Introduction
Platelet cryopreservation technology has been an
area of particular interest for many years. Cryoprotectants and
various additives are necessary for platelets undergoing
cryopreservation, in order to prevent the platelet membrane from
undergoing damage during cryopreservation and affecting the normal
function of the platelets following rewarming. Cryoprotectants may
function by increasing the concentration of solute in the cells and
reducing the number of ice crystals formed at any temperature.
Common cryoprotectants included glycerol, dimethylsulfoxide (DMSO),
ethylene glycol and propylene glycol.
DMSO has been demonstrated to be superior to other
cryoprotectants with regard to the protective effect it exerts on
frozen platelets (1). The addition
of S-nitrosoglutathione (GSNO) to frozen platelets is able to
inhibit platelet activation and maintain their aggregation
activity, indicating the potential of GSNO as a platelet
cryoprotectant (2). The addition
of GSNO (a type of stable nitrosothiol) as a nitric oxide (NO)
donor to frozen platelets as a supplement, when DMSO alone is
insufficient, may prevent platelet consumption and maintain the
function of the platelets.
NO levels are an important factor in blood,
particularly with regard to the efficacy and safety of red blood
cells stored for use in transfusion. The loss of NO in stored blood
has become a concern in blood transfusion safety. NO may inhibit
platelet function, primarily by raising the levels of cyclic
guanosine monophosphate (cGMP). However, non-cGMP-dependent
mechanisms, such as S-nitrosylation, have also been suggested as
alternative NO-mediated signaling pathways (3). The application of NO in platelet
preservation was investigated in a study by Wong and Li, in which
an NO precursor was added to platelets for the in vivo
synthesis of NO using nitric oxide synthetase (NOS); in addition, a
certain quantity of NO solution directly injected into the
platelets was observed to improve the platelet function (4).
Platelet membrane glycoproteins are specific
glycoprotein components that are located inside the platelet
membrane, on the membrane surface and in the plasma. Platelet
membrane glycoproteins are important in initial hemostasis,
platelet adhesion to the extracellular matrix and the subsequent
platelet aggregation process. A lack of platelet membrane
glycoproteins may lead to platelet dysfunction. Platelet function
may be accurately detected using flow cytometry and various
monoclonal antibodies specific to platelet membrane glycoproteins
(5).
Several in vitro functional experiments have
revealed defects in frozen platelets, although the in vivo
hemostatic function of the frozen platelets has been observed to be
markedly enhanced. Furthermore, the immediate hemostatic function
of frozen platelets has been demonstrated to be significantly
improved in comparison with that of liquid-stored platelets
(1). Further studies are required
to confirm whether the significant enhancement of the in
vivo hemostatic function of the cryopreserved platelets is
correlated with changes in the platelet membrane glycoproteins, and
whether the GSNO-mediated inhibition of cryopreserved platelet
aggregation is associated with changes in NO levels and with
alterations in the platelet membrane glycoproteins.
In the present study, we detected the levels of NO
in fresh liquid platelets by the nitrate reductase method. In
addition, the aggregation rate and NO content of frozen platelets
were monitored and compared, prior to and following the addition of
GSNO. Furthermore, in order to explore the possible mechanism
leading to the significantly enhanced in vivo hemostatic
function of cryopreserved platelets, and the inhibitory effect of
GSNO on platelet aggregation, we studied the expression of platelet
membrane glycoproteins prior to and following platelet
cryopreservation, and following treatment with GSNO.
Materials and methods
Specimen collection
Platelets were collected from donors by platelet
apheresis, in accordance with the medical standards of blood
donation in China. The donors did not take aspirin or any similar
anti-platelet/anticoagulant drugs within 2 weeks prior to the
donation. The peripheral blood platelet count of the samples was
>1.5×1011/l. A total of 32 platelet apheresis
donations complying with the previously mentioned conditions were
randomly selected, 12 cases of which were used to determine the
platelet count, membrane glycoprotein expression and aggregation
rates. The study was conducted in accordance with the Declaration
of Helsinki, and with approval from the Ethics Committee of the
Chinese PLA General Hospital (Beijing, China). Written informed
consent was obtained from all participants.
Specimen preparation
Three 1.9 ml samples of each platelet apheresis
donation were collected in Eppendorf tubes under sterile
conditions, following gentle blending. Out of these three samples,
one was used for the determination of NO levels. A total of 100 μl
DMSO (Sigma-Aldrich, St. Louis, MO, USA) was added to the remaining
two samples, respectively, which were then placed on a level
oscillator, with an oscillation frequency of 60–70 times/min. The
final concentration of the DMSO was 5%. Following this, 3.4 μl GSNO
(1 mg/ml, fresh) was added to one of the two samples, at a final
concentration of 10 μM. The two samples were then balanced at 22°C
for 10 min and rapidly cryopreserved at −80°C. One week later, the
two samples, i.e. the frozen platelet group (DMSO-treated) and the
GSNO group (DMSO+GSNO-treated) were removed and rapidly defrosted
at 37°C in order to determine the aggregation rate, membrane
glycoprotein expression and NO content.
Quantitative measurement of platelet
aggregation
The platelet level in each group was adjusted to
(2–3)x106/ml with the original
plasma. The reaction volume was set at 250 μl. Platelet-poor plasma
(PPP) from the same sample was used as the substrate, followed by
pressing the key of PPP. It was not necessary to pre-warm the PPP,
and a magnetic stirring rod was not added to the PPP test cup.
Instead, a magnetic rod was placed into the platelet-rich plasma
(PRP) test cup, which was then added to the test channel and
pre-warmed in the test area for 1 min. Following the prewarming, 10
μl (1 mmol/l) adenosine diphosphate (ADP; Sigma-Aldrich) was added
to each test cup with a micropipette. An SC-2000 platelet
aggregation instrument (Succeeder Technology Development Co., Ltd.,
Beijing, China) was used to measure the platelet aggregation.
Determination and calculation of the NO
content of platelets
An NO kit (comprising nitrate reductase) was
purchased from Nanjing Jiancheng Technology Co., Ltd. (Nanjing,
China). The detector tubes were divided into three groups: blank,
standard and test tubes. The components in each tube are displayed
in Table I. The components were
added to each tube and mixed at 37°C in a water bath for 60 min.
Reagents were added to each tube and fully vortically blended for
30 sec. The tubes were then left to stand at room temperature for
40 min, prior to being centrifuged at 2,740–3,580 × g for 10 min.
Following this, 0.5 ml supernatant was blended into 0.6 ml
chromogenic agent and left to stand at room temperature for 10 min.
Distilled water was used for zero adjustment. The absorbance value
in each tube was measured at 550 nm, with a light path of 0.5
cm.
 | Table IComponents added to each of the
tubes. |
Table I
Components added to each of the
tubes.
| Type of tube |
|---|
|
|
|---|
| Component | Blank | Standard | Test |
|---|
| Double-distilled
water (ml) | 0.1 | - | - |
| 100 μmol/l standard
solution (ml) | - | 0.1 | - |
| Sample (3 types of
platelets, ml) | - | - | 0.1 |
| Mixing reagent
(ml) | 0.4 | 0.4 | 0.4 |
The content of NO (μmol/l) was calculated according
to the following formula, based on the measured absorbance values
of the various tubes: NO content=[(absorbance of test
tube-absorbance of blank tube)/(absorbance of standard
tube-absorbance of blank tube)] × concentration of standard
substance.
Determination of platelet membrane
glycoprotein expression
Peridinin chlorophyll protein complex
(PerCP)-labeled anti-CD61, fluorescein isothiocyanate
(FITC)-labeled PAC-1, phycoerythrin (PE)-labeled CD62P,
allophycocyanin (APC)-labeled CD42b,, PE-labeled immunoglobulin G
(IgG; negative control for CD62P) and APC-labeled IgG (negative
control for CD42b) antibodies were purchased from Becton Dickinson
(BD Biosciences, Franklin Lakes, NJ, USA). RGDS, as a blocker for
PAC-1, combining with PAC-1, was used as negative control for
PAC-1.
Labeled antibody were mixed for control tube and
test tube. Mixed antibody (20 μl) and 10 μl platelets were added to
each test tube, in accordance with Table II, while 20 μl mixed antibody and
10 μl platelets were added to each control tube. The tubes were
kept away from the light at room temperature for 15 min, prior to
the addition of 500 μl 1% paraformaldehyde to the tubes. The tubes
were then stored away from the light at 4°C.
 | Table IICombinations of fluorescent antibodies
in the control and test tubes. |
Table II
Combinations of fluorescent antibodies
in the control and test tubes.
| Quantity added
(μl) |
|---|
|
|
|---|
| Tube | CD61 PerCP | PAC-1 FITC | CD62P PE | CD42b APC | RGDS | IgG PE | IgG APC |
|---|
| Control tube | 100 | 50 | - | - | 50 | 100 | 100 |
| Test tube | 100 | 100 | 100 | 100 | - | - | - |
The analysis of the results from the four-color flow
cytometry was performed as follows: Data were obtained from the
cytometer and CellQuest software (BD Biosciences) was opened
following startup. The control and test tubes were placed in the
correct order and the condition of access (CD61-PerCP positivity)
was chosen. The log pattern was selected in the forward scatter
detector (FSC) and the side scatter detector (SSC), and the
photomultiplier tubes (PMTs) of first fluorescence (FL1) and second
fluorescence (FL2) were adjusted using the control tubes. The
negative population was located on the lower left corner of the FL1
versus FL2 dot plot. The FL2-FL1 and FL1-FL2 compensations were
adjusted using PAC-1 FITC/IgG PE/CD61 PerCP and PAC-1
FITC+RGDS/CD62P PE/CD61 PerCP, respectively. The cytometry data of
various tubes, the contents of which are presented in Table II, were obtained.
The platelet group was identified for gating in the
CD61 versus SSC dot plot. The CD61 versus SSC dot plot displayed
three groups, i.e., the CD61-positive/low-SSC group (predominantly
composed of platelets), the CD61-positive/high-SSC group
(predominantly composed of blood cells adhered to platelets) and
the CD61-positive/lower-scattered light group (predominantly
composed of platelet-derived fragments). Since the size and the
graininess of the platelet and red blood cell groups are similar
under physiological and pathological conditions, it was not
recommend that the FSC-SSC plot was used for gating. Therefore, the
platelet group was identified for gating in the CD61 versus SSC dot
plot (platelets and platelets adhered to white blood cells). The
two-parameter analysis of PAC-1 FITC versus CD62P PE was performed
inside the gating to obtain statistical results.
Statistical analysis
The data are presented as the mean ± standard
deviation. All statistical data were analyzed using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). The results of NO content,
aggregation rate and expression of membrane glycoproteins for the
three groups of platelets (fresh liquid blood platelets, frozen
platelets and frozen platelets treated with GSNO) were compared.
Comparisons were evaluated using an independent sample t-test.
P<0.05 was considered to indicate a statistically significant
result.
Results
Platelet aggregation rate
As demonstrated in Table III, the ADP-induced platelet
aggregation rate was 63.44±2.96 and 35.47±2.93% in the fresh liquid
platelet and frozen platelet groups, respectively. The result for
the frozen platelet group was significantly lower than that for the
fresh liquid platelet group (P=0.000). The ADP-induced platelet
aggregation rate was 24.43±3.07% in the GSNO-treated frozen
platelet group, which was significantly lower than that in the
other two groups. The results indicate that DMSO and GSNO exerted
certain protective functions in platelet aggregation, which was
reflected in the weakening of the aggregation response to the
ADP-induced polymerization.
 | Table IIIAggregation of the three groups of
platelets. |
Table III
Aggregation of the three groups of
platelets.
| Group | n | Aggregation (%) |
|---|
| Fresh liquid
platelets | 12 | 63.44±2.96 |
| Frozen platelets | 12 | 35.47±2.93a |
| Frozen platelets
treated with GSNO | 12 | 24.43±3.07b |
NO level in platelets
Statistical analysis revealed that the NO level in
the fresh liquid platelets from 32 normal blood donors was
31.59±16.88 μmol/l, whereas the NO level in the frozen platelets
from 32 normal blood donors was 22.16±6.38 μmol/l. The NO level in
the GSNO-treated frozen platelets from 32 normal blood donors was
45.64 6.31 μmol/l (Table IV).
 | Table IVNO concentration of the three groups
of platelets. |
Table IV
NO concentration of the three groups
of platelets.
| Group | n | NO concentration
(μmol/l) |
|---|
| Fresh liquid
platelets | 32 | 31.59±16.88 |
| Frozen platelets | 32 | 22.16±6.38a |
| Frozen platelets
treated with GSNO | 32 | 45.64±6.31b |
As demonstrated in Table IV, the NO level in the fresh
liquid platelet group was significantly higher compared with that
in the frozen platelet group (t=2.958, P=0.004), whereas the NO
level in the fresh liquid platelet group was significantly lower
compared with that in the GSNO-treated frozen platelet group
(t=4.289, P=0.000).
Platelet flow cytometry
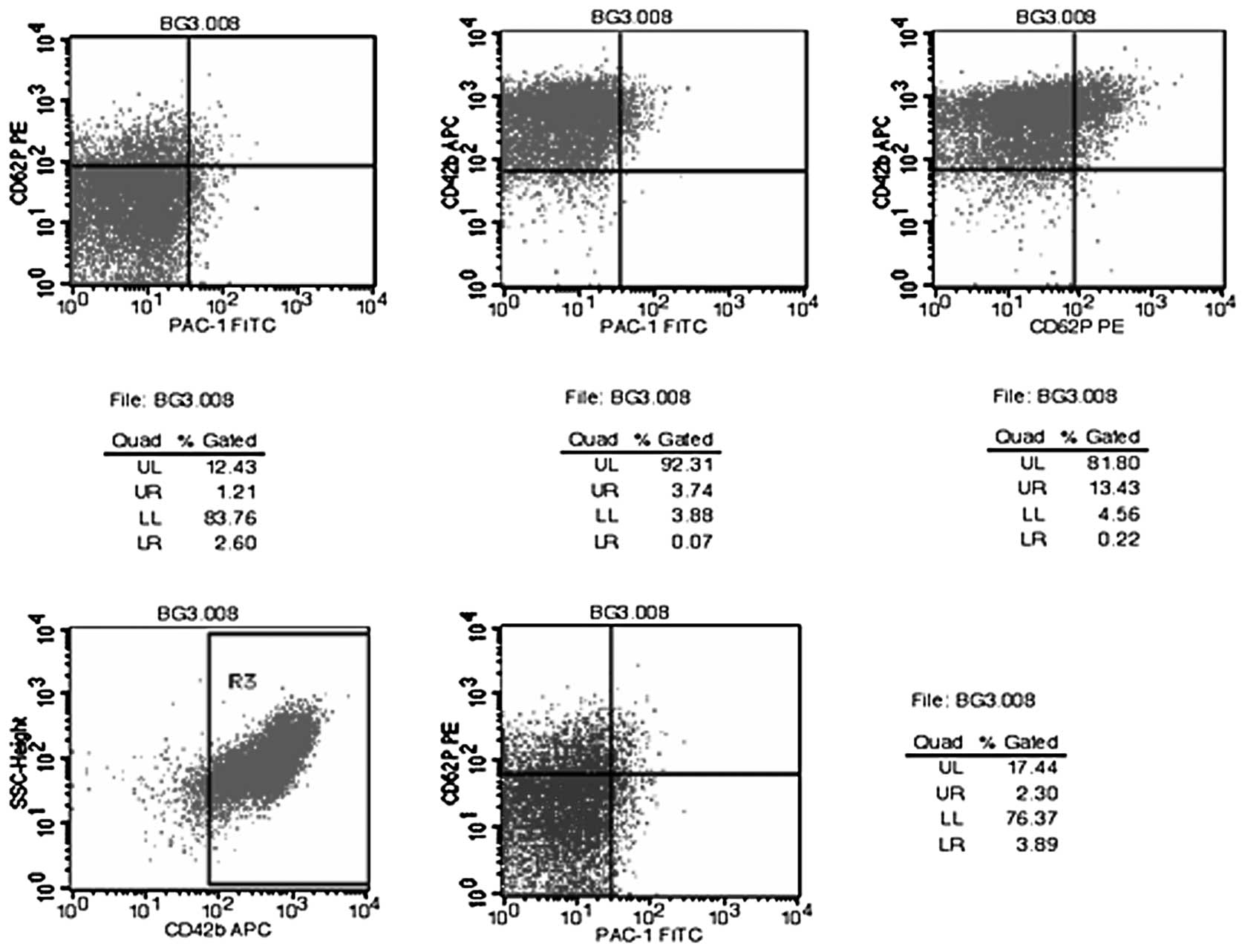
Flow cytometry charts for the tests of random
samples of platelet membrane glycoprotein molecules are displayed
in Figs. 1–4. The results of the flow cytometric
analysis of the fresh liquid platelet, frozen platelet and
GSNO-treated frozen platelet groups are displayed in Table V.
 | Table VFlow cytometry data of platelets in
the three platelet groups. |
Table V
Flow cytometry data of platelets in
the three platelet groups.
| Group | PAC-1
single-positive | CD62P
single-positive | CD42b
single-positive | PAC-1+CD62P
dual-positive | PAC-1+CD42b
dual-positive | CD62P+CD42b
dual-positive | PAC-1+CD62P+CD42b
tri-positive |
|---|
| Fresh liquid
platelets | 8.74±6.51 | 12.74±9.64 | 90.46±6.65 | 3.11±3.66 | 8.98±6.48 | 10.34±7.49 | 4.25±3.07 |
| Frozen platelets | 9.72±6.01 | 31.72±8.20 | 76.94±15.66 | 6.39±4.48 | 9.76±6.07 | 28.49±7.74 | 8.74±5.25 |
| Frozen platelets with
GSNO | 6.60±3.48 | 26.34±9.97 | 78.32±12.54 | 3.75±2.39 | 6.58±3.49 | 23.99±9.26 | 4.91±2.32 |
The differences in membrane glycoprotein expression
in the fresh liquid platelet, frozen platelet and GSNO-treated
frozen platelet groups (groups 1, 2 and 3, respectively) are
demonstrated in Table V. There
were no significant differences in the single-positive PAC-1
expression among the three groups (comparison between groups 1 and
2, P=0.743; comparison between groups 1 and 3, P=0.473; comparison
between groups 2 and 3, P=0.297). However, the single-positive
CD62P expression in group 1 was significantly lower than that of
groups 2 and 3 (P=0.000 and P=0.001, respectively), although there
was no significant difference between groups 2 and 3 (P=0.165).
Furthermore, the expression of single-positive CD42b in group 1 was
significantly higher than that of groups 2 and 3 (P=0.007 and
P=0.015, respectively), but no significant difference was observed
between groups 2 and 3 (P=0.778).
There were no significant differences in the
dual-positive PAC-1+CD62P expression among the three groups (group
1 versus group 2, P=0.125; group 1 versus group 3, P=0.765; group 2
versus group 3, P=0.165), or in the dual-positive PAC-1+CD42b
expression (group 1 and versus group 2, P=0.793; group 1 versus
group 3, P=0.424; group 2 versus group 3, P=0.290). However, the
dual-positive CD62P+CD42b expression in group 1 was significantly
lower to that in groups 2 and 3 (P=0.000 and P=0.000,
respectively). There was no significant difference between groups 2
and 3 (P=0.207).
There were no significant differences in
tri-positive PAC-1+CD62P+CD42b expression among the three groups
(group 1 versus group 2, P=0.114; group 1 versus group 3, P=0.815;
group 2 versus group 3, P=0.176).
Discussion
The enhancement of in vivo hemostatic
function in cryopreserved platelets may be correlated with changes
in the expression of platelet membrane glycoproteins, while the
inhibition of platelet aggregation in cryopreserved platelets by
GSNO may be associated with alterations in the NO levels. The
current study was conducted to further investigate changes in
platelet membrane glycoprotein expression.
The results of the present study revealed that with
regard to the expression of platelet membrane glycoproteins in the
fresh liquid platelet, frozen platelet and GSNO-treated frozen
platelet groups, the differences in the expression of PAC-1 were
not statistically significant, i.e. there were no significant
differences in the single-positive PAC-1, dual-positive
PAC-1+CD62P, dual-positive PAC-1+CD42b and tri-positive
PAC-1+CD62P+CD42b expression levels among the three groups.
However, the CD62P expression was significantly lower in the fresh
liquid platelet group compared with that in the frozen platelet and
GSNO-treated frozen platelet groups (P=0.000 and P=0.001,
respectively), and the CD42b expression in the fresh liquid
platelet group was significantly higher than that in the frozen
platelet and GSNO-treated frozen platelet groups (P=0.007 and
P=0.015, respectively). Furthermore, the CD62P+CD42b expression in
the fresh liquid platelet group was significantly lower than that
in the frozen platelet and GSNO-treated frozen platelet groups
(P=0.000 for each). However, the expression of platelet membrane
glycoproteins in the frozen platelet group was not significantly
different from that in the GSNO-treated frozen platelet group.
When resting platelets are activated, the platelet
membrane glycoproteins rapidly undergo changes in number and
structure. The changes in the platelet membrane are of primary
importance in altering platelet survival. At the time of storage,
the platelet may be activated, injured and cleared (6). Avoiding platelet activation may
improve the recovery rate and prolong the survival time. The injury
occurring to platelets as a result of preservation has been
considered an important factor leading to the failure of platelets
following long-term preservation. Platelet injury during
preservation has been revealed to be correlated with in
vitro platelet activation, i.e., the higher the degree of in
vitro platelet activation, the more serious the platelet
preservation injury (6). The
presence of the glycoprotein GPIIb/IIIa complex is currently
accepted as the ideal evaluation index of platelet activation.
Platelet aggregation function is an important factor that is
closely associated with the platelet hemostatic effect, and is
primarily dependent on the quality and quantity of membrane
glycoproteins GPIIb/IIIa present on the surface of the platelets.
Platelet aggregation rate is an important indicator reflecting the
efficacy of the platelet aggregation function.
PAC-1, a type of monoclonal antibody that binds to
activated human platelets, is only able to combine with the
GPIIb/IIIa compound present on the activated platelet. In the
present study, flow cytometry was used to detect the positive
expression level of PAC-1, which represented the degree of
GPIIb/IIIa activation and reflected the early activation of the
platelets.
Platelet apheresis itself is an important factor
affecting the clinical effect of platelet transfusions. Our results
demonstrated that an extended storage time did not significantly
alter the level of PAC-1 expression, a platelet activation marker,
in the frozen platelet or GSNO-treated frozen platelet groups
(P>0.05).
During in vitro platelet activation,
GPIIb/IIIa translocating from within the platelet to the platelet
surface is degraded by proteases of the immune system, resulting in
the loss of normal aggregation function. By contrast, the
GPIIb/IIIa stored inside the platelets is able to continue to
transfer to the platelet surface to enhance the GPIIb/IIIa levels,
thus maintaining the GPIIb/IIIa presence and the immediate
hemostatic response. However, the finite nature of the GPIIb/IIIa
storage leads to a decline in the polymerization capacity of the
activated platelets in response to the aggregation inducer.
Therefore, while the immediate hemostatic function is maintained,
eventually, the hemostatic function declines following platelet
transfusion (18).
There are numerous membrane glycoproteins in normal
human platelets, of which GPIb/IX and GPIIb/IIIa are the major
glycoproteins. Each platelet comprises ~25,000 GPIb/IX molecules.
GPIb consists of an αβ chain linked by disulfide bonds, with an Mr
of 165,000. The platelet membrane glycoprotein, GPIbA, is one of
the components of the GPIb/IX complex, and participates in numerous
physiological and pathological processes. It is the receptor for
the adhesive protein von Willebrand Factor and for thrombin, in
addition to being one of the key substances mediating the initial
contact between platelets and the vascular walls, and participating
in platelet adhesion, early physiological hemostasis and
pathological thrombosis (7).
During in vitro platelet activation in the
frozen platelet and GSNO-treated frozen platelet groups, the
proteases of the immune system may have damaged the GPIb on the
surface of the platelets, resulting in a loss of normal function
(2). It was observed in the
current study that the expression of CD42b in the fresh liquid
platelet group was significantly different from that in the frozen
platelet and GSNO-treated frozen platelet groups, and that the
expression of CD62P+CD42b in the fresh liquid platelet group was
significantly different from that in the frozen platelet and
GSNO-treated frozen platelet groups. In addition, the
polymerization capacity of the platelets, induced in response to an
aggregation inducer, was reduced, eventually leading to decline in
hemostatic function following platelet transfusion.
In vitro, platelets may be naturally
activated at room temperature, leading to the irreversible
expression of high levels of CD62P on the platelet membrane;
therefore, CD62P is considered to be the best marker and gold
standard of platelet activation. Furthermore, CD62P exists solely
on the surface of activated platelets, a quality making CD62P an
ideal indicator in the detection of platelet activation. As such,
CD62P is attracting an increasing focus. A high expression level of
CD62P in activated platelets may be sensitively and specifically
detected using flow cytometry (8).
It was revealed in the current study that the expression of CD62P
in the fresh liquid platelet group was significantly different to
the expression in the frozen platelet and GSNO-treated frozen
platelet groups and that the expression of CD62P+CD42b in the fresh
liquid platelet group was significantly different from the
expression in the frozen platelet and GSNO-treated frozen platelet
groups. Furthermore, the polymerization capacity of the platelets,
induced in response to an aggregation inducer, was decreased when
CD62P was activated, eventually leading to a decline in hemostatic
function following platelet transfusion. However, the immediate
hemostatic function was maintained due to the fact that the
activation of GPIIb–IIIa was inhibited in the frozen platelets and
GSNO-treated frozen platelets.
The results of this study revealed that the NO level
in the fresh liquid platelet group was significantly higher
compared with that of the frozen platelet group (t=2.958, P=0.004),
whereas the NO level in fresh liquid platelet group was
significantly lower compared with that of the GSNO-treated frozen
platelet group (t=4.289, P=0.000). The level of NO in the fresh
liquid platelet group, which was markedly higher than that in the
frozen platelet group, may have prevented platelet consumption and
maintained platelet function. However, the level of NO in the fresh
liquid platelet group was significantly lower than that in the
GSNO-treated frozen platelet group. Due to the presence of the
external NO donor in the GSNO-treated frozen platelet group, NO may
have been released to improve the inhibition of platelet activation
and aggregation, and to maintain the platelet function. Although
there was a decline in the metabolic function of the frozen
platelets, NO loss was still apparent in the cryopreservation
process. The NO content may therefore be increased by adding an
external NO donor.
In a cardiopulmonary bypass model (9), an external NO polymer, acting as an
NO donor, was able to release NO to prevent platelet consumption
and maintain platelet function.
Although NO is important in many biological
processes, <10% of the studies in the last century in the field
of NO mention the direct measurement of NO. By contrast, the NO
content is often indirectly measured, for example, by the
measurement of S-nitrosothiols in body fluids using
chemiluminescence methods (10). A
type of porphyrin microsensor has also been used for the
measurement of NO released by whole blood platelets and washed
platelet suspensions. The use of these techniques has demonstrated
that NO is produced during the aggregation of human platelets
(11).
The current technologies that are used for the
measurement of NO (12) include
the chemiluminescence and Griess methods, paramagnetic resonance
spectroscopy, paramagnetic resonance imaging, spectrophotometry and
biological assays. Each technology has certain advantages; however,
there are also several shortcomings, such as low sensitivity and a
requirement for complex and expensive laboratory equipment.
S-nitrosothiols are formed by thiols through
S-nitrosyl acylation, in the presence of NO or NO2, and
have been demonstrated to be effective inhibitors of in
vitro platelet aggregation. It has been shown that the
stability of highly reactive and unstable NO may be maintained
through reactions with carrier molecules, such as R-SH, thereby
prolonging the half-life of NO and maintaining its biological
activity. As a stable S-nitrosothiol, GSNO may be synthesized by
the most abundant intracellular thiol, glutathione, in order to
maintain a dynamic equilibrium. As an NO donor, GSNO has the
potential to be added to cryopreserved platelets in order to
release NO and inhibit the adherence of platelets to collagen,
thereby inhibiting platelet activation and maintaining platelet
function (3).
The current methods for the detection of platelet
function include platelet aggregation instruments for the detection
of platelet aggregation and bleeding time and enzyme-linked
immuno-sorbent assay (ELISA) for the detection of thromboxane B2
(TXB2). In the present study, flow cytometry was directly used for
the detection of platelet membrane glycoproteins on the surface of
platelets. This was a good method of detecting platelet activation
as it analyzed the platelets in close to their natural preservation
state, in addition to being easy to operate. Furthermore, the
procedure minimized the changes in platelet status.
The results revealed that the ADP-induced platelet
aggregation rate in the frozen platelet group was significantly
lower than that of the fresh liquid platelet group, and that the
ADP-induced platelet aggregation rate in the GSNO-treated frozen
platelet group was significantly lower than that in the fresh
liquid platelet and frozen platelet groups. These observations
suggest that DMSO and GSNO played certain protective roles in
platelet aggregation, which was reflected in the reduction of the
aggregation response, due to inhibition of the polymerization
induced by ADP.
An NO-releasing polymer has been demonstrated to
inhibit platelet activation (13).
It was observed that NO released by the polymer was able to reduce
platelet activation and reduce thrombosis by affecting the membrane
glycoprotein, P-selectin. NO may lead to molecular changes in the
platelet membrane glycoproteins and inhibit thrombosis, indicating
that NO-releasing polymers may be used to coat ECCs for biological
and medical equipment.
NO may inhibit platelet function through the precise
regulation of the platelet response and the inhibition of platelet
activation via the NO-soluble guanylyl cyclase (sGC)-cGMP signal
pathway (14). NO released by the
endothelium may activate the only receptor of NO that is located
inside platelets (NO-sensitive sGC), produce cGMP and activate
cGMP-dependent protein kinase (PKG), leading to a reduction in
intracellular calcium levels and inhibiting platelet adhesion and
aggregation (14). In one study,
the inhibitory effect of NO donor substances on platelet activation
was observed to be sGC-dependent only at the micromolar, but not at
the millimolar, concentration level (15).
Non-cGMP dependent mechanisms, such as
S-nitrosylation, have also been suggested as alternative
NO-mediated signaling pathways. GSNO, an NO donor, has been
demonstrated to inhibit the adherence of platelets to static
collagen in a concentration-dependent manner. Biotin transformation
analysis of platelets revealed that there were several
S-nitrosylated proteins in the basic state. At concentrations
sufficient to inhibit platelet adhesion, the treatment of platelets
with an exogenous NO donor was able to increase the types of S
nitrosylation and led to the hyper-S-nitrosylation of
S-nitrosylated proteins. The degree of S-nitrosylation caused by
exogenous NO was not affected by platelet activation. Furthermore,
in the absence of exogenous NO, platelet activation did not
increase S-nitrosylation, and the nitrocellulose level remained
below the basic level, indicating that platelet-derived NO was not
able to induce this type of protein modification. The
S-nitrosylation of platelet proteins induced by exogenous NO may be
an important non-cGMP-dependent signaling mechanism, which may
regulate platelet adhesion (3).
S-Nitrosothiols, such as GSNO, have numerous
potential clinical applications, particularly as anti-thrombotic
agents, primarily due to their platelet inhibitory effect and the
fact that they exhibit a certain degree of platelet selectivity. A
recent study revealed that S-nitrosothiols are involved in a
variety of pathways. The delivery of an NO-related signal into
cells by a stable S-nitrosothiol compound was demonstrated to
result in the denitrification of cell surface enzymes, in addition,
to the transportation of intact S-nitroso-cysteine by the amino
acid transport system (L-AT). The different roles of these pathways
in platelets and vascular cells may partially explain the selective
effects on platelets (16).
Low levels of GSNO have been demonstrated to inhibit
platelet aggregation without leading to vasodilation, indicating
that the action of NO is platelet-selective; this selectivity may
involve mercaptan isomerase on the surface of cells, and
particularly the protein disulfide isomerase (csPDI; EC 5.3.4.1).
In a previous study, flow cytometry demonstrated that the positive
expression rate of csPDI in platelets was higher than in blood
vessel cells. Furthermore, the reductase activity associated with
mercaptan isomerase was higher on platelets (P<0.01). Following
the activation of the cell, the activities of csPDI on the
platelets and smooth muscle cells were increased; however, the
activity on the endothelial cells was not increased. GSNO released
NO more inside the platelet cells than inside the vascular cells
(P<0.002). Compared with the vessel wall cells, the activity of
mercaptan isomerase on the surface of the platelets was increased,
which may explain the selective action of GSNO on platelets and aid
the elucidation of its anti-thrombotic ability (17).
DMSO has been widely used in the field of cell
biology, not only a cryoprotectant, but also as a fortifier of cell
fusion and permeability. The protective effect of DMSO on platelets
has been demonstrated to be better than that of other
cryoprotectants, and the function of DMSO in platelet
cryopreservation is not readily replicated by other cryoprotectants
(18).
New preservation solutions have been developed,
which may, alone or in combination with other preservation
solutions, improve frozen platelet function. These solutions have
been expected to further improve the clinical infusion effects of
cryopreserved platelets. However, the inhibition of the activation
of cryopreserved platelets is challenging. The results of the
present study indicated that the expression of platelet membrane
glycoproteins in the GSNO-treated frozen platelet group was not
significantly different from that of the frozen platelet group.
Treatment with GSNO may have altered the molecular arrangement and
structure of the frozen platelets, or affected the platelets
through other mechanisms.
References
|
1
|
Ouyang XL, Zhou D, Wu JH, Wang LH, Hao J
and Liu JH: Cryopreservation strengthens procoagulative activities
of platelets. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 16:930–932.
2008.(In Chinese).
|
|
2
|
Lee JH, Kim JT and Cho YG: Effect of
nitric oxide on the cryopreservation of platelets. Korean J Lab
Med. 28:136–143. 2008.(In Korean).
|
|
3
|
Irwin C, Roberts W and Naseem KM: Nitric
oxide inhibits platelet adhesion to collagen through cGMP-dependent
and independent mechanisms: the potential role for S-nitrosylation.
Platelets. 20:478–486. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wong K and Li X: Nitric oxide infusion
alleviates cellular activation during preparation, leucofiltration
and storage of platelets. Transfus Apher Sci. 30:29–39. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sandgren P, Hansson M, Gulliksson H and
Shanwell A: Storage of buffy-coat-derived platelets in additive
solutions at 4 degrees C and 22 degrees C: flow cytometry analysis
of platelet glycoprotein expression. Vox Sang. 93:27–36. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou J, Liu JH, Jin Y, Ouyang XL and Yang
LG: Protective effects of DMSO on function of lyophilized human
platelets. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 15:1284–1288.
2007.(In Chinese).
|
|
7
|
Gu Y, Ji SD, Zhao YM, Shen F and Ruan CG:
Development, identification and function assay of monoclonal
antibody against platelet membrane glycoprotein Ib. Xi Bao Yu Fen
Zi Mian Yi Xue Za Zhi. 27:650–652. 2011.(In Chinese).
|
|
8
|
Zhao LN, Zhao HS, Li JB, Shan H, Han XG
and Jiao HL: Effects of 25 Gy gamma-ray irradiation on the
expression of CD62p in manually enriched human platelets. Zhongguo
Shi Yan Xue Ye Xue Za Zhi. 18:490–493. 2010.(In Chinese).
|
|
9
|
Skrzypchak AM, Lafayette NG, Bartlett RH,
et al: Effect of varying nitric oxide release to prevent platelet
consumption and preserve platelet function in an in vivo model of
extracorporeal circulation. Perfusion. 22:193–200. 2007. View Article : Google Scholar
|
|
10
|
Nagababu E and Rifkind JM: Determination
of s-nitrosothiols in biological fluids by chemiluminescence.
Methods Mol Biol. 704:27–37. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Malinski T, Radomski MW, Taha Z and
Moncada S: Direct electrochemical measurement of nitric oxide
released from human platelets. Biochem Biophys Res Commun.
194:960–965. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Serpe MJ and Zhang X: The principles,
development and application of microelectrodes for the in vivo
determination of nitric oxide. Electrochemical Methods for
Neuroscience. Michael AC and Borland LM: CRC Press; Boca Raton, FL:
pp. 465–488. 2007, PubMed/NCBI
|
|
13
|
Major TC, Brant DO, Reynolds MM, et al:
The attenuation of platelet and monocyte activation in a rabbit
model of extracorporeal circulation by a nitric oxide releasing
polymer. Biomaterials. 31:2736–2745. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Evora PR, Evora PM, Celotto AC, Rodrigues
AJ and Joviliano EE: Cardiovascular therapeutics targets on the
NO-sGC-cGMP signaling pathway: a critical overview. Curr Drug
Targets. 13:1207–1214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang G, Xiang B, Dong A, et al: Biphasic
roles for soluble guanylyl cyclase (sGC) in platelet activation.
Blood. 118:3670–3679. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gordge MP and Xiao F: S-nitrosothiols as
selective antithrombotic agents - possible mechanisms. Br J
Pharmacol. 159:1572–1580. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiao F and Gordge MP: Cell surface thiol
isomerases may explain the platelet-selective action of
S-nitrosoglutathione. Nitric Oxide. 25:303–308. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xie ZT, Yang LH, Tao ZH, et al: Changes of
platelet aggregation function of apheresis collected platelets and
soluble P-selectin during storage. Zhongguo Shi Yan Xue Ye Xue Za
Zhi. 16:1188–1191. 2008.(In Chinese).
|