Introduction
Titanium (Ti) and its alloys are frequently used as
orthopedic implant materials, due to their mechanical strength,
chemical stability and biocompatibility (1,2). It
has been demonstrated that the biocompatibility of Ti is closely
related to the properties of the surface oxide layer (predominantly
titanium dioxide, TiO2), with regard to its structure,
morphology and composition. However, TiO2 does not
exhibit sufficient bioactivity to form a direct bond with the
juxtaposed bone, and this may translate into a lack of
osseointegration, leading to the long-term failure of the implant
(3–5). Combining the TiO2 with
bioactive materials is recognized to be an effective method of
overcoming this drawback.
There has recently been an increased focus on the
effects of trace elements on biological processes, particularly in
the field of bone formation and in the study of essential elements.
With regard to the divalent cations, there have, to date, been a
variety of studies on manganese (Mn) (6–9). Mn
is an essential trace element in the human body, and it has the
most potent capacity for binding to integrins, and for mediating
the binding of ligands to various integrins, at low concentrations
(6,10–12).
Previous studies have endeavored to use Mn to enhance the
osteoconductivity of a bioinert Ti substrate, by means of its
potent cell adhesion-promoting effect (7,9,13,14).
However, there have not been any studies into Mn-containing
TiO2 ceramics or coatings, and the effects of Mn on the
composition, microstructure and biological responses of
TiO2 have not been elucidated. Moreover, the signal
transduction pathway that mediates the effects of Mn on
osteoblastic adhesion has not been studied. Therefore, the aim of
the present study was to investigate the preparation and
characterization of Mn-containing TiO2 coatings.
Plasma electrolytic oxidation (PEO), also known as
microarc oxidation, is a relatively convenient and effective
technique for the preparation of TiO2-based coatings on
a Ti substrate (15–17). PEO coatings are, in general, porous
and nanostructured, and this has been demonstrated to be beneficial
to osteoblast adhesion and proliferation (15,17).
At present, the PEO process is widely applied to the
biofunctionalization of titanium, in order to create bioactive
porous oxide coatings (18). In
addition, many biological elements, for example calcium (Ca) and
phosphorus (P), may be effectively incorporated into the PEO-evoked
TiO2 coating through the supplementation of the
electrolyte (19,20). In the present study, a porous
Mn-TiO2 coating was prepared by PEO in an electrolyte
containing Ca, P and Mn. In addition, the adhesion behavior of
osteoblast-like MG63 cells onto the Mn-TiO2 coating, and
the corresponding signal transduction pathway, were
investigated.
Materials and methods
Preparation of samples
The study used commercially pure Ti plates with
dimensions of 10×10×1 mm. The Ti was mechanically polished using
silicon carbide (SiC) abrasive sandpaper. For the TiO2
coatings, the Ti plates were anodized in an electrolyte containing
0.05 mol/l glycerophosphate disodium salt pentahydrate
(C3H7Na2O6P.5H2O,
GP) and 0.1 mol/l calcium acetate monohydrate
[(CH3COO)2Ca.H2O, CA], while for
the Mn-TiO2 coatings, 0.04 mol/l manganese acetate
[Mn(CH3COO)2·2H2O] was added into
the electrolyte. The current density, frequency, duty cycle and
duration time were fixed at 16.5 A/dm2, 800 Hz, 10% and
4 min, respectively. Following the PEO treatment, the samples were
washed with deionized water, and dried in air.
The surface characterization of the PEO-treated
samples was performed using scanning electron microscopy (SEM),
with a S-4200 scanning electron microscope (Hitachi, Tokyo, Japan),
X-ray diffraction (XRD), with a D/MAX-2550 diffractometer (Rigaku
Corporation, Tokyo, Japan) and energy-dispersive X-ray spectrometry
(EDS) attached to an electron probe X-ray microanalysis system
(EPMA), using an XA-8100 microprobe (Hitachi, Tokyo, Japan). The
surface roughness (Ra) of the samples was measured using a surface
profiler (Hommel Tester T8000; Hommelwerke GmbH,
Villingen-Schwennigen, Germany) with a scan distance of 4.8 mm and
a scan rate of 0.5 mm/sec. The scan was performed on each sample
three times at different locations on the sample.
Cell culture
The osteoblastic MG63 cells were cultured in
α-minimum essential medium (MEM), supplemented with 100 mg/ml
penicillin G, 50 mg/ml gentamicin, 3 mg/ml amphotericin B and 15%
newborn bovine serum, at 37°C in a humidified atmosphere of 95% air
and 5% CO2. The osteoblasts were trypsinized (0.25%
trypsin, 0.1% glucose, citrate-saline buffer; pH 7.8) prior to
confluent growth, in order to avoid post-confluence differentiation
effects, counted electronically with a cell counter (Coulter
Electronics Ltd., Luton, UK) and plated onto the test and control
surfaces at a density of 30,000–100,000 cells/ml. A total of 31.5
mg/ml sodium-β-glycerol phosphate and 0.58 mg/ml L-ascorbic acid
phosphate magnesium salt n-hydrate were added to the supplemented
α-MEM over the course of the experiments. Cells were not used above
passage number 20.
MTT assay
An MTT assay was used to determine the cell
attachment. The cells were seeded at a concentration of
2×105 cells/cm2 onto the disks of the Ti
plates, and the TiO2 and Mn-TiO2 coatings,
and were cultured on the disks for 1, 8, 16 and 24 h, respectively,
in a 37°C incubator with 5% CO2. At the pre-determined
time points, each disk was transferred to a well in a new 24-well
plate, and 1.5 ml medium was added to each disk. A total of 150 μl
freshly prepared 5 mg/ml MTT was added to each well where a disk
was present. The plates were then placed in an incubator at 37°C
for 3 h, prior to the supernatant of each well being removed, and
replaced with acidified isopropanol (0.04 M HCl in isopropanol).
This was subsequently mixed thoroughly to dissolve the dark-blue
crystals. The absorbance was measured with a spectrophotometer at a
wavelength of 570 nm, with a subtraction of the absorbance at 650
nm. The cell number was determined using a linear correlation
between the absorbance and MG63 cell concentration.
Cell morphology
The cells were seeded onto the Ti plates and the
TiO2 and Mn-TiO2 coatings at a density of
1×104/cm2 in α-MEM supplemented with 10%
phosphate-buffered saline (PBS), and were cultured under standard
cell culture conditions for 16 h. The specimens were then separated
from the medium, washed twice in PBS and fixed with 2.5%
glutaraldehyde in PBS for 1 h at room temperature. Following this,
the samples were dehydrated in a graded ethanol series of 50, 70,
80 and 95% ethanol for 5 min, as well as twice with 100% ethanol
for 15 min. Subsequently, the samples were stored overnight in a
thermo-ventilated oven at 37°C, prior to undergoing gold
metallization using Emscope SC500 apparatus (Quorum Technologies
Ltd., Ashford, UK), and SEM analysis with a Philips XL30 FEG
scanning electron microscope (Philips, Amsterdam, the
Netherlands).
Quantitative polymerase chain reaction
(qPCR) analysis
The gene expression of the integrin subunits
β1, β3, α1 and α3, as
well as FAK, ERK1 and ERK2, were determined using qPCR analysis.
The cells were seeded onto Mn-TiO2 and TiO2
coatings, in addition to Ti plates, in 24-well plates at a density
of 2×104 cells/well, and cultured for 1, 8, 16 and 24 h,
respectively. The total cellular RNA was extracted using
TRIzol® reagent (Invitrogen Life Technologies, Carlsbad,
CA, USA), in accordance with the manufacturer’s instructions. To
obtain first-strand cDNAs, 1 μg total RNA extract was used for
reverse transcription. The reactions were performed using a
RevertAid™ First Strand cDNA Synthesis kit (Thermo Fisher
Scientific, Inc., Waltham, MA, USA), in a final volume of 20 μl, at
42°C for 60 min, and were then terminated by heating at 70°C for 5
min. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as
the housekeeping gene. The primer sequences utilized are displayed
in Table I.
 | Table IPrimer pairs used in the present
study. |
Table I
Primer pairs used in the present
study.
| Target | Primer
sequence |
|---|
| Integrin
α1 | F:
5′-TCGCTCCGTGGCCTTGTGGAA-3′ |
| R:
5′-CCCATTTCAGTAACCACGCCC-3′ |
| Integrin
α3 | F:
5′-TGGGTCATAGACCGGTATAC-3′ |
| R:
5′-ATCCACTCATAGCAAACAGT-3′ |
| Integrin
β3 | F:
5′-TGCTGACGGGAGGAACGGTA-3′ |
| R:
5′-CGGGATCAGATGTGTCTGGG-3′ |
| Integrin
β1 | F:
5′-GTCTTTGCGTAGGCTTACTT-3′ |
| R:
5′-ACCCGTGGGTACGATGCATC-3′ |
| FAK | F:
5′-GCGAGAGGTGTGGTAATATCAGGTGAA-3′ |
| R:
5′-ACACGTATTCACTGTTCAACTATTGAC-3′ |
| ERK1 | F:
5′-ACACACGCCCAAACCATG-3′ |
| R:
5′-TCCACTCGCGCATTCGTA-3′ |
| ERK2 | F:
5′-TTTACCATAGGACTCAACCT-3′ |
| R:
5′-GGGCATGGTGTCCTGCAGAA-3′ |
| GAPDH | F:
5′-CGCGTCGCGCTCGATGTCAC-3′ |
| R:
5′-GGTAAGTGACATGCTGAGTT-3′ |
The amplification process was performed using a
Maxima SYBR-Green qPCR Master Mix in an Applied Biosystems 7500
RT-PCR system (Applied Biosystems, Foster City, CA, USA). The
reaction volume was 25 μl, containing 12.5 μl SYBR-Green qPCR
Master Mix, 1 μl each primer (0.3 μM), 2 μl template DNA and 8.5 μl
nuclease-free water. The initial denaturation was carried out at
95°C for 600 sec, with denaturation at 95°C for 15 sec, annealing
at 60°C for 30 sec and then 40 cycles of extension at 72°C for 30
sec. At the end of the PCR cycles, a melting curve analysis was
performed to determine the specificity of the PCR product. The mRNA
content of each gene was normalized to the quantity of GAPDH mRNA,
and the average threshold cycle (CT) values were used to quantify
the gene expression in each sample: ΔCT =
ΔCT(experiment)-ΔCT(control). The relative gene expression (fold
change) was obtained by transforming the logarithmic values into
absolute values using 2−ΔΔct.
Statistical analysis
One way analysis of variance (ANOVA) and Tukey’s
multiple comparison tests were performed to detect any significant
effects that occurred as a result of the experimental variables.
The results were analyzed using the Student’s t-test, with a sample
number including ≥4 samples. The error bars represent mean ±
standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
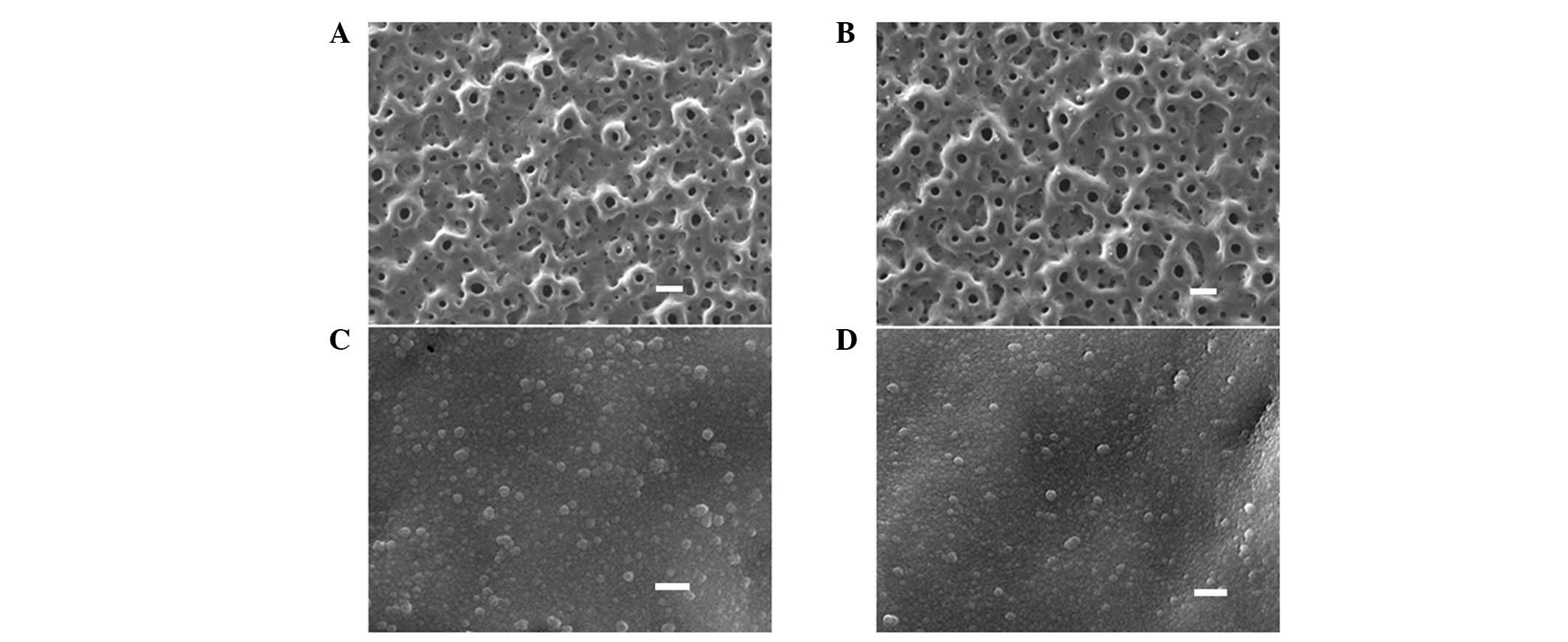
As demonstrated in Fig.
1, the low magnification views (x1,000) revealed that the
TiO2 (Fig. 1A) and
Mn-TiO2 (Fig. 1B)
coatings were porous, with a pore size of <5 μm. The pores were
well separated, and homogeneously distributed over the coating
surfaces. The high magnification views (x50,000) indicated that the
TiO2 (Fig. 1C) and
Mn-TiO2 (Fig. 1D)
coatings were covered by nanograins of ~30–50 nm. No obvious
differences in morphology were observed between the TiO2
and Mn-TiO2 coatings. Fig.
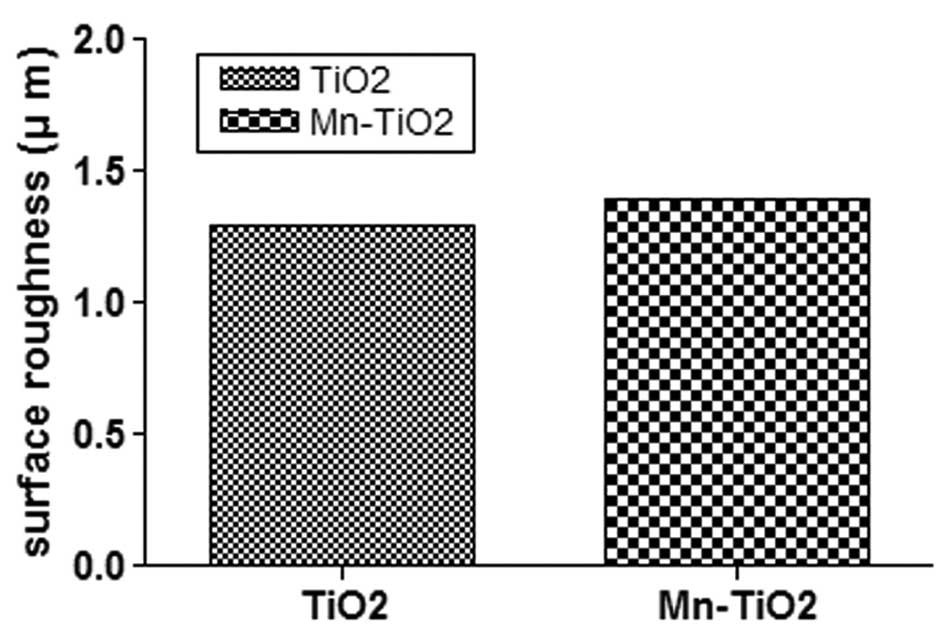
2 indicates that there was no significant difference between
the Ra of the TiO2 and Mn-TiO2 coatings
(1.3±0.1 μm and 1.4±0.2 μm, respectively).
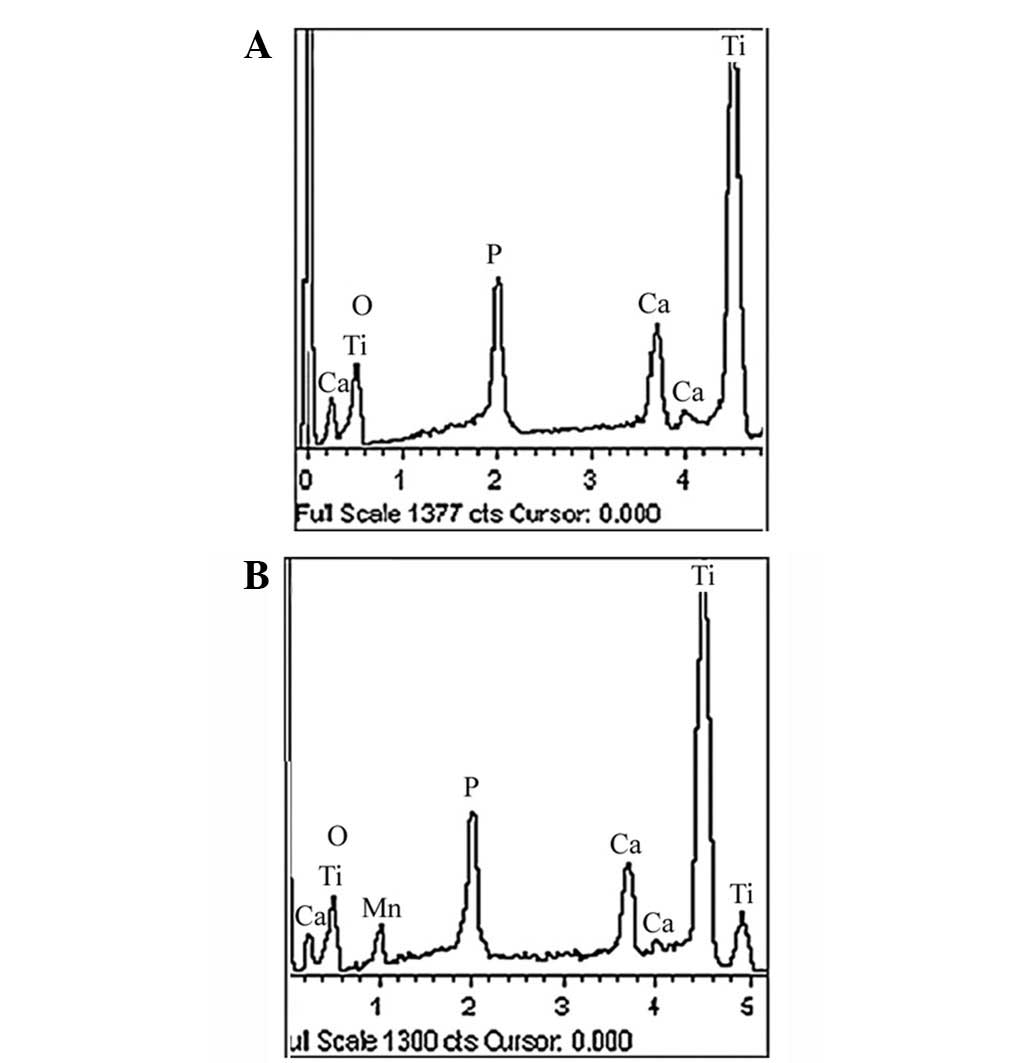
Fig. 3 displays the
elemental compositions of the surfaces of the TiO2 and
Mn-TiO2 coatings, as determined by EDS. Ti, oxygen (O),
Ca and P were detected in the TiO2 coating, while in the
Mn-TiO2 coating, Mn was detected in addition to Ti, O,
Ca and P. This indicated that Mn had been successfully incorporated
into the coating. Table II
summarizes the elemental compositions of the TiO2 and
Mn-TiO2 coatings, and reveals that the Mn content was
2.41±0.08 wt% in the Mn-TiO2 coating. Following the Mn
incorporation, the P content increased from 7.47±0.25 to 8.06±0.24
wt%, while the Ca content decreased from 7.09±0.21 to 6.04±0.19
wt%.
 | Table IIElemental compositions of the
TiO2 and Mn-TiO2 coatings, detected by
EDS. |
Table II
Elemental compositions of the
TiO2 and Mn-TiO2 coatings, detected by
EDS.
| Elemental
composition (weight %) |
|---|
|
|
|---|
| Samples | Ca | P | Ti | O | Mn |
|---|
|
TiO2 | 7.09±0.21 | 7.47±0.25 | 43.55±0.44 | 41.89±0.42 | - |
|
Mn-TiO2 | 6.04±0.19 | 8.06±0.24 | 37.31±0.36 | 46.18±0.49 | 2.41±0.08 |
The XRD patterns of the TiO2 and
Mn-TiO2 coatings are displayed in Fig. 4. The two coatings primarily
consisted of the anatase phase, while small peaks of the rutile
phase were also detected in the XRD patterns of the Mn-incorporated
sample. The incorporation of Mn only marginally altered the phase
compositions of the TiO2 coating.
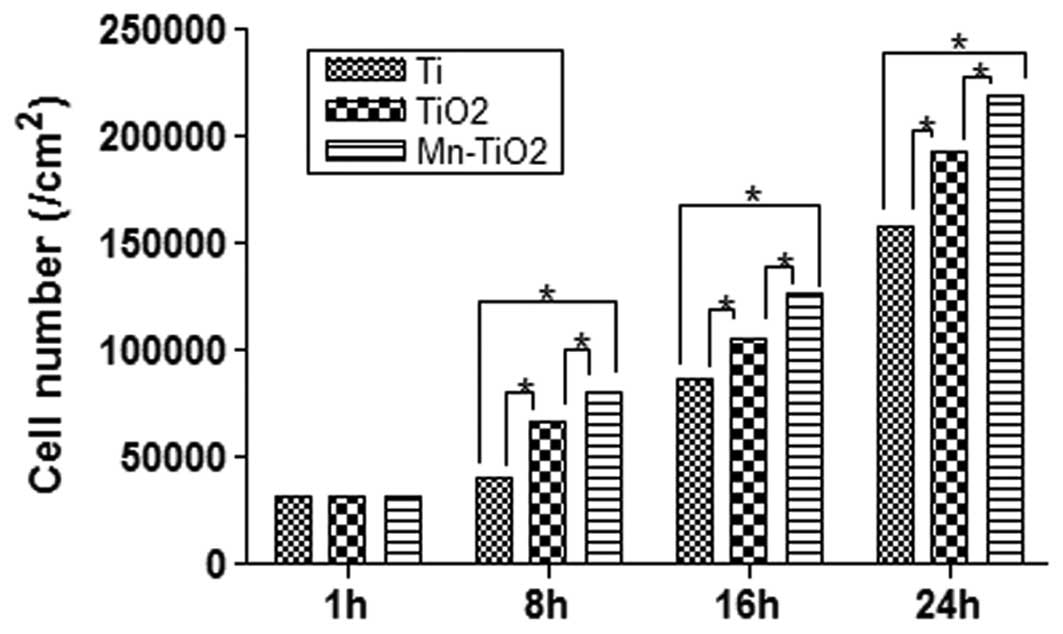
Fig. 5 displays the
results of the MTT assay used to determine the attachment of the
MG63 cells cultured on the Ti plates, and the TiO2 and
Mn-TiO2 coatings at 1, 8, 16 and 24 h. During the
initial 1 h of the incubation, the majority of the cells attached
onto each of the substrates, and no significant differences were
detected between them (P>0.05). However, the MTT assay indicated
that there was a more rapid increase in cell attachment on the
Mn-TiO2 coating, as compared with the Ti plates and
TiO2 coating. At 8, 16 and 24 h, it was observed that
the number of cells on the TiO2 coating was
significantly greater than that on the Ti plates (P<0.05), but
significantly less than that on the Mn-TiO2 coating
(P<0.05). Therefore, the Mn-TiO2 coating appeared to
provide a more favorable surface for the attachment of
osteoblasts.
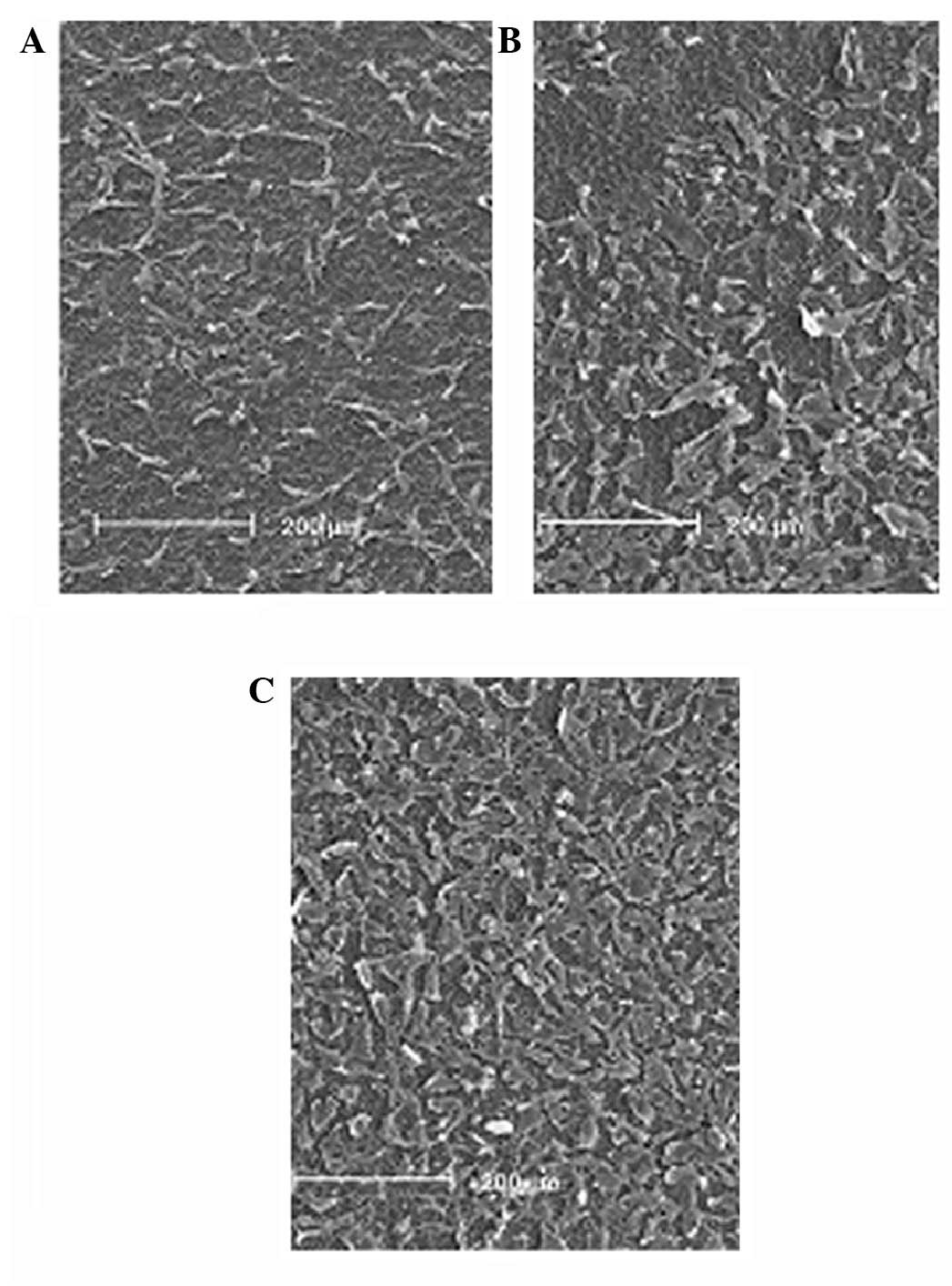
Fig. 6 reveals the
osteoblastic morphology of the cells on the three substrates. The
micrographs demonstrate that, following 16 h of culture on the
Mn-TiO2 and TiO2 coatings, and the Ti plates,
the MG63 cells exhibited different morphologies. In comparison with
the cells seeded on the TiO2 coating and the Ti plates,
those seeded on the Mn-TiO2 coating displayed a
particularly spread-out morphology, with numerous connections to
the surface.
The expression of adhesion-specific genes, including
integrins (subunits β1, β3, α1 and
α3), FAK and ERK (ERK1 and −2), were measured at 1, 8,
16 and 24 h using qPCR. It was observed that the MG63 cells
cultured on the Mn-TiO2 coating expressed higher levels
of the mRNA of integrin (subunits β1 and α1),
FAK and ERK2 compared with the cells seeded on the TiO2
coating and Ti plates. Integrins, as transmembrane heterodimeric
receptors consisting of an α- and β-subunit, are important in
mediating osteoblast adhesion onto biomaterials. Fig. 7 displays the integrin gene
expression of MG63 cells cultured on Ti plates, and TiO2
and Mn-TiO2 coatings. The integrin β1
(Fig. 7A) and α1
(Fig. 7C) gene expression of the
MG63 cells increased gradually with culture time, on each of the
substrates. During the initial 8 h, the MG63 cells seeded on the
three substrates demonstrated no significant differences in
integrin β1 and α1 gene expression
(P>0.05). When the MG63 cells were cultured on the substrates
for ≥16 h, the gene expression of integrin β1 and
α1 became significantly different, depending on the
substrate. The Mn-TiO2 coating appeared to promote
integrin β1 and α1 gene expression more
effectively than the TiO2 coating and Ti plates, and
resulted in the highest level of gene expression (P<0.05). There
were no significant differences in the gene expression of integrin
β3 or α3 between the cells seeded on the
three substrates at any time; however, as the experiment
progressed, the cells on each of the substrates demonstrated a
marked upregulation in gene expression, which persisted throughout
the course of the experiment (Figs. 7B
and D).
FAK, an integrin receptor, is important in the
integrin-mediated signal transduction pathway, which mediates
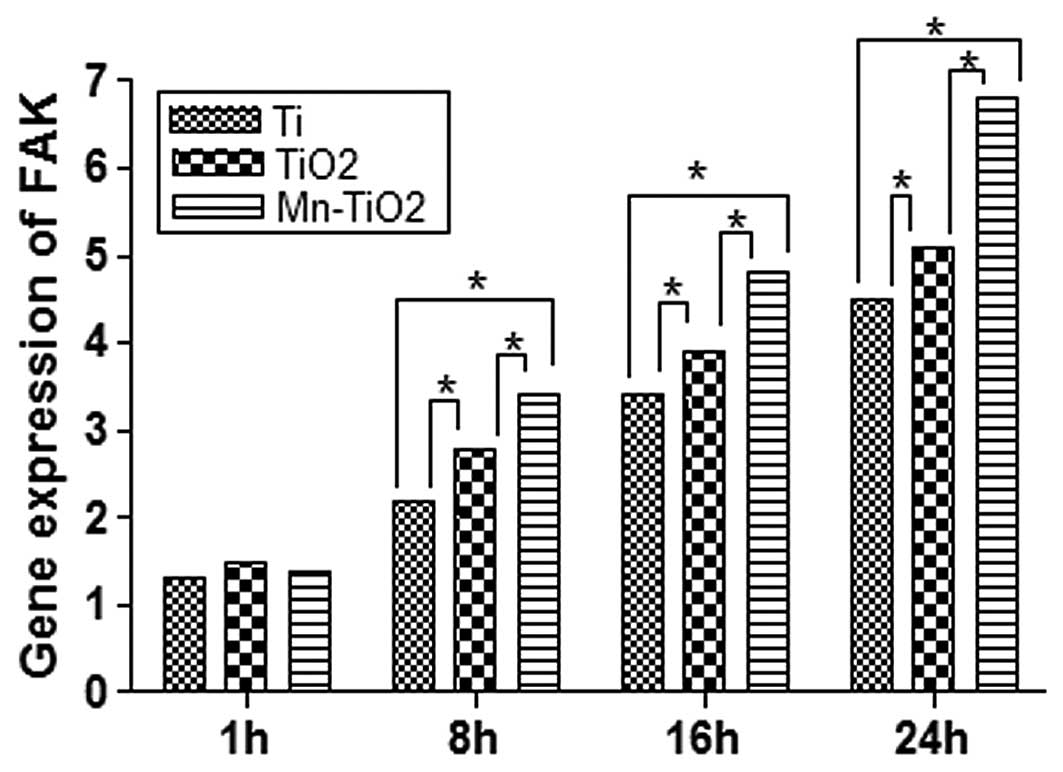
osteoblastic adhesion onto biomaterials. Fig. 8 demonstrates that at 1 h, the
differences in FAK gene expression were not statistically
significant (P>0.05). When the incubation time was ≥8 h, the FAK
gene expression of the MG63 cells seeded on the TiO2 and
Mn-TiO2 coatings was markedly upregulated. It was
observed that the MG63 cells seeded on the Mn-TiO2
coating had a significantly higher level of FAK gene expression
than those cultured on the TiO2 coating and Ti plates
(P<0.05). The trend in FAK gene expression was similar to that
of the cell attachment activity indicated in Fig. 5.
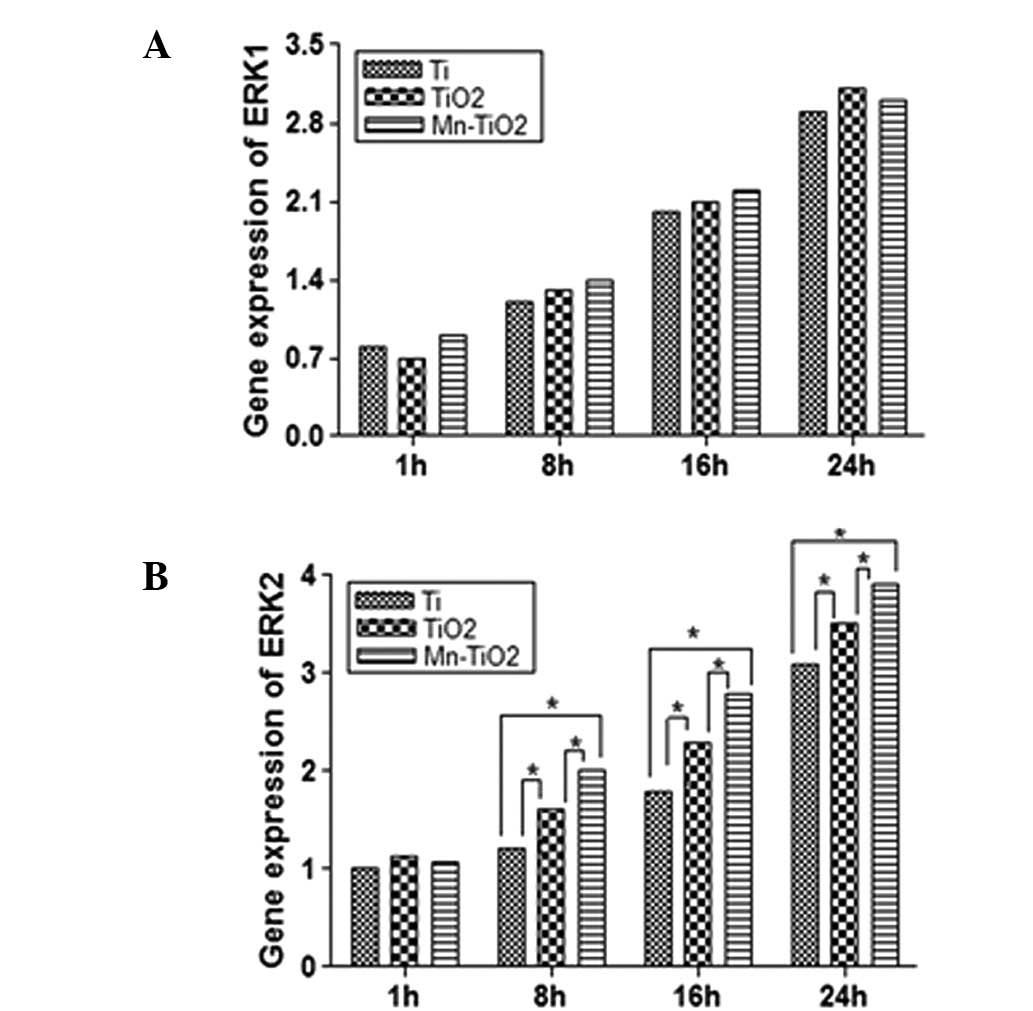
ERK (including ERK1 and −2), a member of the
mitogen-activated protein kinase (MAPK) family, is essential in the
regulation of osteoblastic adhesion, and may be stimulated as a
result of FAK activation. The gene expression of ERK1 and −2 is
displayed in Fig. 9. The results
demonstrated that the ERK2 gene expression of the MG63 cells
cultured on the Mn-TiO2 coating was upregulated at all
time points, and became significantly greater than that of the
cells on the TiO2 coating and Ti plates at 8, 16 and 24
h (Fig. 9B). With regard to the
ERK1 gene expression, there were no significant differences between
the three substrates at any of the time points, although the gene
expression was upregulated continuously throughout the experiment
(Fig. 9A).
Discussion
The initial adhesion and spreading activities of
osteoblasts characterize the first phase of cell-material
interactions, and the quality of this stage influences the capacity
of the cells to proliferate and differentiate on contact with the
implant (21,22). Preclinical and clinical studies
have demonstrated that osteoblastic adhesion on implant contact is
significantly influenced by the surface properties of the implant,
including the surface chemistry and topography (2,21,23).
In the present study, we evaluated the adhesion of MG63 cells on
three substrates (Ti plates, and TiO2 and
Mn-TiO2 coatings), and investigated the relationship
between the material of the substrate and the cell behavior.
As indicated by the MTT assay (Fig. 5), the attachment of a significantly
greater number of cells to the Mn-TiO2 coating, compared
with the TiO2 coating and the Ti plates, has certain
implications for the long-term success of this material, due to the
fact that the cell-surface integration is critical for the
incorporation of the material into the new bone. Surface chemistry
is an important factor affecting osteoblastic attachment to
biomaterials (2,21). The Mn released from
Mn-TiO2 coatings has been demonstrated to contribute to
extracellular pH changes, which alter the structure of
transmembrane proteins (9,10,11,14).
This alteration of the transmembrane proteins has been revealed to
facilitate the interaction and bonding of the transmembrane
proteins with proteins adsorbed onto the Mn-TiO2
coatings from the culture medium, which promotes the attachment of
MG63 cells onto the surface (7,10,11).
In addition to surface chemistry, the attachment of osteoblasts is
also affected by surface topography (21,24–27).
Although a variety of studies (21,25,27–30)
have revealed few consistent trends in the effects of surface
topography on initial osteoblastic attachment, the most commonly
observed trend has been that a porous structure is beneficial to
cell attachment (15,17, 21,25,28,29).
When the Mn-TiO2 coating is placed into the culture
medium, the porous nanostructured surface facilitates the
adsorption of proteins from the culture medium, by providing a
larger contact area at the sample-medium interface. This promotes
cell recruitment, and may be one of the reasons for the enhanced
osteoblastic attachment on Mn-TiO2 coatings.
Osteoblastic spreading on the biomaterial is a
process that is essential in establishing the biological properties
of the cells (21,31,32),
and may be significantly influenced by the surface chemistry of the
biomaterial (2,21,33–35).
It has been observed that incorporating Mn into a hyaluronan
coating may promote the spreading of osteoblasts (8,9). In
addition, Li et al(8)
demonstrated that osteoblasts displayed a significantly flatter
morphology on an Mn-containing coating. In the present study, we
observed that following seeding on a Mn-TiO2 coating for
16 h, MG63 cells displayed a flatter morphology, and exhibited
numerous connections to the surface, in comparison with cells on a
TiO2 coating and Ti plates (as indicated in Fig. 6). The Mn released from the
Mn-TiO2 coating may have bonded to oxygen, forming a Mn
network structure on the surface of the coating, which was capable
of holding elements of the proteins together in an organized
fashion, thus contributing to the architecture of the connective
tissue (6,8,10–12).
It is possible that these proteins may have been adsorbed onto the
Mn network structure, promoting enhanced osteoblastic spreading via
interactions with the integrins on the MG63 cells and, in turn,
triggering certain specific signals, which may have then had a
stimulatory effect on the bone mineralization process. However,
further investigation into the precise mechanism by which Mn
affects osteoblastic spreading is required. The surface topography
of the biomaterial is another factor that is important in
osteoblastic spreading (21,24,26,27).
Several studies have observed that bone cells spread and flattened
with greater efficacy on nanostructured porous coatings, compared
with rough coatings (21,36,37).
However, contrary conclusions have been drawn in other studies
(38,39), and, as a result, it has been
difficult to establish a simple conclusion with regard to the
correlation between the surface topography and osteoblastic
spreading. In the present study, we demonstrated that the spreading
of MG63 cells cultured on porous TiO2 and
Mn-TiO2 coatings for 16 h was more pronounced than that
on polished Ti plates (Fig. 6),
suggesting that the spreading was promoted by the surrounding
porous nanostructures.
When a biomaterial is placed in culture medium,
proteins adsorb to its surface. This protein layer subsequently
regulates the interaction of the biomaterial with the osteoblasts
arriving from the surrounding tissue. Integrins are essential
transmembrane molecules that are involved in the process of
osteoblastic adhesion (1). It has
been demonstrated that Mn increases the ligand-binding affinity of
integrins, thus affecting cellular interactions with the
extracellular matrix (ECM), and activating cell adhesion (10,11,13).
The present study investigated the gene expression of the integrin
subunits β1, β3, α1 and
α3. We observed a significantly higher expression of the
integrin β1 and α1 genes in the cells on the
Mn-TiO2 coating compared with those on the
TiO2 coating and Ti plates (Figs. 7A and C), following 16 h of
culture, while no significant differences were observed with regard
to the integrin β3 and α3 gene expression
(Figs. 7B and D). The results
indicated that the integrin β1 and α1
subunits may have been partially responsible for the enhanced
osteoblastic adhesion on the Mn-TiO2 coating. However, a
comparison of Fig. 5 and Fig. 7, revealed that although at 8 h a
significantly greater number of MG63 cells were attached on the
Mn-TiO2 coating than on the TiO2 coating or
Ti plates, the levels of integrin β1 and α1
gene expression were approximately equal, irrespective of the
substrate. This implied that alternative integrin subunits, in
addition to β1 and α1, may have had an
earlier effect on the process of MG63 cell attachment onto
Mn-TiO2 coatings.
Following the binding of a ligand, integrins cluster
together into focal contacts (1,40,41).
This is an area of close contact between a cell and the ECM, and
consists of additional cytoskeletal proteins, adapter molecules,
and kinases (42). Subsequent to
the clustering, cytoskeletal elements and signaling molecules are
recruited and activated, in a process known as outside-in signaling
(41,42). The signaling pathway inside the
cell is complex, and involves the accumulation of several proteins,
including FAK, Src, and cytoskeletal proteins (43). The activation of FAK initiates
intracellular signal transduction cascades, including those
involved in the MAPK effector cascades and the remodeling of the
cytoskeleton. These effects, in turn, regulate cellular processes
such as adhesion, growth, and differentiation (43). In the present study, it was
observed that the FAK gene expression in the MG63 cells cultured on
the Mn-TiO2 coating was significantly higher compared
with that of the cells cultured on a TiO2 coating or Ti
plates at 8, 16, and 24 h (Fig.
8). This revealed a similar trend to that of the gene
expression of the integrin β1 and α1
subunits, indicating that these subunits may have been involved in
stimulating FAK activation at 16 and 24 h. It is possible that
alternative integrin subunits may have contributed to the
activation of FAK, leading to the higher expression of FAK in the
cells on the Mn-TiO2 coating at 8 h.
Integrin and FAK molecules may provide a platform
for intracellular signaling; however, they are not able to exhibit
intrinsic enzymatic activity in their cytoplasmic domains.
Downstream signaling, following integrin binding, is regulated by
non-receptor tyrosine kinases (43), and one such pathway is the ERK/MAPK
signaling cascade (44). Several
studies have demonstrated that integrin engagement and FAK
activation stimulated ERK expression (43,44).
In the current study, we observed that the gene expression of ERK2
(Fig. 9B) was similar to that of
the integrin β1, α1 subunits (Fig. 7A and C) and FAK (Fig. 8), which implied that integrin
β1 and α1-mediated activation of FAK may have
been responsible for the enhanced ERK2 expression on the
Mn-TiO2 coating at 16 and 24 h. The increased ERK2 gene
expression at 8 h on the Mn-TiO2 coating may have been
induced by alternative integrin subunits, in addition to
β1 and α1.
In conclusion, the present study prepared a porous
and nanostructured Mn-TiO2 coating by PEO, using a novel
Mn-containing electrolyte, which successfully incorporated Mn into
the coating. The microstructure, Ra and phase composition of the
TiO2 coating were not altered following the
incorporation of Mn; however, the Mn-incorporated TiO2
coating was demonstrated to exhibit biological activity in
promoting the adhesion of MG63 cells. Moreover, the present study
indicated that Mn-TiO2 coatings may modulate
osteoblastic proliferation and differentiation, a process regulated
by the ERK/MAPK signaling pathway, through integrin-FAK mediated
cellular adhesion. There are limitations to using an evaluation of
initial cell adhesion as an end point for a screening assay of
potential material surfaces; however, further studies involving
in vitro cellular calcification and mineralization assays,
along with in vivo histological observations are currently
in progress.
References
|
1
|
Hamilton DW and Brunette DM: The effect of
substratum topography on osteoblast adhesion mediated signal
transduction and phosphorylation. Biomaterials. 28:1806–1819. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen J, Ulerich JP, Abelev E, Fasasi A, et
al: An investigation of the initial attachment and orientation of
osteoblast-like cells on laser grooved Ti-6Al-4V surfaces. Mat Sci
Eng C. 29:1442–1452. 2009. View Article : Google Scholar
|
|
3
|
von Wilmowsky C, Bauer S, Lutz R, Meisel
M, et al: In vivo evaluation of anodic TiO2 nanotubes:
an experimental study in the pig. J Biomed Mater Res B Appl
Biomater. 89:165–171. 2009.PubMed/NCBI
|
|
4
|
Aita H, Hori N, Takeuchi M, Suzuki T, et
al: The effect of ultraviolet functionalization of titanium on
integration with bone. Biomaterials. 30:1015–1025. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Advincula MC, Rahemtulla FG, Advincula RC,
Ada ET, et al: Osteoblast adhesion and matrix mineralization on
sol-gel-derived titanium oxide. Biomaterials. 27:2201–2212. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bracci B, Torricelli P, Panzavolta S,
Boanini E, et al: Effect of Mg2+, Sr2+, and
Mn2+ on the chemico-physical and in vitro biological
properties of calcium phosphate biomimetic coatings. J Inorg
Biochem. 103:1666–1674. 2009.
|
|
7
|
Park JW, Kim YJ and Jang JH: Surface
characteristics and in vitro biocompatibility of a
manganese-containing titanium oxide surface. Appl Surf Sci.
258:977–985. 2011. View Article : Google Scholar
|
|
8
|
Li Y, Widodo J, Lim S and Ooi CP:
Synthesis and cytocompatibility of manganese (II) and iron (III)
substituted hydroxyapatite nanoparticles. J Mater Sci. 47:754–763.
2012. View Article : Google Scholar
|
|
9
|
Paluszkiewicz C, Œlósarczyk A, Pijocha D,
Sitarz M, et al: Synthesis, structural properties and thermal
stability of Mn-doped hydroxyapatite. J Mol Struct. 976:301–309.
2010. View Article : Google Scholar
|
|
10
|
Dormond O, Ponsonnet L, Hasmim M, Foletti
A and Rüegg C: Manganese-induced integrin affinity maturation
promotes recruitment of alpha V beta 3 integrin to focal adhesions
in endothelial cells: evidence for a role of phosphatidylinositol
3-kinase and Src. Thromb Haemost. 92:151–161. 2004.
|
|
11
|
Legler DF, Wiedle G, Ross FP and Imhof BA:
Superactivation of integrin αvβ3 by low antagonist concentrations.
J Cell Sci. 114:1545–1553. 2001.
|
|
12
|
Lüthen F, Lange R, Becker P, Rychly J, et
al: The influence of surface roughness of titanium on beta1- and
beta3-integrin adhesion and the organization of fibronectin in
human osteoblastic cells. Biomaterials. 26:2423–2440.
2005.PubMed/NCBI
|
|
13
|
Lüthen F, Bulnheim U, Müller PD, Rychly J,
et al: Influence of manganese ions on cellular behavior of human
osteoblasts in vitro. Biomol Eng. 24:531–536. 2007.PubMed/NCBI
|
|
14
|
Zreiqat H, Howlett CR, Zannettino A, Evans
P, et al: Mechanisms of magnesium-stimulated adhesion of
osteoblastic cells to commonly used orthopaedic implants. J Biomed
Mater Res. 62:175–184. 2002. View Article : Google Scholar
|
|
15
|
Hu H, Zhang W, Qiao Y, Jiang X, et al:
Antibacterial activity and increased bone marrow stem cell
functions of Zn-incorporated TiO2 coatings on titanium.
Acta Biomater. 8:904–915. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Y, Lee IS, Cui FZ and Choi SH: The
biocompatibility of nanostructured calcium phosphate coated on
micro-arc oxidized titanium. Biomaterials. 29:2025–2032. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Han Y, Chen DH, Sun J, Zhang Y and Xu K:
UV-enhanced bioactivity and cell response of micro-arc oxidized
titania coatings. Acta Biomater. 4:1518–1529. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sul YT, Johansson C, Byon E and
Albrektsson T: The bone response of oxidized bioactive and
non-bioactive titanium implants. Biomaterials. 26:6720–6730. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee JM, Lee JI and Lim YJ: In vitro
investigation of anodization and CaP deposited titanium surface
using MG63 osteoblast-like cells. Appl Surf Sci. 256:3086–3092.
2010. View Article : Google Scholar
|
|
20
|
Wei D, Zhou Y and Yang C: Characteristic,
cell response and apatite-induction ability of microarc oxidized
TiO2-based coating containing P on Ti6Al4V before and
after chemical-treatment and dehydration. Ceram Int. 35:2545–2554.
2009. View Article : Google Scholar
|
|
21
|
Anselme K: Osteoblast adhesion on
biomaterials. Biomaterials. 21:667–681. 2000. View Article : Google Scholar
|
|
22
|
Silva GA, Coutinho OP, Ducheyne P, Shapiro
IM and Reis RL: The effect of starch and starch-bioactive glass
composite microparticles on the adhesion and expression of the
osteoblastic phenotype of a bone cell line. Biomaterials.
28:326–334. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kennedy SB, Washburn NR, Simon CG Jr and
Amis EJ: Combinatorial screen of the effect of surface energy on
fibronectin-mediated osteoblast adhesion, spreading and
proliferation. Biomaterials. 27:3817–3824. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kang IC, Kim TS, Ko KK, Song HY, et al:
Microstructure and osteoblast adhesion of continuously porous
Al2O3 body fabricated by fibrous monolithic
process. Mater Lett. 59:69–73. 2005. View Article : Google Scholar
|
|
25
|
Linez-Bataillon P, Monchau F, Bigerelle M
and Hildebrand HF: In vitro MC3T3 osteoblast adhesion with respect
to surface roughness of Ti6Al4V substrates. Biomol Eng. 19:133–141.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zinger O, Anselme K, Denzer A, Habersetzer
P, et al: Time-dependent morphology and adhesion of osteoblastic
cells on titanium model surfaces featuring scale-resolved
topography. Biomaterials. 25:2695–2711. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bacakova L, Grausova L, Vacik J, Fraczek
A, et al: Improved adhesion and growth of human osteoblast-like MG
63 cells on biomaterials modified with carbon nanoparticles. Diam
Relat Mater. 16:2133–2140. 2007. View Article : Google Scholar
|
|
28
|
Rodil SE, Ramírez C, Olivares R, Arzate H,
et al: Osteoblasts attachment on amorphous carbon films. Diam Relat
Mater. 15:1300–1309. 2006. View Article : Google Scholar
|
|
29
|
Huang HH, Ho CT, Lee TH, Lee TL, et al:
Effect of surface roughness of ground titanium on initial cell
adhesion. Biomol Eng. 21:93–97. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dalby MJ, Kayser MV, Bonfield W and Di
Silvio L: Initial attachment of osteoblasts to an optimised HAPEX
topography. Biomaterials. 23:681–690. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Finke B, Luethen F, Schroeder K, Mueller
PD, et al: The effect of positively charged plasma polymerization
on initial osteoblastic focal adhesion on titanium surfaces.
Biomaterials. 28:4521–4534. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tang ZG and Hunt JA: The effect of PLGA
doping of polycaprolactone films on the control of osteoblast
adhesion and proliferation in vitro. Biomaterials. 27:4409–4418.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hillberg AL, Holmes CA and Tabrizian M:
Effect of genipin cross-linking on the cellular adhesion properties
of layer-by-layer assembled polyelectrolyte films. Biomaterials.
30:4463–4470. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pallu S, Bourget C, Bareille R, Labrugère
C, et al: The effect of cyclo-DfKRG peptide immobilization on
titanium on the adhesion and differentiation of human
osteoprogenitor cells. Biomaterials. 26:6932–6940. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Feng B, Weng J, Yang BC, Qu SX and Zhang
XD: Characterization of titanium surfaces with calcium and
phosphate and osteoblast adhesion. Biomaterials. 25:3421–3428.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xue WC, Krishna BV, Bandyopadhyay A and
Bose S: Processing and biocompatibility evaluation of laser
processed porous titanium. Acta Biomater. 3:1007–1018. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yun KD, Yang YZ, Lim HP, Oh GJ, et al:
Effect of nanotubular-micro-roughened titanium surface on cell
response in vitro and osseointegration in vivo. Mat Sci Eng C.
30:27–33. 2010. View Article : Google Scholar
|
|
38
|
Randeniya LK, Bendavid A, Martin PJ, Amin
MS, et al: Thin-film nanocomposites of diamond-like carbon and
titanium oxide; Osteoblast adhesion and surface properties. Diam
Relat Mater. 19:329–335. 2010. View Article : Google Scholar
|
|
39
|
Liu XM, Lim JY, Donahue HJ, Dhurjati R, et
al: Influence of substratum surface chemistry/energy and topography
on the human fetal osteoblastic cell line hFOB 1.19: Phenotypic and
genotypic responses observed in vitro. Biomaterials. 28:4535–4550.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rouahi M, Champion E, Hardouin P and
Anselme K: Quantitative kinetic analysis of gene expression during
human osteoblastic adhesion on orthopaedic materials. Biomaterials.
27:2829–2844. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rubin J, Rubin C and Jacobs CR: Molecular
pathways mediating mechanical signaling in bone. Gene. 367:1–16.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Siebers MC, ter Brugge PJ, Walboomers XF
and Jansen JA: Integrins as linker proteins between osteoblasts and
bone replacing materials. A critical review. Biomaterials.
26:137–146. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wilson CJ, Clegg RE, Leavesley DI and
Pearcy MJ: Mediation of biomaterial-cell interactions by adsorbed
proteins: a review. Tissue Eng. 11:1–18. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Prasadam I, Friis T, Shi W, van Gennip S,
et al: Osteoarthritic cartilage chondrocytes alter subchondral bone
osteoblast differentiation via MAPK signalling pathway involving
ERK1/2. Bone. 46:226–235. 2010. View Article : Google Scholar : PubMed/NCBI
|