Introduction
Ultraviolet (UV) irradiation, particularly UVB, has
suppressive effects on the immune system. UVB-induced
immunosuppression and photodamage are risk factors for skin cancer
development in animals and humans (1–3). The
activation of the p53 gene is important for photodamage/repair,
cell cycle arrest, cellular apoptosis and photocarcinogenesis
(4). It has been demonstrated that
numerous cytokines are involved in the process of UVB-induced
inflammation and/or immunosuppression (5,6).
With regard to the T-helper (Th)1/Th2 balance, Th1 cells secrete
interferon (IFN)-γ and IL-2, and Th2 cells secrete IL-4, IL-5 and
IL-10. Certain potential mechanisms for UVB-induced photodamage and
immunosuppression involve the expression and activation of the p53
protein, as well as an alteration in the balance of Th1- and
Th2-associated cytokines (7,8).
There has been a particular focus on the
chemoprevention of photodamage, which is considered as a less toxic
and more effective approach than traditional methods. Ginsenoside
is extracted from the root, stem and leaves of ginseng, and
consists of three major moieties: Rg, Rb and Rh (9). Ginsenoside Rg1 is a member of the
protopanaxatriol group of compounds, which exhibit multiple
pharmacological effects (10). A
recent study observed that ginsenoside Rg1 attenuated the
UVB-induced G1 arrest in HaCaT cells and dermal fibroblasts through
downregulating the expression of p16, p21 and p53 (11). Consistent with other studies, our
previous studies have shown that 8-methoxypsoralen
(8-MOP)/UVA-irradiated fibroblasts pretreated with ginsenoside Rg1
demonstrated a reduction in the expression of senescence-associated
β-galactosidase (SA-β-gal), a down-regulation in the level of
senescence-associated proteins and a deceleration in telomere
shortening (12). These results
suggest that ginsenoside Rg1 significantly antagonizes premature
senescence induced by 8-MOP/UVA in fibroblasts. However, it has not
yet been elucidated whether the photoprotective and
immunoregulatory mechanisms of ginsenoside Rg1 are correlated with
these cytokines in BALB/c mice. This study focused on the
photoprotective capacity of ginsenoside Rg1 and its
immunoregulatory capacity on the changes in IFN-γ, IL-10 and TNF-α
cytokines induced by chronic UVB irradiation in BALB/c mouse
skin.
Materials and methods
Experimental animals
Female BALB/c mice, aged 6–8 weeks old and weighing
∼20–25 g, were obtained from the Chinese Academy of Science,
Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China). A
total of six mice were used in each group. Mice were housed in a
pathogen-free barrier facility in the Experimental Animal Center of
Nanjing Medical University (Nanjing, China) and all experiment
protocols were approved by The Animal Care and Use Committee of
Nanjing Medical University.
Reagents
Purified ginsenoside Rg1 was purchased from Sigma
(St. Louis, MO, USA) and the ginsenoside Rg1 solution was prepared
as follows: 3 mg ginsenoside Rg1 was dissolved in 100 μl
acetone (3.0 mg, 6.5 μmol ginsenoside Rg1 in 100 μl
acetone/3 cm2 mouse skin). Polyclonal antibodies to
mouse p53 (sc-6243) were purchased from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA).
UVB irradiation protocols
UVB (280–320 nm) radiation was used, according to
the experiment. The UVB source (Spectronics Corp., Lincoln, NE,
USA) emitted an average irradiation of 1,243 mW/cm2.
BALB/c mice were randomly divided into groups as follows: (i)
sham-irradiated group, mice subjected to a sham UVB irradiation
procedure; (ii) UVB irradiation only; (iii) ginsenoside Rg1
pretreatment plus UVB exposure; and (iv) acetone pretreatment plus
UVB exposure, as the control. Prior to UVB exposure, all mice were
shaved with electric clippers and were treated topically with
ginsenoside Rg1 solution, acetone or nothing for 30 min, and then
certain dosages of UVB (30, 60 and 120 mJ/cm2) were
delivered at each exposure, respectively, for 30 consecutive
days.
Skin tissue treatment
At the end of experiment, the treated skin of the
ear and dorsal areas were obtained. For histopathological
examination and immunohistochemical analysis, the ear biopsies were
placed in 10% phosphate-buffered formalin, then dehydrated in
ascending concentrations of ethanol, cleared in xylene and embedded
in paraffin. Following conventional treatment, 4 mm-thick tissue
sections were prepared for regular hematoxylin and eosin staining
or immunohisto-chemical staining. For reverse transcription
polymerase chain reaction (RT-PCR) detection, the dorsal tissue was
preserved in freezing conditions (−70°C). Total RNA was isolated
using a total RNA extraction kit (TRIzol® reagent;
Molecular Research Centre, Inc., Cincinnati, OH, USA). The quality
of the RNA was confirmed by measuring the optical density (OD)
260/280 ratio.
Immunohistochemical analysis of p53
protein expression
The sections were treated with 0.01 M sodium citrate
buffer, prior to incubation with primary antibodies against p53
(Santa Cruz Biotechnology, Inc.) and incubation in a moist chamber
overnight at 4°C. After being washed in phosphate-buffered saline
(PBS), the sections were incubated in secondary antibody,
immunoglobulin (Ig)G, for 20 min and then were incubated with the
streptavidin-biotin-peroxidase complex (SABC) and
3,3′-diaminobenzidine (DAB) substrate solution, respectively.
Subsequently, the slides were counterstained with hematoxylin and
observed under a light microscope. A positive result was shown as a
light to dark brown staining/precipitate in the nuclei of the
cells. The p53+ nuclei in the epidermis among each 200
basal cells were counted in 10 randomly selected visual fields at
×400 magnification.
RT-PCR measurement
The mRNA expression levels of the IFN-γ, IL-10 and
TNF-α genes were detected by RT-PCR. In brief, cDNAs were
synthesized from 1 μl total RNA using avian myeloblastosis
virus (AMV) reverse transcriptase (Takara Co., Ltd., Shiga, Japan)
and random oligo (dT) primers. PCR amplification was performed with
a thermal cycler (Geneamp® PCR System 2400,
Perkin-Elmer, Norwalk, CT, USA). Thirty-five PCR cycles were run
under the following conditions: DNA denaturation at 94°C for 1 min;
primer annealing at 60°C for IFN-γ and 56°C for IL-10 for 1 min and
at 57°C for TNF-α for 40 sec; and DNA extension at 72°C for 1 min.
The housekeeping gene β-actin was amplified as an internal control
from the same cDNA product in a separate reaction. The primer
sequences (Shanghai BioAsia Co. Ltd, Shanghai, China) specific for
the previously mentioned cytokines (IFN-γ, IL-10, TNF-α and
β-actin) were as follows: IFN-γ gene (426 bp), 5′-GGCTGTTTCTGGCTG
TTACTGC-3′ (upstream) and 5′-GACTCCTTTTCCGCT TCCTGA-3′
(downstream); IL-10 gene (394 bp), 5′-CAA TAACTCACCCACTTCC-3′
(upstream) and 5′-CAT GGCCTTGTAGACACCTT-3′ (downstream); TNF-α gene
(212 bp), 5′-TCTCATCAGTTCTATGGCCC-3′ (upstream) and
5′-GGGAGTAGACAAGGTACAAC-3′ (downstream); and β-actin gene (222 bp),
5′-TGACCGGCTTGTATGCTATC-3′ (upstream) and
5′-CAGTGTGAGCCAGGATATAG-3′ (downstream).
All the PCR products were subjected to
electrophoresis in a 2.0% agarose gel containing 0.5 mmol/l
ethidium bromide and photographic images were subsequently
captured. The density of each band was analyzed using a gel imaging
system densitometer (Bio-Rad, Hercules, CA, USA).
Statistical analysis
The results are expressed as the mean ± standard
deviation. The statistical significance of the difference between
two independent groups was determined using a t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Ginsenoside Rg1 protects against
photodamage caused by UVB irradiation in the skin of BALB/c
mice
The histological changes in the irradiated and
non-irradiated control mouse skin samples were examined 30 days
subsequent to the UVB-irradiation at different doses. Fig. 1 demonstrates the pathological
results of the hematoxylin and eosin-stained specimens observed in
the different groups of BALB/c mice. The UVB-exposed mouse skin
appeared thicker compared with that of the mice in the control and
ginsenoside Rg1-pretreated groups, with hyperkeratosis, acanthosis,
sponge-like edematization and sunburn occurring in the epidermis.
In addition, edema in the papillary layer of the dermis,
telangiectasis, an intense diffuse inflammatory leukocyte
infiltration (predominantly monocytes/macrophages and neutrophils)
and tissue necrosis were observed in the UVB-irradiated mice.
Following chronic UVB irradiation, infiltrating cells were present
in the dermis in higher numbers, compared with those in the
non-UVB-exposed skin of the control mice. The damage was more
marked in the mice exposed to a higher dosage of UVB
irradiation.
The application of ginsenoside Rg1 protected against
UVB-induced damage and maintained the normal structure of the
epidermis and dermis. A significant alleviation of skin swelling,
slight epidermal injury and a reduced lymphocyte and
polymorphonuclear cell infiltration with minimal dermal tissue
destruction were observed in the groups pretreated with ginsenoside
Rg1 compared with that in the UVB-irradiated mice that did not
receive pretreatment. Therefore, morphological differences between
the irradiation-only mouse skin samples and samples pretreated with
ginsenoside Rg1 were able to be distinguished in the
histopathological images.
In addition, to examine whether ginsenoside Rg1
exerted a toxic effect on the skin of BALB/c mice, one group was
treated with ginsenoside Rg1 without UVB irradiation. The result
demonstrated that the morphology of sham-irradiated skin treated
with ginsenoside Rg1 for 1 month appeared similar to the skin of
the normal control mice. Thus, it was concluded that 30 mg/ml
ginsenoside Rg1 did not affect the histopathology of the mouse
skin. For the group treated with acetone plus UVB irradiation, the
pathological changes appeared to be similar to those in the
UVB-irradiated mouse skin, which suggested that the acetone solvent
possessed no marked photoprotective capacity on the UVB-irradiated
mouse skin.
Ginsenoside Rg1 downregulates p53 protein
expression induced by UVB irradiation in the skin of BALB/c
mice
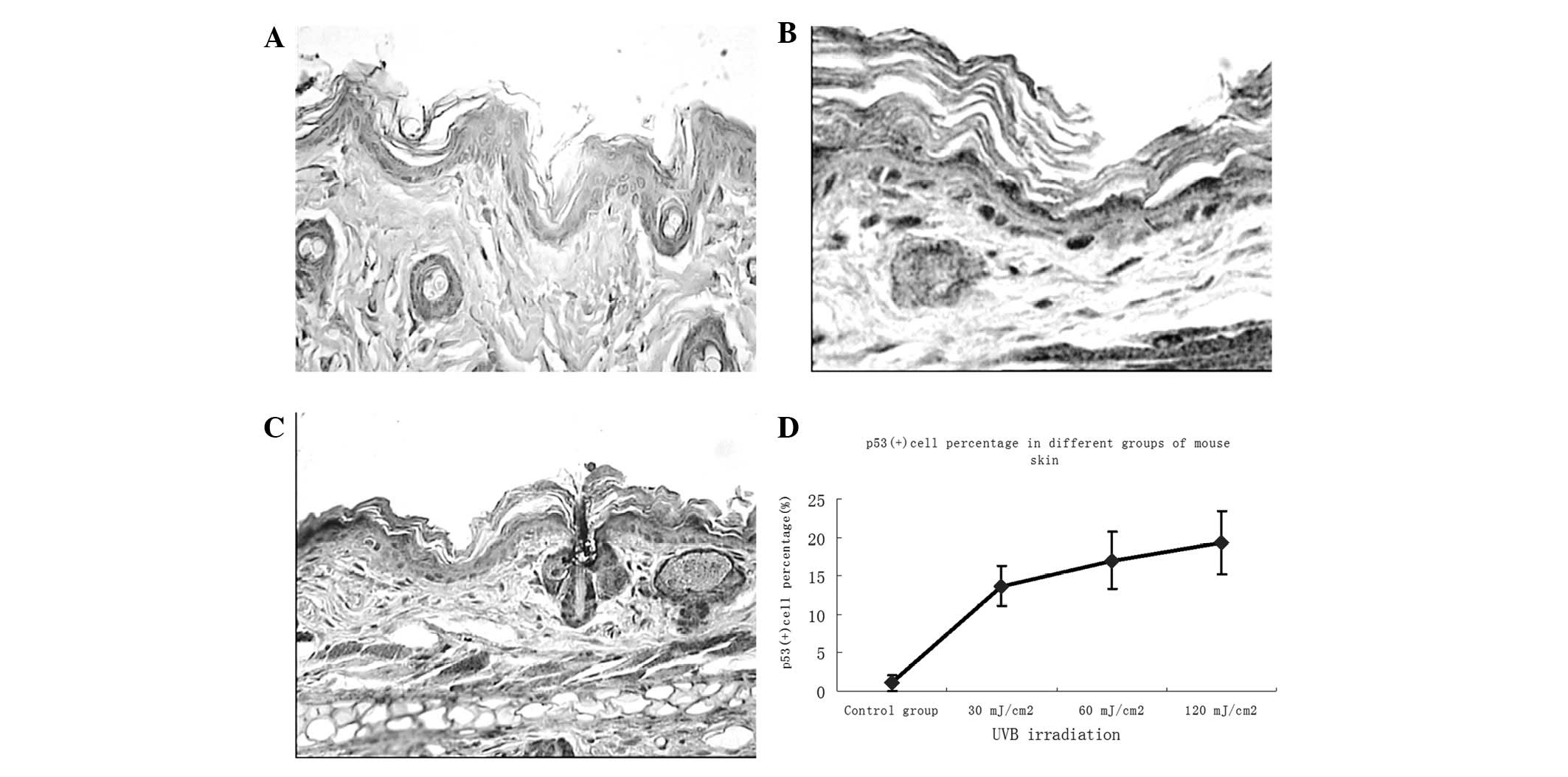
To examine the effects of different doses of UVB
irradiation and ginsenoside Rg1 on local p53 expression in BALB/c
mice, skin biopsies were performed following 30 days of consecutive
specific treatments and immunohistochemical analysis was used to
determine the number of p53+ stained cells (which were
stained brown). Subsequent to UVB exposure, the majority of the
p53+ cells appeared in the basal layer of the epidermis,
while some were present around the hair follicles in the skin of
irradiated mice (Fig. 2). The
number of p53+ cells was markedly increased in
comparison with that in the non-UVB-exposed skin, with a noticeable
upregulation at 30 mJ/cm2 and marginal increases at 60
and 120 mJ/cm2. Abundant p53+ cells were
observed in the skin of the UVB-irradiated groups; however, only a
few p53+ cells remained in the group pretreated with
ginsenoside Rg1. Significant differences between the ginsenoside
Rg1-pretreated and the UVB irradiation-only groups were observed at
each parallel control under 30, 60 and 120 mJ/cm2 UVB
irradiation (Fig. 3). With regard
to the p53 protein expression induced by UVB irradiation,
pretreatment with ginsenoside Rg1 resulted in marked reductions of
69.50, 23.53 and 12.93% at 30, 60 and 120 mJ/cm2,
respectively. However, in the acetone-pretreated mice, the number
of p53+ nuclei was 78% of the number in the UVB
irradiation group (data not shown), which further indicated that
acetone did not have any photoprotective capacity under such
circumstances.
Different doses of multiple UVB
irradiation modulate the mRNA expression of three types of
cytokines, leading to the downregulation of IFN-γ and upregulation
of IL-10 and TNF-α
To examine the effects of different doses of
multiple UVB irradiation on the local expression of the IFN-γ,
IL-10 and TNF-α genes, skin samples were collected following 30
consecutive days of irradiation and processed for mRNA detection by
RT-PCR. There were statistically significant differences in the
mRNA expression of cytokines between mice irradiated with different
doses of UVB and non-irradiated control mice (Fig. 4). Compared with the sham-irradiated
group, the mRNA expression of the IFN-γ cytokine was downregulated
by 19.6, 36.3 and 39.6% following 30, 60 and 120 mJ/cm2
UVB irradiation, respectively, and the difference was statistically
significant. In addition, the levels of IL-10 mRNA in the
irradiated groups demonstrated a dose-dependent induction and
increased by 40.1, 71.0 and 89.4% following 30, 60 and 120
mJ/cm2 UVB irradiation, respectively, and the
upregulation was statistically significant at each dose of UVB.
Furthermore, the expression of TNF-α mRNA in each UVB-irradiated
group was upregulated by 36.4, 18.4 and 8.6% versus the
sham-irradiated group, respectively. The expression level of TNF-α
mRNA peaked significantly at 30 mJ/cm2 and fell
marginally at 60 and 120 mJ/cm2.
 | Figure 4.Effects of different doses of
ultraviolet B (UVB) irradiation on the mRNA expression levels of
three types of cytokines. BALB/c mice were irradiated with
different doses of UVB irradiation (30, 60 and 120
mJ/cm2) for 30 consecutive days, skin samples were
collected and the mRNA expression of interferon (IFN)-γ,
interleukin (IL)-10 and tumor necrosis factor (TNF)-α genes was
detected by a reverse transcription polymerase chain reaction
(RT-PCR). The mRNA expression of the IFN-γ cytokine was
downregulated by 19.6, 36.3 and 39.6% following 30, 60 and 120
mJ/cm2 UVB irradiation, respectively (P<0.05). The
levels of IL-10 mRNA in the UVB-irradiated group were upregulated
in a dose-dependent manner, showing 40.1, 71.0 and 89.4% increases
following 30, 60 and 120 mJ/cm2 UVB irradiation,
respectively (P<0.05). An upregulation of TNF-α mRNA was
observed in the UVB-irradiated groups by 36.4% at 30
mJ/cm2 (P<0.05) and 18.4 and 8.6% at 60 and 120
mJ/cm2 (P>0.05), respectively, compared with the
control group. The combined data of three representative
experiments are shown and expressed as the mean band density value
± standard deviation. |
Pretreatment with ginsenoside Rg1 prior
to UVB exposure may reverse UVB-induced mRNA expression levels of
IFN-γ, IL-10 and TNF-α
While UVB exposure reduced IFN-γ mRNA expression and
induced IL-10 and TNF-α mRNA expression in the skin of BALB/c mice
(Fig. 4), pretreatment with
ginsenoside Rg1 prior to UVB exposure resulted in an attenuation of
the effects on the expression of the cytokines caused by 30
mJ/cm2 UVB irradiation (Fig. 5). The conditioned skin samples were
collected and the RT-PCR results revealed that ginsenoside Rg1
increased the UVB-reduced IFN-γ mRNA level by 19.7% and led to
significant reductions in the mRNA expression levels of IL-10 and
TNF-α of 25.7% and 20%, respectively. Unlike ginsenoside Rg1,
acetone had no such effect on the mRNA expression of the three
cytokines.
 | Figure 5.Effect of ginsenoside Rg1 pretreatment
on the ultraviolet B (UVB)-induced mRNA expression levels of three
cytokines. BALB/c mice were pretreated with 3.0 mg/100 μl
ginsenoside Rg1 and then irradiated with 30 mJ/cm2 UVB
for 30 consecutive days. A reverse transcription polymerase chain
reaction (RT-PCR) was used to detect the mRNA expression of
inter-feron (IFN)-γ, interleukin (IL)-10 and tumor necrosis factor
(TNF)-α genes for the conditioned skin samples. (A) RT-PCR results
for IFN-γ, IL-10 and TNF-α mRNA expression on agarose gel
electrophoretograms from the different conditioned skin samples of
BALB/c mice: Lane M, DNA marker; lane 1, 120 mJ/cm2 UVB
irradiation; lane 2, 60 mJ/cm2 UVB irradiation; lane 3,
acetone plus 30 mJ/cm2 UVB irradiation; lane 4,
ginsenoside Rg1 plus 30 mJ/cm2 UVB irradiation; lane 5,
30 mJ/cm2 UVB irradiation; lane 6, sham-irradiated
group. (B) Ginsenoside Rg1 increased the mRNA level of IFN-γ by
19.7% and inhibited the UVB-induced IL-10 and TNF-α mRNA expression
by 25.7 and 20%, respectively. However, acetone had no significant
effect on the mRNA expression of the three cytokines (P>0.05).
The combined data of three representative experiments are shown and
expressed as the mean band density value ± standard deviation. |
Discussion
UVB irradiation is the major environmental
carcinogen for human skin. The biologically active UVB wavelengths
are predominantly absorbed in the epidermis, where UVB directly
damages DNA via formation of mutagenic photoproducts (13). It has been demonstrated that
leukocyte infiltration into UVB-irradiated skin is critical in
UVB-induced inflammation and the immunological response (14). In order to understand the mechanism
by which ginsenoside Rg1 affects p53 protein expression and certain
cytokine changes induced by UVB irradiation, the present study was
conducted on BALB/c mice by pretreating the mice with ginsenoside
Rg1 prior to 30 days of UVB exposure. The histopathological results
suggested that ginsenoside Rg1 protected against UVB-induced
photodamage to the skin, and resulted in an alleviation of skin
swelling, a relief of epidermal injury and dermal destruction and a
reduction in the inflammatory cell infiltration. Of particular
importance was the fact that the number of p53+ cells in
the UVB-exposed epidermis was higher than that in the control,
showing a noticeable upregulation at a dose of 30 mJ/cm2
and marginal increases at doses of 60 and 120 mJ/cm2.
This type of curved pattern indicated that particularly high doses
of UVB irradiation injured the epidermis, leading to cell death and
broken cells, with the result that p53+ cells were not
able to be stained and detected. This further suggested that UVB
irradiation damaged the epidermal cells and upregulated p53 protein
expression in intact cells. Pretreatment with ginsenoside Rg1
significantly reduced the number of p53+ cells in the
epidermis, particularly in the low dose UVB-irradiated group.
Ginsenoside Rg1 treatment resulted in a marked reduction in p53
protein expression by 69.50% at a dose of 30 mJ/cm2 and
smaller reductions for the groups irradiated at doses of 60 and 120
mJ/cm2, which may indicate that the protection of
ginsenoside Rg1 is limited, since high doses of UVB irradiation
destroy epidermal cells. These data suggest that a possible
mechanism of the photoprotective capacity of ginsenoside Rg1 may be
associated with the downregulation of p53 protein expression.
UVB irradiation impairs the cell-mediated immune
response by means of reducing the levels of Th1 cytokines (such as
IFN-γ, IL-2 and IL-12) and inducing Th2 cytokines (such as IL-10
and IL-4). An imbalance of the Th1 versus Th2 cell cytokines may be
responsible for the development of UVB-induced immunosuppression
(15). The aim of the study was to
clarify in an in vivo experiment whether UVB-induced
immunosuppression was associated with the downregulation of IFN-γ
and/or the upregulation of IL-10 and TNF-α, and whether the topical
application of ginsenoside Rg1 prior to UVB irradiation was able to
reverse the immunosuppressive changes caused by UVB irradiation.
The study demonstrated that UVB exposure led to a reduction in
IFN-γ mRNA expression levels and increases in IL-10 and TNF-α mRNA
expression levels and that ginsenoside Rg1 was able to attenuate
these phenomena to a certain degree, i.e. by upregulating IFN-γ
and/or downregulating IL-10 and TNF-α in the skin of BALB/c
mice.
With regard to the three types of cytokines, IFN-γ
and IL-10 represent Th1/Th2 development and perform different
functions to maintain the balance of Th1/Th2 immunity (16,17).
TNF-α is not only associated with immuno-suppression, but also acts
as an inflammatory mediator (18,19).
IFN-γ, serving as one type of Th1 cytokine, is able to stimulate
antigen presenting cells (APCs) and promote the cell-mediated
immune response (20). IL-10,
which is identified as a Th2 cell product, is an important
regulator of cutaneous immune function and has been demonstrated to
be involved in UVB-induced immunosuppression (15,21,22).
In addition, IL-10 inhibits Th1 cell clones by downregulating IFN-γ
expression and it also reduces antigen presentation by APCs,
including epidermal Langerhans cells (23). With regard to the immunity balance
in the skin, IFN-γ and IL-10, are involved in modulating the
immunity of the skin. It has been demonstrated that numerous
factors are able to stimulate TNF-α production, and UVB is one of
the major stimuli for TNF-α production in the surrounding
environment (24,25). In addition, the results of the
present study indicated that TNF-α and IL-10 signaling was involved
in UVB-induced immunosuppression, and was important in the
UVB-induced immunosuppressive mechanism.
An additional aim of the study was to explore
whether ginsenoside Rg1 application at the site of UVB irradiation
was capable of repairing the impaired immune response. On the basis
of previous results, it was speculated that the immunoprotective
effect of ginsenoside Rg1 resulted from the enhancement of host
immunity through the upregulation of Th1 cytokines and the
prevention of the UVB-induced cytokines from decreasing. In the
present animal model, ginsenoside Rg1 application prior to UVB
exposure exerted an effect on the immune response in favor of Th1
cytokine production. Our data showed that it was likely that the
immunoprotective capacity of ginsenoside Rg1 was mediated by the
upregulation of IFN-γ and the downregulation of IL-10 mRNA
expression, thereby acting against the effects induced by UVB
irradiation. In addition, ginsenoside Rg1 inhibited UVB-induced
TNF-α mRNA expression in BALB/c mice, which led to protection
against UVB-induced inflammation. It was concluded that the
anti-inflammatory effect of ginsenoside Rg1 was mediated in part
through the downregulation of TNF-α mRNA expression. In
combination, the data strongly indicated that ginsenoside Rg1 may
be a potential immunomodulator and anti-inflammatory substance
against UVB-induced immuno-suppression and inflammation. The study
demonstrated that the local application of ginsenoside Rg1 may
provide a novel method for cutaneous photoprotection and the
treatment of immune-mediated skin diseases. The data also showed
that it was ginsenoside Rg1 itself, rather than acetone, that
provided the photoprotective effects against UVB-induced
photo-damage and photoimmunological alteration, since there was no
significant difference between the acetone pretreatment and UVB
groups. This implied that unlike ginsenoside Rg1, acetone did not
demonstrate any photoprotective capacity.
In conclusion, the present study demonstrated that
chronic UVB irradiation induced dose-dependent histopathological
changes and affected the levels of p53 protein expression in the
skin of BALB/c mice, and that pretreatment with topical ginsenoside
Rg1 was able to protect the mouse skin from photodamage and p53
expression. In addition, pretreatment with ginsenoside Rg1 resulted
in an attenuation of the up- or downregulation of the mRNA
expression of three cytokines (IFN-γ, IL-10 and TNF-α) induced by
UVB exposure, suggesting a potential mechanism by which ginsenoside
Rg1 prevents UVB-induced local immunosuppression. The results
suggest that ginseng may be a potential photocarcinogenesis
inhibitor for humans.
Acknowledgements
This study was supported by grants
from the China National Natural Science Foundation (grant no.
81000700), the science project from the Traditional Chinese
Medicine Bureau of Jiangsu Province (grant no. LZ11084) and the
Jiangsu National Natural Science Foundation (grant no.
BK2012877).
References
|
1.
|
Marrot L and Meunier JR: Skin DNA
photodamage and its biological consequences. J Am Acad Dermatol.
58(Suppl 2): S139–S148. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Seité S, Fourtanier A, Moyal D and Young
AR: Photodamage to human skin by suberythemal exposure to solar
ultraviolet radiation can be attenuated by sunscreens: a review. Br
J Dermatol. 163:903–914. 2010.PubMed/NCBI
|
|
3.
|
Afaq F, Adhami VM and Mukhtar H:
Photochemoprevention of ultraviolet B signaling and
photocarcinogenesis. Mutat Res. 571:153–173. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Benjamin CL, Ullrich SE, Kripke ML and
Ananthaswamy HN: p53 tumor suppressor gene: a critical molecular
target for UV induction and prevention of skin cancer. Photochem
Photobiol. 84:55–62. 2008.PubMed/NCBI
|
|
5.
|
Granstein RD and Matsui MS: UV
radiation-induced immuno-suppression and skin cancer. Cutis.
74(Suppl): 4–9. 2004.
|
|
6.
|
Schwarz T: Mechanisms of UV-induced
immunosuppression. Keio J Med. 54:165–171. 2005. View Article : Google Scholar
|
|
7.
|
Katiyar SK: UV-induced immune suppression
and photocarcino-genesis: chemoprevention by dietary botanical
agents. Cancer Lett. 255:1–11. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Katiyar SK: Grape seed proanthocyanidines
and skin cancer prevention: inhibition of oxidative stress and
protection of immune system. Mol Nutr Food Res. 52(Suppl 1):
S71–S76. 2008.PubMed/NCBI
|
|
9.
|
Christensen LP: Ginsenosides chemistry,
biosynthesis, analysis, and potential health effects. Adv Food Nutr
Res. 55:1–99. 2009.PubMed/NCBI
|
|
10.
|
Cheng Y, Shen LH and Zhang JT:
Anti-amnestic and anti-aging effects of ginsenoside Rg1 and Rb1 and
its mechanism of action. Acta Pharmacol Sin. 26:143–149. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Wang XY, Wang YG and Wang YF: Ginsenoside
Rb1, Rg1 and three extracts of traditional Chinese medicine
attenuate ultra-violet B-induced G1 growth arrest in HaCaT cells
and dermal fibroblasts involve down-regulating the expression of
p16, p21 and p53. Photodermatol Photoimmunol Photomed. 27:203–212.
2011. View Article : Google Scholar
|
|
12.
|
Zhou BR, Xu Y, Wu D, Permatasari F, Gao YY
and Luo D: Ginsenoside Rg1 protects human fibroblasts against
psoralen- and UVA-induced premature senescence through a telomeric
mechanism. Arch Dermatol Res. 304:223–228. 2012. View Article : Google Scholar
|
|
13.
|
Cadet J, Mouret S, Ravanat JL and Douki T:
Photoinduced damage to cellular DNA: direct and photosensitized
reactions. Photochem Photobiol. 88:1048–1065. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Byrne SN, Beaugie C, O’Sullivan C,
Leighton S and Halliday GM: The immune-modulating cytokine and
endogenous Alarmin interleukin-33 is upregulated in skin exposed to
inflammatory UVB radiation. Am J Pathol. 179:211–222. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Leitenberger J, Jacobe HT and Cruz PD Jr:
Photoimmunology - illuminating the immune system through
photobiology. Semin Immunopathol. 29:65–70. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Ding W, Beissert S, Deng L, et al: Altered
cutaneous immune parameters in transgenic mice overexpressing viral
IL-10 in the epidermis. J Clin Invest. 111:1923–1931. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Lewis W, Simanyi E, Li H, et al:
Regulation of ultraviolet radiation induced cutaneous
photoimmunosuppression by toll-like receptor-4. Arch Biochem
Biophys. 508:171–177. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Ihnatko R and Kubes M: TNF signaling:
early events and phosphorylation. Gen Physiol Biophys. 26:159–167.
2007.PubMed/NCBI
|
|
19.
|
Mathew SJ, Haubert D, Krönke M and Leptin
M: Looking beyond death: a morphogenetic role for the TNF
signalling pathway. J Cell Sci. 122:1939–1946. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Donnelly RP and Kotenko SV:
Interferon-lambda: a new addition to an old family. J Interferon
Cytokine Res. 30:555–564. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Yoshiki R, Kabashima K, Sakabe J, et al:
The mandatory role of IL-10-producing and OX40 ligand-expressing
mature Langerhans cells in local UVB-induced immunosuppression. J
Immunol. 184:5670–5677. 2010. View Article : Google Scholar
|
|
22.
|
Schwarz T: 25 years of UV-induced
immunosuppression mediated by T cells-from disregarded T suppressor
cells to highly respected regulatory T cells. Photochem Photobiol.
84:10–18. 2008.PubMed/NCBI
|
|
23.
|
Katiyar SK, Challa A, McCormick TS, Cooper
KD and Mukhtar H: Prevention of UVB-induced immunosuppression in
mice by the green tea polyphenol (-)-epigallocatechin-3-gallate may
be associated with alterations in IL-10 and IL-12 production.
Carcinogenesis. 20:2117–2124. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Faurschou A: Role of tumor necrosis
factor-α in the regulation of keratinocyte cell cycle and DNA
repair after ultraviolet-B radiation. Dan Med Bull.
57:B41792010.
|
|
25.
|
Bashir MM, Sharma MR and Werth VP:
TNF-alpha production in the skin. Arch Dermatol Res. 301:87–91.
2009. View Article : Google Scholar : PubMed/NCBI
|