large,
advanced-stage tumor, which has extensively invaded the surrounding
tissues; ii) perigastric major blood vessels that are encapsulated
by the tumor or metastatic lymph nodes; iii) a loss of normal
anatomical spaces; iv) obesity and extensive adhesions; v)
suspected metastases to substantial organs with poor classification
and local invasion during surgery (which are observable, but not
palpable under the laparoscope, and are thus prone to be missed);
and vi) uncontrolled bleeding and injury during the surgery.
The patient survival rate is used as the main
measure of the efficacy of treatments for malignant tumors. It is a
current aim to achieve an enhanced survival rate following
laparoscopy-assisted radical gastrectomy for gastric cancer that is
comparable to that of open surgery. In the present study, no
significant differences in the 1- and 3-year cumulative survival
rates or the mean survival time, were observed between the
laparoscopy and the laparotomy groups. With prompt preoperative
determination of the indications for surgery, and a detailed
intraoperative protocol, the survival time following
laparoscopy-assisted D2 radical gastrectomy was similar to that
following open surgery, which is consistent with the findings of a
previous study (38).
In conclusion, laparoscopy-assisted D2 radical
gastrectomy for locally advanced gastric cancer is safe and
effective. Provided that the surgeons are experienced at the
laparoscopic technique, particularly in D2 radical gastrectomy, and
are fully aware of the indications for surgery, laparoscopic D2
radical gastrectomy for gastric cancer may achieve the same
mid-term result as the laparotomy, with the additional advantage of
minimal invasion. In the present study, the follow-up period was
short and the number of cases was small. Therefore, confirmation of
the long-term efficacy of laparoscopic D2 radical gastrectomy for
gastric cancer remains to be achieved. We propose that, with
experience, laparoscopic D2 radical gastrectomy for locally
advanced gastric cancer may achieve the same long-term treatment
effects as laparotomy.
References
|
1
|
Jemal A, Center MM, DeSantis C and Ward
EM: Global patterns of cancer incidence and mortality rates and
trends. Cancer Epidemiol Biomarkers Prev. 19:1893–1907. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sankaranarayanan R, Swaminathan R, Brenner
H, et al: Cancer survival in Africa, Asia, and Central America: a
populationbased study. Lancet Onco. 11:165–173. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Coleman MP, Quaresma M, Berrino F, et al:
Cancer survival in five continents: a worldwide population-based
study (CONCORD). Lancet Oncol. 2008.9:730–756
|
|
4
|
Goh PM, Khan AZ, So JB, et al: Early
experience with laparoscopic radical gastrectomy for advanced
gastric cancer. Surg Laparosc Endosc Percutan Tech. 11:83–87. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bouras G, Lee SW, Nomura E, et al:
Comparative analysis of station-specific lymph node yield in
laparoscopic and open distal gastrectomy for early gastric cancer.
Surg Laparosc Endosc Percutan Tech. 21:424–428. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Montesani C, D'Amato A, Santella S, et al:
Billroth I versus Billroth II versus Roux-en-Y after subtotal
gastrectomy. Prospective [correction of prespective] randomized
study. Hepatogastroenterology. 49:1469–1473. 2002.PubMed/NCBI
|
|
7
|
Kitano S, Iso Y, Moriyama M and Sugimachi
K: Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc
Endosc. 4:146–148. 1994.PubMed/NCBI
|
|
8
|
Kitano S, Shiraishi N, Kakisako K, et al:
Laparoscopy-assisted Billroth-I gastrectomy (LADG) for cancer: our
10 years' experience. Surg Laparosc Endosc Percutan Tech.
12:204–207. 2002.PubMed/NCBI
|
|
9
|
Huscher CG, Mingoli A, Sgarzini G, et al:
Laparoscopic versus open subtotal gastrectomy for distal gastric
cancer: five-year results of a randomized prospective trial. Ann
Surg. 241:232–237. 2005.PubMed/NCBI
|
|
10
|
Weber KJ, Reyes CD, Gagner M and Divino
CM: Comparison of laparoscopic and open gastrectomy for malignant
disease. Surg Endosc. 17:968–971. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee SI, Choi YS, Park DJ, et al:
Comparative study of laparoscopy-assisted distal gastrectomy and
open distal gastrectomy. J Am Coll Surg. 202:874–880. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fujiwara M, Kodera Y, Misawa K, et al:
Longterm outcomes of early-stage gastric carcinoma patients treated
with laparoscopy-assisted surgery. J Am Coll Surg. 206:138–143.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim MC, Jung GJ and Kim HH: Learning curve
of laparoscopy-assisted distal gastrectomy with systemic
lymphadenectomy for early gastric cancer. World J Gastroenterol.
11:7508–7511. 2005.PubMed/NCBI
|
|
14
|
Jin SH, Kim DY, Kim H, et al:
Multidimensional learning curve in laparoscopy-assisted gastrectomy
for early gastric cancer. Surg Endosc. 21:28–33. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tokunaga M, Hiki N, Fukunaga T, et al:
Quality control and educational value of laparoscopy-assisted
gastrectomy in a high-volume center. Surg Endosc. 23:289–295.
2009.PubMed/NCBI
|
|
16
|
Minutolo V, Gagliano G, Rinzivillo C, et
al: Usefullness of the ultrasonically activated scalpel in
laparoscopic cholecystectomy: our experience and review of
literature. G Chir. 29:242–245. 2008.PubMed/NCBI
|
|
17
|
Tucker RD: Laparoscopic electrosurgical
injuries: survey results and their implications. Surg Laparosc
Endosc. 5:311–317. 1995.PubMed/NCBI
|
|
18
|
Zeng YK, Yang ZL, Peng JS, et al:
Laparoscopy-assisted versus open distal gastrectomy for early
gastric cancer: evidence from randomized and nonrandomized clinical
trials. Ann Surg. 256:39–52. 2012. View Article : Google Scholar
|
|
19
|
Oz BS, Mataraci I, Iyem H, et al:
Comparison of ultrasonically activated scalpel and traditional
technique in radial artery harvesting: clinical research. Thorac
Cardiovasc Surg. 55:104–107. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Satoh S, Okabe H, Kondo K, et al: Video. A
novel laparoscopic approach for safe and simplified suprapancreatic
lymph node dissection of gastric cancer. Surg Endosc. 23:436–437.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ogura G, Nakamura R, Muragaki Y, et al:
Development of an articulating ultrasonically activated device for
laparoscopic surgery. Surg Endosc. 23:2138–2142. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Martínez-Ramos D, Miralles-Tena JM, Cuesta
MA, et al: Laparoscopy versus open surgery for advanced and
resectable gastric cancer: a meta-analysis. Rev Esp Enferm Dig.
103:133–141. 2011.PubMed/NCBI
|
|
23
|
Varela JE, Hiyashi M, Nguyen T, et al:
Comparison of laparoscopic and open gastrectomy for gastric cancer.
Am J Surg. 192:837–842. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Usui S, Yoshida T, Ito K, et al:
Laparoscopy-assisted total gastrectomy for early gastric cancer:
comparison with conventional open total gastrectomy. Surg Laparosc
Endosc Percutan Tech. 15:309–314. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim YW, Han HS and Fleischer GD:
Hand-assisted laparoscopic total gastrectomy. Surg Laparosc Endosc
Percutan Tech. 13:26–30. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Reymond MA, Bien N, Pross M and Lippert H:
The status of port-site recurrence. Kongressbd Dtsch Ges Chir
Kongr. 118:187–191. 2001.(In German).
|
|
27
|
Stocchi L and Nelson H: Wound recurrences
following laparoscopic-assisted colectomy for cancer. Arch Surg.
135:948–958. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Person B and Cera SM: Prolonged
postlaparoscopy carbon dioxide pneumoperitoneum. Surg Laparosc
Endosc Percutan Tech. 18:114–117. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fuganti PE, Rodrigues AJ Júnior, Rodrigues
CJ and Sato M: A comparison of the effects of pneumoperitoneum and
laparotomy on natural killer cell mediated cytotoxicity and Walker
tumor growth in Wistar rats. Surg Endosc. 20:1858–1861. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kosugi C, Ono M, Saito N, et al: Port site
recurrence diagnosed by positron emission tomography after
laparoscopic surgery for colon cancer. Hepatogastroenterology.
52:1440–1443. 2005.PubMed/NCBI
|
|
31
|
Hirabayashi Y, Yamaguchi K, Shiraishi N,
et al: Port-site metastasis after CO2 pneumoperitoneum:
role of adhesion molecules and prevention with antiadhesion
molecules. Surg Endosc. 18:1113–1117. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Iwanaka T, Arya G and Ziegler MM:
Mechanism and prevention of port-site tumor recurrence after
laparoscopy in a murine model. J Pediatr Surg. 33:457–461. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Reymond MA, Wittekind C, Jung A, et al:
The incidence of port-site metastases might be reduced. Surg
Endosc. 11:902–906. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ziprin P, Ridgway PF, Peck DH and Darzi
AW: The theories and realities of port-site metastases: a critical
appraisal. J Am Coll Surg. 195:395–408. 2002.PubMed/NCBI
|
|
35
|
Dulucq JL, Wintringer P, Stabilini C, et
al: Laparoscopic and open gastric resections for malignant lesions:
a prospective comparative study. Surg Endosc. 19:933–938. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pugliese R, Maggioni D, Sansonna F, et al:
Total and subtotal laparoscopic gastrectomy for adenocarcinoma.
Surg Endosc. 21:21–27. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shimizu S, Noshiro H, Nagai E, et al:
Laparoscopic gastric surgery in a Japanese institution: analysis of
the initial 100 procedures. J Am Coll Surg. 197:372–378. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kitano S, Shiraishi N, Uyama I, et al: A
multicenter study on oncologic outcome of laparoscopic gastrectomy
for early cancer in Japan. Ann Surg. 245:68–72. 2007. View Article : Google Scholar : PubMed/NCBI
|