Introduction
Diabetic nephropathy (DN), a major complication of
diabetes, is the leading cause of end-stage renal disease worldwide
(1). The appearance of
microalbuminuria is a detectable early marker of DN.
Microalbuminuria may develop into proteinuria and hyperfiltration,
followed by reductions in the glomerular filtration rate. The
detailed molecular mechanisms underlying the correlation between
albuminuria and DN remain elusive. However, functional and
structural abnormalities in glomerular podocytes have been recently
observed to be one of the earliest events during the development of
diabetic glomerular injury (2,3).
Podocyte loss and injury are frequently observed in patients with
DN at very early stages, which may contribute to the development of
severe proteinuria and renal lesions (4–6).
The pathogenesis of DN involves numerous factors,
such as metabolic disturbance, abnormal renal hemodynamics and
chronic inflammatory factors, including chemokines, adhesion
molecules and proinflammatory cytokines (7–9).
Chronic inflammation is closely associated with permeability
changes in the glomerular filtration barrier and proteinuria in DN
(10). Podocytes are located at
the outer layer of the filtration barrier, and injury to podocytes
is involved in the inflammatory processes of DN (2). It is
important to investigate how to prevent podocyte injury in patients
with DN; furthermore, novel drugs are required to improve the
treatment of podocyte injury in patients with DN.
Extracts of Tripterygium wilfordii Hook F
(TwHF) have been used in the treatment of glomerulonephritis for
>30 years in China. The TwHF extracts have been shown to have
significant proteinuria-reducing effects in patients with focal
segmental glomerular sclerosis and membranous nephropathy.
Triptolide, a diterpene triepoxide, has been identified to be one
of the major active components in the TwHF extract. Previous
studies have shown that triptolide exerts potent immunosuppressive,
anti-inflammatory, anti-proliferative and anti-oxidative effects
(11–13). Our previous study and an
investigation from another laboratory have indicated that
triptolide may inhibit inflammatory responses, thereby reducing
albuminuria and improving renal functions in type II diabetic rats
and patients with type II diabetes (14,15).
However, the possible effects of triptolide on podocytes have yet
to be elucidated. In the current study, it was observed that
triptolide markedly attenuated albuminuria and improved podocyte
injury in a rat model of DN, possibly due to its inhibitory effects
on inflammation and macrophage infiltration in the kidneys.
Materials and methods
Reagents
Triptolide (molecular formula,
C20H24O6) was obtained from the
Jiahe Medicine Technology Development Co. Ltd, (Shanghai, China).
The purity of triptolide, detected by high-performance liquid
chromatography, was 99%. The triptolide was reconstituted in 0.01%
dimethyl sulfoxide (DMSO) and freshly diluted with culture medium,
prior to use. The final DMSO concentration used in the present
study was <0.002% (v/v), which was not harmful to cells.
Streptozocin was purchased from Sigma (St. Louis, MO, USA) and then
dissolved in citrate buffer (0.01 mol/l, pH 4.5).
Animals
Fifty 8-week-old male Wistar rats (weight, ~200 g)
were purchased from the Laboratory Animal Center of the Qingdao
Institute for Drug Control (Qingdao, China). The rats were housed
in individual cages in a temperature-controlled room with a 12/12-h
light-dark cycle, and were left to acclimatize for 1 week prior to
the initiation of dietary intervention. All animal experiments were
conducted in accordance with the ethical guidelines of the National
Defense Medical College (Qingdao, China) and the EthicsCommittee of
Qingdao University Medical College (Qingdao, China) for the care
and use of laboratory animals in research.
The rats with type II diabetes were modeled
according to the methods previously described by Danda et al
(17) and Guo et al (18). Rats were randomly assigned to
regular rat chow [n=10, control (NC) group] or a high-fat,
high-sucrose diet (n=40; 10% animal fat, 20% cane sugar, 2.5 %
cholesterol, 1% cholate and 66.5% regular chow). After 8 weeks, the
rats fed on the high-fat, high-sucrose diet were injected
intraperitoneally with a low dose of streptozocin (30
mg.kg−1). Following this, the type II diabetic rats were
divided into two groups, specifically a group without triptolide
treatment (n=14, DM group) and a group with triptolide treatment
(100 μg.kg−1.day−1; n=14, DT group).
Triptolide was administered intragastrically with a volume <1
ml/day. The drug vehicle DMSO was used as a control. Eight weeks
subsequent to treatment, the body weights (BWs) of the rats were
examined and urine samples were collected. Following this, the rats
were sacrificed and blood samples and kidneys were collected.
Blood glucose, hemoglobin A1c (HbA1c) and
insulin measurements
Blood samples were obtained from the tail veins.
Fasting blood glucose (FBG) was determined at 1–2-week intervals in
all groups using a glucometer (One Touch™ Surestep™; Lifescan,
Inc., Milpitas, CA, USA). The serum insulin level and HbA1c were
determined by enzyme-linked immunosorbent assay (ELISA) using
antibodies against insulin and HbA1c, respectively (Aquatic
Diagnostic Ltd., Glasgow, Scotland).
Noninvasive blood pressure
measurement
Blood pressure was measured using the tail-cuff
method and an LE5002 noninvasive blood pressure detecting
instrument (Diagnostic Systems Laboratories, Inc., Webster, TX,
USA) under resting, conscious conditions in a climate-controlled
room (23°C). Five consecutive systolic blood pressure (SBP)
measurements were taken.
Determination of urine albumin and
creatinine concentrations
Rats were placed in metabolic cages and their urine
was collected for 24 h every 4 weeks for the determination of
albumin and creatinine excretion. Urine albumin excretion was
determined using a turbidimetric immunoassay kit (Shibayagi Co.,
Ltd., Shibukawa, Japan). The urine creatinine (Ucr) level was
determined using an automatic biochemistry analyzer (model no.
7600-020, Hitachi Ltd., Tokyo, Japan). The urine albumin/Ucr ratio
was subsequently calculated.
Determination of biochemical
parameters
The biochemical parameters were determined from the
blood samples obtained at the end of 8 and 17 weeks. Serum
creatinine (Scr), urea nitrogen, total cholesterol (CH),
triglyceride (TG), aspartate transaminase (AST) and alanine
transaminase (ALT) levels were determined using the automatic
biochemistry analyzer. The creatinine clearance (Ccr) was
calculated using the following formula: Ccr = UCr/Scr × V [V,
volume of urine per min (ml/min)]. The insulin sensitivity index
(ISI) was calculated based on the levels of FBG and INS. The
formula was ISI = 22.5/(FBG × INS).
Transmission electron microscopy
Renal cortex samples, measuring 1 mm3,
were fixed, embedded and cut into 50-nm sections. The specimens
were examined and photographed with a JEM-1200 transmission
electron microscope (Jeol Ltd., Tokyo, Japan). The microscopic
evaluations conducted are described in the following sections.
Immunohistochemical staining for nephrin,
podocin and ED-1
Renal tissue sections (3 μm) were used for the
immunohistochemical staining of nephrin, podocin and ED-1.
Deparaffinized sections were stained with primary antibodies,
specifically, rabbit-anti-rat nephrin antibody (1:200), podocin
antibody (1:200) and goat-anti-rat ED-1 antibody (1:50) at room
temperature for 1 h. All antibodies were purchased from Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA). The color was developed
by incubating with diaminobenzidine (Santa Cruz Biotechnology,
Inc.) and counterstaining with hematoxylin. Controls were obtained
by replacing the primary antibody with phosphate-buffered saline.
In total >50 glomeruli and 20 non-overlapping interstitial areas
from each section were assessed under high power magnification
(x400). The numbers of ED-1 positive cells in each glomerulus and
interstitial area were counted and averaged in each group.
Quantitative polymerase chain reaction
(qPCR) analysis
Frozen renal tissues were homogenized and total RNA
was extracted with TRIzol reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA). The extracted RNA was measured by agarose gel
electrophoresis for quality and spectrometry for quantity. A
reverse transcription reaction kit [Cat. no. DRR035A; TaKaRa
Biotechnology (Dalian) Co. Ltd., Dalian, China] was used.
For fluorescence qPCR, 1,000 ng mRNA sample was
added to the reverse transcription system. This was diluted with
EASY dilution [TaKaRa Biotechnology (Dalian) Co. Ltd.] in a 10-fold
series to generate a standard curve. The qPCR process was carried
out in an ABI Prism® 7000 HT sequence detection system
(cat. no. 11744-100; Applied Biosystems, Invitrogen Life
Technologies). Primers for nephrin, podocin, osteopontin (OPN) and
transforming growth factor (TGF)-β1 were designed and synthesized
by Shanghai Sangon Biological Engineering Technology Co., Ltd.
(Shanghai, China). Each experiment was performed at least three
times. The primers used in the PCR process are presented in
Table I. A two-step PCR procedure
was applied and standard curves for the target genes and an
internal reference gene were made under the same conditions. The
cycle threshold (Ct) values of the samples were used to calculate
the corresponding gene copy number. The results are presented as
the ratio of the target gene copy over the housekeeping gene
[glyceraldehyde 3-phosphate dehydrogenase (GAPDH)] copy (16).
 | Table IPrimers used in the study. |
Table I
Primers used in the study.
| Primer | Sequence | Length (bp) |
|---|
| Nephrin (F) | 5′-AAGTACGAATGGAC
CCCTATGAC-3′ | 176 |
| Nephrin (R) |
5′-CAGGGCTGTAGGAAACGGGTG-3′ | |
| Podocin (F) |
5′-CACGGTAGTGAATGTGGACGA-3′ | 145 |
| Podocin (R) | 5′-GAGGA CAAGAAGCCA
CTCGCAGGCC-3′ | |
| OPN (F) | 5′-TCAGCATTTC
GCTTCTGTTCT-3′ | 111 |
| OPN (R) |
5′-CTGTAAGTTTGCCTGCCTCTA-3′ | |
| TGF-β (F) | 5′-GCCTGAG
TGGCTGTCTTTTGA-3′ | 197 |
| TGF-β (R) | 5′-GGAAGG
GTCGGTTCATGTCAT-3′ | |
| GAPDH (F) | 5′-TTCTAGAGACAGCC
GCATCT-3′ | 106 |
| GAPDH (R) | 5′-TGGTAACCAGG
TGTCCGATA-3′ | |
Western blot analysis
Cells were lysed in cold cell lysis buffer (50 mM
Tris, 150 mM NaCl, 10 mM ethylenediaminetetraacetic acid and 1%
Triton X-100) containing protease and phosphatase inhibitors.
Briefly, the proteins were separated on 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels, and
subsequently transferred to nitrocellulose membranes. The primary
antibodies were nephrin (rabbit anti-rat, 1:4,000), podocin
(1:4,000), OPN (goat anti-rat, 1:1,000 and TGF-β1 (mouse anti-rat,
1:1,000) antibodies (Santa Cruz Biotechnology, Inc.). Horseradish
peroxidase-conjugated anti-immunoglobulin (Ig) G was used as a
secondary antibody (Beijing Zhongshan Golden Bridge Biotechnology
Co., Ltd., Beijing, China). The blots were detected using an
enhanced chemiluminescence (ECL) system (Beijing Zhongshan Golden
Bridge Biotechnology Co., Ltd.).
Statistical analysis
The data are presented as the mean ± standard
deviation of at least three independent experiments. The
significance of the differences between the groups was determined
by multiple sample comparison methods analysis of variance (ANOVA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Triptolide significantly decreases
urinary albumin levels, kidney weight (KW)/BW and total CH and TG
levels
At 8 weeks subsequent to treatment, the numbers of
live rats in the NC, DM and DT groups were 10, 11 and 12,
respectively (Table II). The
renal function and the general parameters of the three groups were
evaluated, including urinary albumin, KW/BW, CH levels, TG levels,
SBP, FBG and Ccr. There were no statistically significant
differences in levels of urea nitrogen, AST and ALT following
triptolide treatment. The results from each group are summarized in
Table II.
 | Table IIGeneral data of the rats used in the
study. |
Table II
General data of the rats used in the
study.
| Group |
|---|
|
|
|---|
| Variable | NC | DM | DT |
|---|
| UAL (mg/mg.Cr) | 0.98±0.42 | 6.36±0.92a | 2.48±0.57ab |
| KW/BW (g/kg) | 2.05±0.32 | 5.06±0.57a | 2.75±0.61ab |
| SBP (mmHg) | 111.0±3.8 | 136.5±5.6a | 133.8±4.2 |
| FBG (mM) | 5.07±1.73 | 16.41±3.12a | 18.70±3.56a |
| INS (ng/ml) | 18.83±6.20 | 20.51±7.14 | 19.26±5.29 |
| ISI | 0.182±0.010 | 0.068±0.015a | 0.056±0.019a |
| HbA1c | 2.89±0.31 | 6.52±0.48a | 6.73±0.50a |
| CH (mM) | 1.80±0.47 | 3.87±0.98a | 2.80±0.56ab |
| TG (mM) | 0.83±0.31 | 3.52±0.56a | 1.62±0.28ab |
| Ccr (ml/min) | 1.57±0.33 | 2.28±0.35a | 1.96±0.57 |
| Urea nitrogen
(mM) | 8.23±1.25 | 9.25±2.02 | 8.28±1.37 |
| AST (U/l) | 64.3±12.4 | 61.6±13.2 | 60.9±12.6 |
| ALT (U/l) | 46.2±7.5 | 56.2±8.4 | 58.4±8.7 |
There was no significant change in the BWs of the
type II diabetic rats following 8 weeks of triptolide treatment;
however, the KWs and the KW/BW in the DM rats were significantly
increased compared with those in the NC rats. During the
experiment, no significant changes in food and water intake were
observed and no diarrhea was noted.
As shown in Table
II, the FBG, HbA1c, SBP, insulin sensitivity index, total CH
and TG levels in the DM group were higher than those in the NC
group (P<0.01–0.05). Moreover, the urinary albumin levels and
Ccr in the DM rats were significantly increased compared with those
of the rats in the NC group.
Triptolide significantly decreased the urinary
albumin levels, KW/BW ratio and total CH and TG levels compared
with those in the DM rats (P<0.05). However, no statistically
significant differences were observed in SBP, FBG or Ccr between
the untreated diabetic and triptolide-treated groups (P>0.05;
Table II).
Triptolide attenuates podocyte injury in
rats with DN
To determine the effects of triptolide on renal
ultramicrostructure, glomerular podocytes were examined by
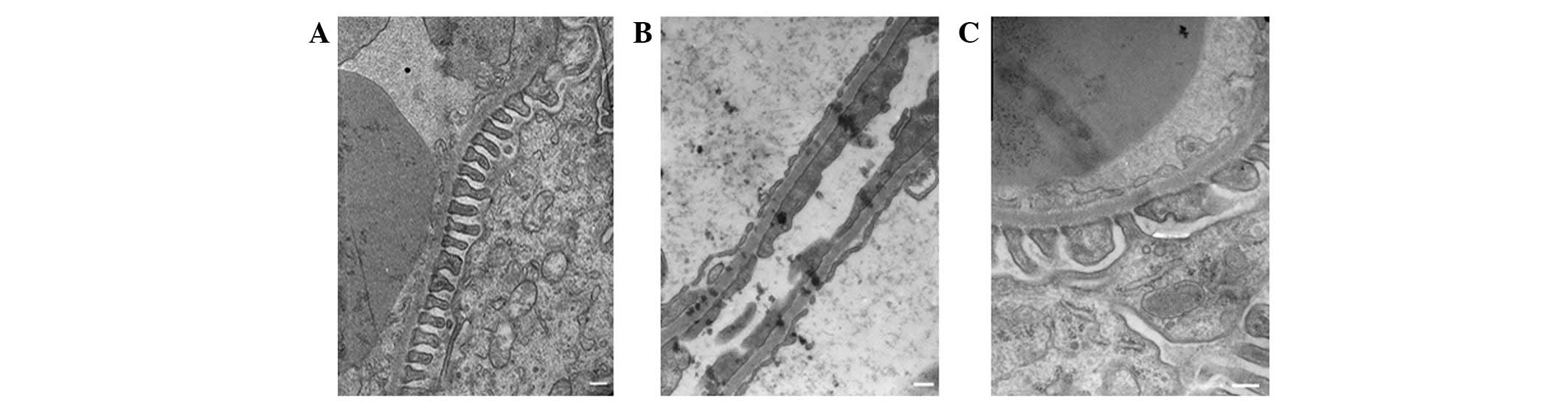
transmission electron microscopy. As shown in Fig. 1, the foot processes of neighboring
podocytes were interdigitated, and the glomerular basement membrane
(GBM) thickness was uniform in the NC group (Fig. 1A). However, significant increases
in GBM thickness (812.40±65.49 versus 238.64±33.69; P<0.01),
foot process fusion rate (56.73±8.40 versus 0; P<0.01) and foot
process width (517.87±25.39 versus 261.18±17.55; P<0.01) were
observed in the DM rats compared with the NC group (Fig. 1B), which was consistent with the
severe albuminuria observed in these rats. Treatment with
triptolide was observed to inhibit the thickening of the GBM
(276.64±45.42 versus 812.40±65.49; P<0.01), the foot process
fusion rate (15.76±6.27 versus 56.73±8.40; P<0.01) and the foot
process width (323.14±19.86 versus 517.87±25.39; P<0.01),
compared with their values in the DM group, which predominantly
normalized the glomerular filtration barrier structure (Fig. 1C).
Triptolide restores the distribution of
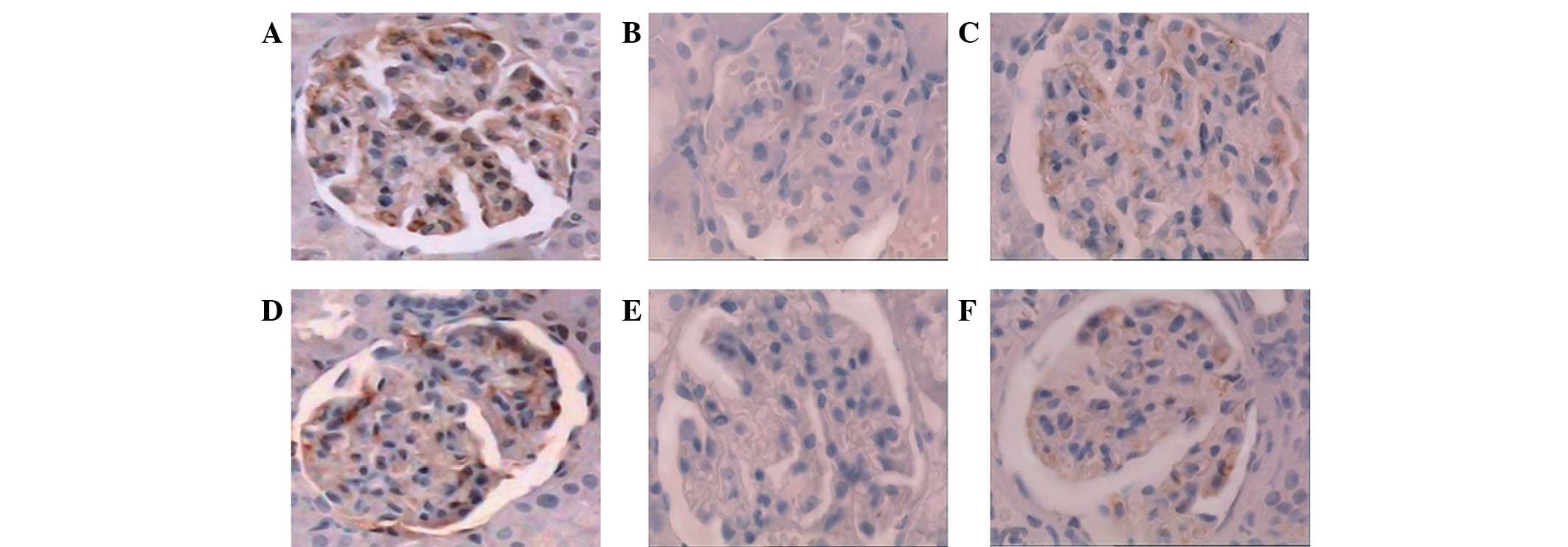
nephrin and podocin in rats with DN
The expression intensity and distribution pattern of
nephrin and podocin in glomeruli were observed by
immunohistochemical staining. The staining of nephrin and podocin
was revealed to form a linear pattern along the glomerular
capillary wall in the NC rats (Fig. 2A
and D). By contrast, in the DM rats, the expression of nephrin
and podocin was decreased significantly (Fig. 2B and E). However, the expression
levels of nephrin and podocin in the DT rats were similar to the
levels in the NC rats, indicating that triptolide significantly
improved the expression of nephrin and podocin in diabetic rats and
restored the distribution of nephrin and podocin (Fig. 2C and F).
Triptolide restores the mRNA expression
of nephrin and podocin in renal tissue
The mRNA expression of nephrin and podocin was
determined by qPCR. Consistent with the expression intensity of
nephrin and podocin, as shown in Fig.
2, the expression levels of nephrin and podocin mRNA were
decreased significantly in the kidney cortex of the untreated DM
rats compared with the NC rats (Fig.
3A and B, P<0.01). The downregulated expression levels of
nephrin and podocin mRNA in the DM rats were restored significantly
by the treatment with triptolide (P<0.01; Fig. 3A and B). Thus triptolide treatment
restored the mRNA expression of nephrin and podocin in the renal
tissue of diabetic rats.
Triptolide restores the protein
expression of nephrin and podocin
Western blot analysis indicated that the expression
level of nephrin in the rat kidneys was lower in the DM group than
in the NC group (1.63±0.06 versus 2.17±0.02; P<0.05; Fig. 4); similarly, the expression level
of podocin in the rat kidneys was also significantly lower in the
DM group than in the NC group (Fig.
4B). However, the expression levels of nephrin and podocin in
the DT group were significantly higher than those in the DM group
(Fig. 4B). Therefore, triptolide
restored the expression of nephrin and podocin in the kidneys of
rats with DN.
Triptolide decreases the number of
ED-1-positive cells in the glomeruli and interstitium
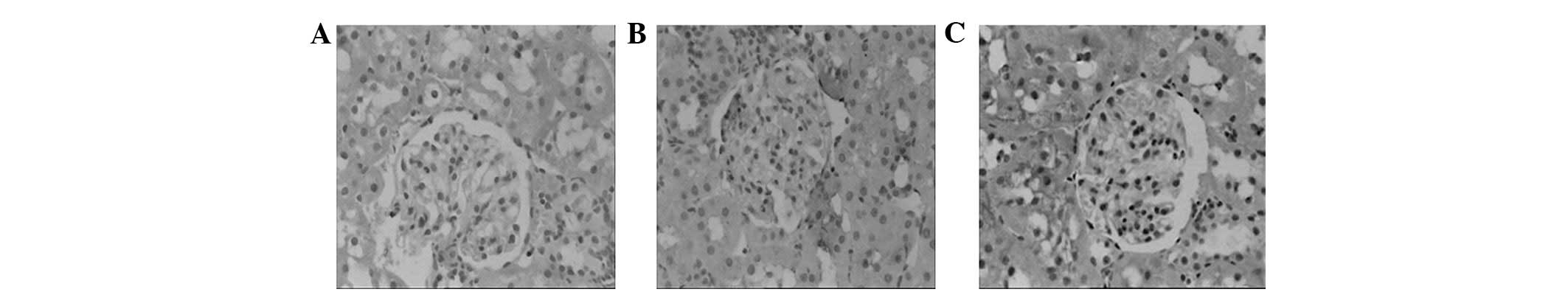
Using immunohistochemical staining, the ED-1
expression was examined in each group. The numbers of ED-1-positive
cells were significantly increased in the glomeruli of DM rats
compared with those of the NC rats (1.96±0.12 versus
0.23±0.06/glomerular cross-section (gcs); P<0.05; Fig. 5). The numbers of the ED-1-positive
cells in the interstitium of DM rats were also increased [3.02±0.20
versus 0.41±0.07/high-power field (hpf); P<0.05; Fig. 5]. The increased prevalence of
ED-1-positive cells in the glomeruli and interstitium of the DM
rats was significantly suppressed by treatment with triptolide
(1.32±0.10/gcs in the glomeruli and 2.05±0.19/hpf in the
interstitium; Fig. 5). In the DM
rats, the correlation between the mRNA expression of nephrin and
the number of ED-1-positive cells in the glomeruli was significant
(r=−0.696, P<0.05). A similar correlation between interstitial
fibrosis and the number of ED-1-positive cells in the interstitium
was also observed (r=−0.756, P<0.01). These data suggest that
the number of ED-1 positive cells in the glomeruli and interstitium
was decreased by triptolide treatment.
Triptolide suppresses the mRNA expression
of TGF-β1 and OPN
The mRNA expression levels of TGF-β1 and OPN in the
renal cortex were measured by qPCR. The OPN mRNA expression in the
untreated DM rats was increased in comparison with that in the NC
rats (P<0.01; Fig. 3C).
Treatment with triptolide reduced the OPN mRNA expression by 40%
(Fig. 3C). In addition, the TGF-β1
mRNA expression in the renal cortex was significantly increased in
the untreated DM rats compared with the NC rats (P<0.01;
Fig. 3D). However, this
overexpression of TGF-β1 mRNA in the DM rats was significantly
suppressed by 56% with triptolide treatment (P<0.01, Fig. 3D). There were significant negative
correlations between the mRNA expression of nephrin and OPN
(r=−0.794, P<0.01) and between TGF-β1 and nephrin (r=−0.847,
P<0.01) in the renal cortex of DM rats. The mRNA levels of
TGF-β1 and OPN in the renal cortex of diabetic rats were therefore
suppressed by triptolide treatment.
Triptolide inhibits the protein
expression of TGF-β1 and OPN in diabetic rats
Western blot analysis was performed to determine the
protein level of TGF-β1 and OPN. The results showed that the
protein expression of OPN in the rat kidneys was increased in the
DM group, compared with the NC group (Fig. 4B). The overexpression of OPN
protein in the diabetic rats was significantly suppressed by
treatment with triptolide. The TGF-β1 protein expression in the NC
rats was moderate; however, there was an increased expression in
the DM rats (1.02±0.03 versus 1.44±0.02; P<0.05; Fig. 4B). The administration of triptolide
significantly suppressed the TGF-β1 protein expression. These
results suggested that triptolide suppressed the overexpression of
OPN and TGF-β1 proteins in the kidneys of diabetic rats.
Discussion
In the present study, we induced a rat model of DN
and studied the effects of triptolide on podocyte injury following
8 weeks of treatment. The results showed that triptolide treatment
effectively reduced albuminuria, and ameliorated podocyte foot
process effacement and glomerular hypertrophy in diabetic rats. In
addition, the recovery from podocyte injury was further
demonstrated by increases in nephrin and podocin expression in
diabetic rats, following treatment with triptolide. Furthermore, it
was observed that triptolide notably decreased macrophage
accumulation and the expression of OPN and TGF-β1 in the renal
tissue of diabetic rats. The administration of triptolide to the
diabetic rats was shown to be safe. During the experiment, the drug
did not demonstrate any effect on serum creatinine or urea nitrogen
levels in the diabetic rats, and did not exert any adverse effects,
such as changes in blood leucocytes, hematocrit or AST or ALT
levels, which suggested that triptolide treatment in diabetic
patients may be safe, without marrow, liver or kidney toxicity. In
combination, the results suggested that the therapeutic effect of
triptolide may be achieved by the suppression of macrophage
infiltration and OPN and TGF-β1 expression.
It has been suggested that podocyte injury is a
major contributor to severe proteinuria in DN. Clinically, the
principal features of diabetic podocytopathy manifest as
albuminuria and proteinuria (2,3),
with the albuminuria and proteinuria resulting from reductions in
podocyte number and/or density. These reductions occur due to
apoptosis or detachment, GBM thickening with altered matrix
composition and a reduction in the expression of nephrin protein in
the slit diaphragm, with podocyte foot process effacement. Since
podocytes demonstrate a limited ability to proliferate, they are
not able to restore the architecture of the GBM, and this condition
leads to glomerular sclerosis and tubulointerstitial fibrosis.
Previous studies have revealed that the slit
diaphragm protein complex (nephrin-podocin-CD2AP) is an important
component in maintaining the glomerular filtration barrier. It has
been shown that these podocyte proteins and the onset of
proteinuria in type II DN are closely interrelated (19,20).
Furthermore, the abnormal expression and distribution of nephrin
and podocin have been observed prior to the onset of proteinuria in
rat models of DN. A study by Baelde et al showed that
podocyte loss and significant reductions in the expression of
nephrin and podocin in the glomerulus were closely correlated with
the occurrence and development of proteinuria (21). In the present study, foot process
denudation, GBM exposure and shed podocytes were observed in
diabetic rats using transmission electron microscopy. The greater
the extent of the podocyte injury, the more the nephrin and podocin
expression was downregulated. As a consequence of nephrin and
podocin expression downregulation, the structure of the slit
diaphragm between the podocytes was destroyed, leading to the onset
of proteinuria and subsequent deterioration. All these factors
demonstrated that the disorganization of the slit diaphragm between
podocytes was capable of accelerating the development of DN. The
concept that podocytes are crucial factors in the development of
proteinuria and the progression of glomerulosclerosis has indicated
that novel approaches are required to treat podocyte lesions. These
efforts have led to numerous studies showing that certain reagents,
such as angiotensin-converting enzyme inhibitors (ACEIs),
angiotensin II receptor blockers (ARBs), sirolimus and darbepoetin,
decrease proteinuria by ameliorating podocyte injury (22–24).
However, in some patients with severe albuminuria or proteinuria,
treatment with ACEI/ARB therapy is not able to produce a
satisfactory efficacy. Particular attention has been focused on
anti-inflammatory treatments and immunotherapy, which have been
shown to attenuate podocyte injury and alleviate albuminuria
(25). Chen et al(26) demonstrated that triptolide
effectively reduced proteinuria and alleviated glomerular immune
injuries; furthermore, it markedly improved podocyte lesions and
aided the restoration of the normal slit diaphragm structure in
rats with passive Heymann nephritis (PHN) (26). In addition to its immunosuppressive
and anti-inflammatory activities, the therapeutic effect of
triptolide may be due to its direct activity on podocyte injury.
Zheng et al(27) observed
that triptolide protected podocytes from puromycin aminonucleoside
(PAN)-induced injury in vivo and in vitro(27). Furthermore, Gao et
al(28) revealed that
triptolide treatment reduced monocyte chemottractant protein-1
(MCP-1) expression and the number of CD68+ macrophages
in db/db diabetic mice, with a greater efficacy than valsartan.
These studies demonstrated that, as a novel drug, triptolide
exerted comprehensive protective effects in the prevention of DN
progression (28). In a previous
study, we demonstrated that triptolide effectively attenuated
proteinuria, suppressed the expression of MCP-1 and OPN and
inhibited the infiltration of macrophages in rats with type II DN
(15). However, the protective
effects of triptolide on podocytes in type II DN remain poorly
understood. In the present study, it was demonstrated that
triptolide exhibited a favorable anti-albuminuric efficiency and
potent therapeutic effect in the recovery from podocyte injury in
DN.
In the current study, a type II diabetic rat model
was constructed using a high-fat, high-sugar diet, followed by the
administration of a low dose of streptozocin (30
mg.kg−1) to investigate the mechanism(s) of DN. This rat
model successfully imitated human type II diabetes, with moderate
hyperglycemia, hypertension, dyslipidemia and insulin resistance.
Therefore, it was also an ideal model for studying DN (17,18).
In the current study, urinary albumin excretion was significantly
higher in the DM and DT groups than in the NC group. In addition,
the KW/BW was significantly higher in the DM and DT groups than in
the NC group. Podocyte foot process fusion and effacement, in
addition to markedly reduced nephrin and podocin expression, were
the most predominant characteristics in the DM group, compared with
NC group. These results demonstrated that albuminuria and podocyte
injuries were closely correlated. In comparison, following 8 weeks
of triptolide treatment in the DT group, it was observed that there
was a significant reduction in the albuminuria, accompanied by
improvements in podocyte foot process effacement and the
restoration of nephrin and podocin expression. In combination,
these results indicated that the anti-proteinuric effect of
triptolide was strongly correlated with recovery from podocyte
injury. It has previously been observed that while treatment with
an ACEI or an ARB reduced proteinuria, the treatment resulted in
significantly decreased SBP and Ccr. By contrast, it was observed
in the present study that triptolide treatment had no marked
effects on SBP and Ccr levels at the administered dose. Therefore,
the results suggested that triptolide reduced proteinuria by the
protection of podocytes and the reversal of podocyte injuries in DN
rats, rather than by decreasing Ccr, FBP or SBP. This further
demonstrates the independent action of triptolide in the reduction
of proteinuria. In addition, the correlation between protein intake
and proteinuria is a well-known phenomenon in rats. In the present
study, triptolide did not affect food intake, which suggested that
low protein intake was not responsible for the anti-proteinuric
effect.
In this study, we further explored the underlying
mechanism by which triptolide exerted the protective effect on
podocytes. Previous studies have shown that the progression of DN
may be a result of an inflammatory reaction. Furthermore, studies
have shown that chemokines, such as interleukin (IL)-1 and TGF-β1,
are predominantly synthesized in podocytes and that there are
numerous chemokine receptors on the membranes of podocytes. These
data suggest that podocytes are important in the inflammatory
reaction (29–31). Studies by Takano et
al(32) and Zhang et
al(25) showed that active
macrophages produced proinflammatory factors, such as IL-1 and
tumor necrosis factor (TNF-α), and MCP-1, which reduced the
expression of nephrin through the PI3K/protein kinase B (PK-B) and
TGF-β signaling pathways, thus influencing podocyte survival
(25,32). In the present study, it was shown
that triptolide inhibited the production of TGF-β1 and an
additional monocyte chemotactic factor, OPN. Moreover, triptolide
suppressed the renal infiltration of ED-1-positive cells and
decreased urinary albumin excretion in rats with type II DN. These
results were consistent with the restoration of podocyte
ultramicrostructure and the associated protein expression. In
addition, the results indicated that triptolide may alleviate
albuminuria and protect podocytes from injury by suppressing the
mRNA and protein expression of TGF-β1 and OPN, and inhibiting
macrophage infiltration. Although these results suggested that the
protective effects of triptolide on podocytes in type II DN may be
achieved through an anti-inflammatory mechanism, further studies
are required to elucidate the precise signaling pathways, such as
the RhoA-and p38 mitogen-activated protein kinase (MAPK)-signaling
pathways (33,34). Furthermore, the results of the
present study indicate that triptolide is able to attenuate
glomerular hypertrophy and improve hyperlipidemia, which may be a
contributory factor in the protection of podocytes in DN.
In conclusion, triptolide showed a prominent
anti-albuminuric effect in DN. This effect was characterized by an
improvement in foot process effacement and the recovery of podocyte
injury markers, nephrin and podocin. Notably, triptolide did not
ameliorate hyperglycemia, HbA1c levels or SBP in the rats. The
therapeutic effect of triptolide may be due to the inhibition of
macrophage infiltration, in addition to the decreased secretion of
inflammatory cytokines. The results of the study demonstrate that
triptolide may provide protective effects in the prevention of DN
progression.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 30470642, 30670780,
31071014 and 81100260); the Shandong Province Tackle Key Problems
in Science and Technology Program (grant no. 2008GG10002006); the
Qingdao Municipal Science and Technology Commission [grant nos.
07-2-1-4-nsh-2 and 11-2-3-3-(2)-nsh] and the Shandong Province
Health Department 266021 (grant no. 2007-37). The authors would
like to thank Dr Wei Zhimin (Department of Pathology, Affiliated
Hospital of Qingdao University School of Medicine) for her help
with processing tissues for histology. In addition, the authors
would like to thank Li Yushan and Dr Tian Fen (Qingdao University
School of Medicine) for their assistance with the animal
experiments and statistical tests.
References
|
1
|
Murphy M, Crean J, Brazil DP, Sadlier D,
Martin F and Godson C: Regulation and consequences of differential
gene expression in diabetic kidney disease. Biochem Soc Trans.
36:941–945. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stitt-Cavanagh E, MacLeod L and Kennedy C:
The podocyte in diabetic kidney disease. ScientificWorldJournal.
14:1127–1139. 2009. View Article : Google Scholar
|
|
3
|
Wolf G, Chen S and Ziyadeh FN: From the
periphery of the glomerular capillary wall toward the center of
disease: podocyte injury comes of age in diabetic nephropathy.
Diabetes. 54:1626–1634. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mitu GM, Wang S and Hirschberg R: BMP7 is
a podocyte survival factor and rescues podocytes from diabetic
injury. Am J Physiol Renal Physiol. 293:F1641–F1648. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Patrakka J and Tryggvason K: Nephrin - a
unique structural and signaling protein of the kidney filter.
Trends Mol Med. 13:396–403. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Marshall SM: The podocyte: a potential
therapeutic target in diabetic nephropathy? Curr Pharm Des.
13:2713–2720. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wolf G and Ziyadeh FN: Cellular and
molecular mechanisms of proteinuria in diabetic nephropathy.
Nephron Physiol. 106:26–31. 2007. View Article : Google Scholar
|
|
8
|
Kikuchi Y, Imakiire T, Yamada M, et al:
Mizoribine reduces renal injury and macrophage infiltration in
non-insulin-dependent diabetic rats. Nephrol Dial Transplant.
20:1573–1581. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Williams MD and Nadler JL: Inflammatory
mechanisms of diabetic complications. Curr Diab Rep. 7:242–248.
2007. View Article : Google Scholar
|
|
10
|
Ziyadeh FN and Wolf G: Pathogenesis of the
podocytopathy and proteinuria in diabetic glomerulopathy. Curr
Diabetes Rev. 4:39–45. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wei X, Gong J, Zhu J, et al: Therapeutic
effects of triptolide on interleukin-10 gene-deficient mice with
colitis. Int Immunopharmacol. 8:1808–1812. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chan SC, Shum DK, Tipoe GL, Mak JC, Leung
ET and Ip MS: Upregulation of ICAM-1 expression in bronchial
epithelial cells by airway secretions in bronchiectasis. Respir
Med. 102:287–298. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin N, Liu C, Xiao C, et al: Triptolide, a
diterpenoid triepoxide, suppresses inflammation and cartilage
destruction in collagen-induced arthritis mice. Biochem Pharmacol.
73:136–146. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song HX, Gong J and Chen W: Effect of
triptolide on urinary monocyte chemottractant protein-1 in patients
with diabetic nephropathy. Zhongguo Zhong Xi Yi Jie He Za Zhi.
25:416–418. 2005.(In Chinese).
|
|
15
|
Ma RX, Liu LQ, Xu Y and Jiang W:
Protective effect of triptolide on renal tissues in type 2 diabetic
rats. Chinese Journal of Hypertension. 16:1120–1124. 2008.(In
Chinese).
|
|
16
|
Yamamoto Y, Maeshima Y, Kitayama H, et al:
Tumstatin peptide, an inhibitor of angiogenesis, prevents
glomerular hypertrophy in the early stage of diabetic nephropathy.
Diabetes. 53:1831–1840. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Danda RS, Habiba NM, Rincon-Choles H, et
al: Kidney involvement in a nongenetic rat model of type 2
diabetes. Kidney Int. 68:2562–2571. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo XH, Liu ZH, Li H, et al: A novel rat
model of type 2 diabetes mellitus. Chinese Journal of Nephrology
Dialysis & Transplantation. 9:351–355. 2000.(In Chinese).
|
|
19
|
Jefferson JA, Shankland SJ and Pichler RH:
Proteinuria in diabetic kidney disease: a mechanistic viewpoint.
Kidney Int. 74:22–36. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hauser PV, Collino F, Bussolati B and
Camussi G: Nephrin and endothelial injury. Curr Opin Nephrol
Hypertens. 18:3–8. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Baelde HJ, Eikmans M, Lappin DW, Doran PP,
Hohenadel D, Brinkkoetter PT, van der Woude FJ, et al: Reduction of
VEGF-A and CTGF expression in diabetic nephropathy is associated
with podocyte loss. Kidney Int. 71:637–645. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mathieson PW: Update on the podocyte. Curr
Opin Nephrol Hypertens. 18:206–211. 2009. View Article : Google Scholar
|
|
23
|
Wittmann S, Daniel C, Stief A, Vogelbacher
R, Amann K and Hugo C: Long-term treatment of sirolimus but not
cyclosporine ameliorates diabetic nephropathy in the rat.
Transplantation. 87:1290–1299. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eto N, Wada T, Inagi R, et al: Podocyte
protection by darbepoetin: preservation of the cytoskeleton and
nephrin expression. Kidney Int. 72:455–463. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang Y, Chen B, Hou XH, et al: Effects of
mycophenolate mofetil, valsartan and their combined therapy on
preventing podocyte loss in early stage of diabetic nephropathy in
rats. Chin Med J (Engl). 120:988–995. 2007.PubMed/NCBI
|
|
26
|
Chen ZH, Qin WS, Zeng CH, et al:
Triptolide reduces proteinuria in experimental membranous
nephropathy and protects against C5b-9-induced podocyte injury in
vitro. Kidney Int. 77:974–988. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zheng CX, Chen ZH, Zeng CH, Qin WS, Li LS
and Liu ZH: Triptolide protects podocytes from puromycin
aminonucleoside induced injury in vivo and in vitro. Kidney Int.
74:596–612. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gao Q, Shen W, Qin W, et al: Treatment of
db/db diabetic mice with triptolide: a novel therapy for diabetic
nephropathy. Nephrol Dial Transplant. 25:3539–3547. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tarabra E, Giunti S, Barutta F, et al:
Effect of the monocyte chemoattractant protein-1/CC chemokine
receptor 2 system on nephrin expression in streptozotocin-treated
mice and human cultured podocytes. Diabetes. 58:2109–2118. 2009.
View Article : Google Scholar
|
|
30
|
Lee EY, Chung CH, Khoury CC, et al: The
monocyte chemoattractant protein-1/CCR2 loop, inducible by
TGF-beta, increases podocyte motility and albumin permeability. Am
J Physiol Renal Physiol. 297:F85–F94. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tossidou I, Starker G, Kruger J, et al:
PKC-alpha modulates TGF-beta signaling and impairs podocyte
survival. Cell Physiol Biochem. 24:627–634. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Takano Y, Yamauchi K, Hayakawa K, et al:
Transcriptional suppression of nephrin in podocytes by macrophages:
roles of inflammatory cytokines and involvement of the PI3K/Akt
pathway. FEBS Lett. 581:421–426. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim YH, Lee SH, Lee, Choi SW, Park JW and
Kwon TK: Triptolide inhibits murine inducible nitric oxide synthase
expression by down-regulating lipopolysaccharide-induced activity
of nuclear factor-kappa B and c-Jun NH2-terminal kinase.
Eur J Pharmacol. 494:1–9. 2004. View Article : Google Scholar
|
|
34
|
Zhou Y, Ling EA and Dheen ST:
Dexamethasone suppresses monocyte chemoattractant protein-1
production via mitogen activated protein kinase phosphatase-1
dependent inhibition of Jun N-terminal kinase and p38
mitogen-activated protein kinase in activated rat microglia. J
Neurochem. 102:667–678. 2007. View Article : Google Scholar
|