Introduction
Fat transplantation is extensively applied in
cosmetic and reconstructive surgery as fat is an autologous
material, with specific applications in mammaplasty, such as for
treating breast deformity and asymmetry (1,2). At
present, breast cancer is the second most common type of cancer and
the preferred treatment is surgical therapy (3). However, conservative surgery that
treats breast cancer by local excision has been demonstrated to be
associated with anxiety and depression in patients with breast
cancer and secondary breast deformity. In such cases, autologous
fat grafting enables repair and augmentation of soft tissues, with
the advantages of biocompatibility, versatility, natural appearance
and low donor site morbidity (4).
Although recovery of the fat implant and hyperplasia of adipocytes
(ACs) are directly associated with human adipose-derived stem cells
(hADSCs) (2,5), whether fat granule transplantation
may be applied to patients following mammary cancer surgery and
whether hADSCs enhance the growth and invasive ability of residual
cancer remain unknown.
The transcription factors peroxisome
proliferator-activated receptor γ (PPARγ) and activating protein 2
(aP2), are involved in the adipogenic differentiation of hADSCs and
the occurrence, progression and prognosis of cancer (6–8).
PPARγ is expressed in breast, pancreatic, testicular and other
types of tumor cells (9). The low
level of PPARγ expression observed in breast cancer tissue has been
suggested to be a possible therapeutic target for the prevention of
breast cancer progression (10). A
previous study demonstrated that the activation of PPARγ is able to
inhibit cancer cell growth (11),
and this may be due to the inhibition of angiogenesis (12–14).
aP2 is critical for regulating gene expression during early
development and in breast cancer (15).
Therefore, the present study investigated the
dynamic behavior of relevant transcription factors and the
paracrine effects on MCF-7 human breast cancer cells during hADSC
adipogenesis, providing a basis for the clinical application of fat
transplantation.
Materials and methods
Cell culture and conditioned media
Adipose tissue samples were obtained from the
subcutaneous abdominal fat tissues of three patients (age, 2–4
years) from the Department of Plastic Surgery of Nanfang Hospital
(Guangzhou, China) who had been submitted for surgical treatment of
a cicatrix by dermoplasty with a full-thickness skin graft.
Patients with inflammatory or malignant diseases were not included
in the study. This study was approved by the Research Ethics
Committee of Southern Medical University (Guangzhou, China). All
guardians provided informed consent.
Adipose tissue was obtained from the subcutaneous
fat tissue at the donor site of a full-thickness skin graft taken
during the dermoplastic treatment of a cicatrix. Cell isolation and
culture were performed according to the method described by Zuk
et al (16) with certain
modifications. Briefly, following the removal of all fibrous
material and visible blood vessels, adipose tissue samples were cut
into small pieces (10–15 mm) and digested in 10 mM
phosphate-buffered saline (PBS; Sigma, St. Louis, MO, USA)
containing 0.75% collagenase I (Sigma) for 30–60 min in a shaking
water bath at 37°C. The dispersed material was centrifuged (170 ×
g, 25°C) for 5 min, and the pellet was resuspended and seeded in
flasks. After 24 h, the medium was replaced with fresh medium.
Cells were cultured for up to three to four passages
in triplicate. For each passage, 1×106 cells were seeded
in 75-cm2 culture flasks for 7–10 days. When the cells
attached to the flask reached ∼80% confluence, subculture (passage)
was performed by enzymatic digestion (0.25% trypsinization). Cells
in the second to fourth passage were used for experiments. MCF-7
cells were obtained from the Cell Bank at the Chinese Academy of
Sciences (Shanghai, China). Cell lines used in this study were
maintained in a humidified (5% CO2) incubator. Cells
were cultured in Dulbecco’s modified Eagle’s medium (DMEM)
containing 10% fetal bovine serum (FBS; Gibco-BRL, Carlsbad, CA,
USA) and 2 mM L-glutamine (Gibco-BRL).
Expansion and differentiation of
hADSCs
The hADSCs were seeded by suspending
2–3×105 cells/ml in 24-well plates, and cultured in
osteogenic, adipogenic or chondrogenic induction medium (Table I). The medium was replaced every 3
days. Adipocytes induced for 6 or 12 days were termed as the AC-6d
and AC-12d groups, respectively.
 | Table I.Components of the culture medium. |
Table I.
Components of the culture medium.
| Induction type | Components | Concentration |
|---|
| Osteogenic | DMEM | - |
| FBS | 10% |
| Dexamethasone | 0.1 μM |
| β-glycerophosphate
disodium | 10 mM |
| Vitamin C | 50 μg/ml |
| Adipogenic | DMEM | - |
| FBS | 8% |
| Dexamethasone | 1 μM |
| Insulin | 10 μM |
| Indomethacin | 200 μM |
| Isobutyl
methyl-xanthine | 0.5 mM |
| Chondrogenic | FBS | 1% |
| TGF-β1 | 10 ng/ml |
| Insulin | 6.25
μg/ml |
| Siderophilin | 6.25
μg/ml |
| Dexamethasone | 0.1 μM |
| Vitamin C | 50 μg/ml |
Immunohistochemical staining
Cells were cultured and fixed after 14 days. ACs
were identified as red lipid droplets upon staining with Oil Red O.
Differentiated osteogenic cells were stained with Alizarin Red-S,
alkaline phosphatase and Von Kasso. All staining markers were
purchased from (Genmed Scientifics Inc., Arlington, MA, USA).
Flow cytometric analysis
Cell aliquots (2×106 cells/ml) were
incubated with monoclonal antibodies (Caltag, Carlsbad, CA, USA):
fluorescein isothiocyanate (FITC)-conjugated anti-human-CD29,
-CD34, -CD44, -CD45 and -CD105, respectively, for 30 min, and
washed with PBS prior to analysis.
Cell invasion assay
For the invasion assay, 2.5×105 MCF-7
cells were seeded in the upper well of each transwell chamber
(Corning Inc., Tewksbury, MA, USA). Conditioned culture medium (300
μl; Table I) and
300μl DMEM containing 20% FBS were placed in the lower
compartment of the chemo-taxis chamber as a source of
chemoattractants. Cells were incubated for 24 h at 37°C with 5%
CO2. Cells that had invaded the lower surface of the
membrane were fixed with methanol and stained with
hexamethylpararosaniline (GenMed). Using light microscopy, at least
four random fields were selected and the cells in each field were
counted. Subsequently, the cells were eluted in 600 μl 33%
acetic acid (Shanghai Sangon Biological Engineering Technology and
Services Co., Ltd., SongJiang, China) for 10 min and the optical
density (OD) of the final cells through the matrigel (R&D
Systems, Minneapolis, MN, USA) was determined at 570 nm. The MCF-7
cells grown in standard medium were set as the control group.
Cytokine measurement
The concentrations of vascular endothelial growth
factor (VEGF), matrix metalloproteinase 2 (MMP-2) and MMP-9 were
detected with Quantikine ELISA kits (R&D systems, Minneapolis,
MN, USA). The concentrations of urokinase-type plasminogen
activator (uPA) were measured with uPA Activity Assay kit (EMD
Millipore Corporation, Billerica, MA, USA. The OD was read using a
spectrophotometer set at a wavelength of 450 nm within 30 min.
Western blot analysis
Cells were centrifuged at 240 × g for 1 min at room
temperature. Following centrifugation, cells were washed twice with
PBS, and the supernatant containing proteins was extracted and
quantified on ice. Each lane was loaded with samples on 8 and 12%
vertical sodium dodecyl sulfate-polyacrylamide gel electrophoresis
gel, separated and electro-transferred to polyvinylidene fluoride
ultrafiltration membranes (Millipore, Billerica, MA, USA). The
primary antibodies against aP2 (1:200), PPARγ (1:200), and GAPDH
(1:1,000), as well as the secondary antibodies (1:10,000), were
purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA,
USA).
Statistical analysis
Protein levels were determined by western blot
analysis with Quantity One software (Bio-Rad, Hercules, CA, USA).
SPSS software, version 13.0 (SPSS, Inc., Chicago, IL, USA) was used
for the Student’s unpaired t-test and rank-sum test. Data are
expressed as the mean ± standard deviation (SD). P≤0.05 was
considered to indicate a statistically significant difference.
Results
Characterization of differentiated
hADSCs
To confirm that the culture-expanded cells were true
stem cells, the original phenotype and mesodermal differentiation
potential upon exposure to chondrogenic, osteogenic and adipogenic
specific agents were examined. Compared with the third generation
of hADSCs (Fig. 1A), the presence
of lipid droplets characteristic of adipogenic cells (Fig. 1B), and calcium deposits
characteristic of osteogenic cells (Fig. 1C) was observed, and was confirmed
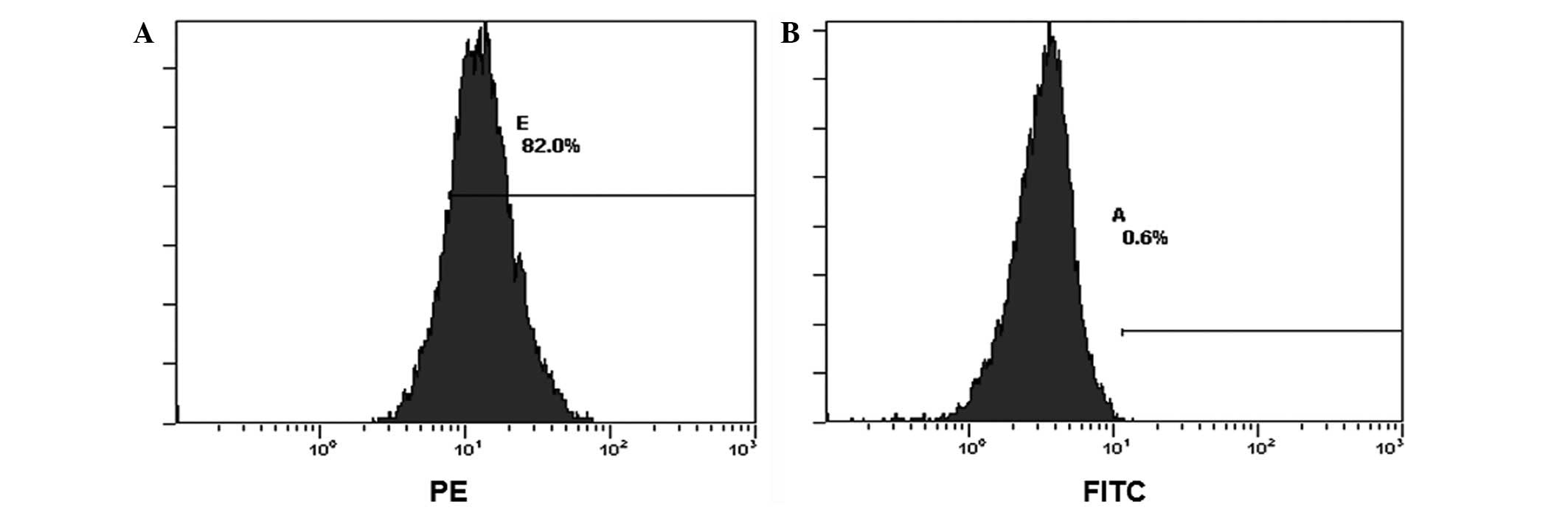
by Oil Red O and Alizarin Red-S staining, respectively (Fig. 1D and E). Flow cytometry analysis
revealed expression of CD29, CD44 and CD105, but no expression of
CD34 and CD45 in the third generation (Fig. 2). In conclusion, these results
indicated that the expanded cells possessed the basic properties of
differentiation.
Invasion ability assay of MCF-7 in
vitro
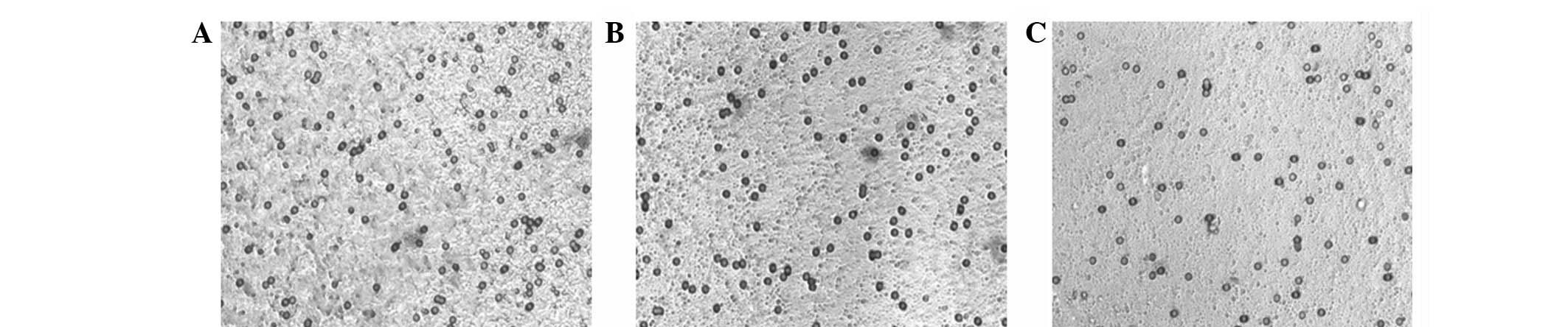
A transwell assay was performed to evaluate the
invasion activity of MCF-7 cells. Morphological invasive features
of MCF-7 in the differently conditioned media are shown in Fig. 3. MCF-7 invasion was markedly
enhanced by the inductive effects of ACs (Table II) for 12 days (AC-12d) and a
greater invasive MCF-7 migration through the matrigel was detected
compared with that of the control group. Moreover, the conditioned
media for hADSCs and adipogenic induction both increased the level
of MCF-7 invasion to a significant level (Table II). These results suggest that
hADSCs enhance the invasive activity of MCF-7 cells.
 | Table II.Detected MCF-7 cell numbers at 450 nm
in the presence of differently conditioned media. |
Table II.
Detected MCF-7 cell numbers at 450 nm
in the presence of differently conditioned media.
| Group | OD |
|---|
| hADSC-induced | 0.263±0.009a |
| AC-12d-induced | 0.202±0.004a |
| Control | 0.184±0.003 |
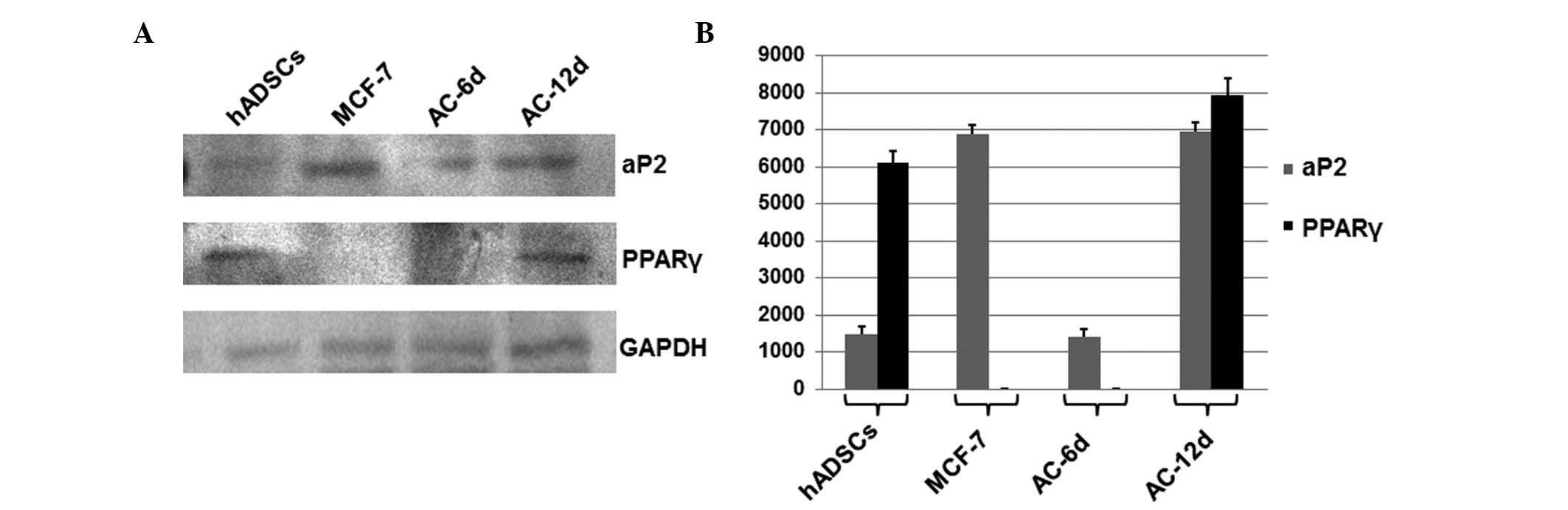
Expression of PPARγ and aP2
To investigate the dynamic behavior of transcription
factors associated with paracrine regulation, the expression levels
of PPARγ and aP2 in MCF-7 cells, hADSCs, and AC-6d and AC-12d group
cells during the entire adipogenic process were analyzed by western
blotting. GAPDH served as a loading control. As shown in Fig. 4, no significant differences were
observed in aP2 expression between the hADSC and AC-6 d groups;
however, a significant increase in the AC-12 d group was observed
when compared with that of the hADSC group (P<0.05). This
indicated that the increased expression of aP2 was accompanied by
adipogenic differentiation. Therefore, this suggests that the high
expression level of aP2 in MCF-7 and the AC-12 d group may be
closely associated with cell growth, invasion and metastasis.
No significant differences in the PPARγ expression
level were detected between the hADSCs and AC-12 d groups, and no
expression was found in the AC-6 d group and MCF-7 cells. The
absence of PPARγ indicates that it may be associated with fatty
synthesis during adipogenic initiation and following adipogenic
differentiation, and may possibly act as a protection factor
resulting in cell maturation and differentiation.
ELISA measurements for selected
cytokines
To evaluate the paracrine effects on secreted
cytokines, the VEGF, MMP-2, MMP-9 and uPA levels were determined in
the differently conditioned media. As shown in Table III, the VEFG concentration in the
hADSC induction group was markedly lower than that in the control
and AC induction groups. However, no significant difference was
identified between the control and the AC induction groups. The
MMP-2 level in the control group was too low to be detected, and no
significant difference was observed between the MMP-2 levels in the
hADSC and AC induction groups. By contrast, the MMP-9 level in the
hADSC induction group was markedly higher than that of the AC
induction group and marginally higher than that of the control
group. The concentration of uPA in the hADSC induction group was
similar to that in the AC induction group and the two groups showed
a significantly higher concentration of uPA in comparison with the
control group. These results indicate that VEGF expression is
markedly inhibited by hADSCs, while the expression levels of MMP-2
and uPA are increased to a significant extent.
 | Table III.Concentrations of VEGF, MMP-2, MMP-9
and uPA under differently conditioned media by ELISA analyses. |
Table III.
Concentrations of VEGF, MMP-2, MMP-9
and uPA under differently conditioned media by ELISA analyses.
| Group | VEGF (pg/ml) | MMP-2 (pg/ml) | MMP-9 (pg/ml) | uPA (pg/ml) |
|---|
| hADSC-induced | 187.450±20.61a |
(4.77×104)±30a |
(1.930×103)±190.00 |
(4.80×103)±266.67a |
| AC-12d-induced | 278.970±56.89 |
(4.93×104)±22a |
(0.618×103)±156.00a |
(5.10×103)±91.30a |
| Control | 320.945±28.03 | 0 |
(1.370×103)±186.67 |
(1.70×104)±566.67 |
Discussion
In this study, the hADSCs of the third generation
showed significantly higher chemotaxis and invasive effects on
MCF-7 cells than the cells treated with adipogenic induction.
Breast cancer cells have been demonstrated to be closely associated
with the expression of uPA, MMP-2, MMP-9 and VEGF (17,18).
uPA enables extracellular matrix degradation by catalyzing the
metalloproteinase precursors to activate MMP-9 (19,20)
and enhancing endotheliocyte proliferation for blood vessel
formation. VEGF may also be activated by uPA, allowing the
infiltration and proliferation of cancer cells (21,22).
In the present study, ELISA demonstrated that the levels of uPA
were parallel with those of VEGF in breast cancer cells. The uPA
levels in the hADSC and AC induction groups were lower compared
with those of the control; however, the invasive ability of MCF-7
remained significantly increased under the same condition, which
may be explained by the functional overlap of uPA. Therefore, it
may be possible that the improvement of tumor invasion and
metastasis occurs during fat implantation. Notably, compared with
hADSC induction, the secreted VEGF level was higher in the
adipogenic induction, which may be due to vasoformation during
adipose tissue growth in the late adipogenesis stage.
As members of the metalloproteinase family, MMP-2
and -9 are associated with breast cancer invasion and metastasis,
as well as bone destruction (23,27). MMP-2 is associated with local
infiltration, while MMP-9 is the main participant in cancer
recurrence and metastasis (19,25).
The present study demonstrated that the levels of MMP-2 and MMP-9
in the hADSC and adipogenic induction groups were different from
each other. The higher expression of MMP-9 in the hADSCs suggests a
strong capability for matrix degradation. During this period, the
presence of hADSCs enables growth and expansion at an early stage
following fat implantation. Shortly after two weeks of adipogenic
induction, subsequent adipogenesis occurs, resulting in a reduction
of the level of MMP-9 and reduced expansion in the late stage
following fat implantation. Therefore, recurrence and metastasis
requires increased attention at the early stage, in which period
the effect of hADSCs on breast cancer cells mainly occurs.
Additionally, the increased level of MMP-2 in the hADSC and AC
induction groups, which is consistent with the results from the
transwell assay, provides further support for the theory that MMP-2
is closely associated with local infiltration.
The current study demonstrated that the level of
PPARγ was higher in the hADSCs and 12 days following adipogenic
differentiation, but that PPARγ was not expressed in MCF-7 cells or
6 days following adipogenic differentiation. This difference
indicates that PPARγ is mainly involved in the early and midterm
stages of the adipogenic differentiation of hADSCs, which is in
accordance with a previous study whereby PPARγ regulated
adipogenesis initiation (26).
However, the inhibitory effect of PPARγ on breast cancer cells was
weakened in the midterm stage. In the late-stage adipogenic
differentiation of hADSCs, the level of VEGF was increased along
with an increase in the level of PPARγ, which may be regulated by
other pathways involved in adipocyte growth and angiogenesis.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (grant no.
81071589).
References
|
1.
|
Bucky LP and Percec I: The science of
autologous fat grafting: views on current and future approaches to
neoadipogenesis. Aesthet Surg J. 28:313–321. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Hsu VM, Stransky CA, Bucky LP and Percec
I: Fat grafting’s past, present, and future: why adipose tissue is
emerging as a critical link to the advancement of regenerative
medicine. Aesthet Surg J. 32:892–899. 2012.
|
|
3.
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
4.
|
Trojahn Kølle SF, Oliveri RS, Glovinski
PV, Elberg JJ, Fischer-Nielsen A and Drzewiecki KT: Importance of
mesenchymal stem cells in autologous fat grafting: a systematic
review of existing studies. J Plast Surg Hand Surg. 46:59–68.
2012.PubMed/NCBI
|
|
5.
|
Philips BJ, Marra KG and Rubin JP: Adipose
stem cell-based soft tissue regeneration. Expert Opin Biol Ther.
12:155–163. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Watanabe M, Inukai K, Katagiri H, Awata T,
Oka Y and Katayama S: Regulation of PPAR gamma transcriptional
activity in 3T3-L1 adipocytes. Biochem Biophys Res Commun.
300:429–436. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Moldes M, Zuo Y, Morrison RF, et al:
Peroxisome-proliferator-activated receptor gamma suppresses
Wnt/beta-catenin signalling during adipogenesis. Biochem J.
376:607–613. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Wu Z, Rosen ED, Brun R, et al:
Cross-regulation of C/EBP alpha and PPAR gamma controls the
transcriptional pathway of adipogenesis and insulin sensitivity.
Mol Cell. 3:151–158. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Prusty D, Park BH, Davis KE and Farmer SR:
Activation of MEK/ERK signaling promotes adipogenesis by enhancing
peroxisome proliferator-activated receptor gamma (PPARgamma) and
C/EBPalpha gene expression during the differentiation of 3T3-L1
preadipocytes. J Biol Chem. 277:46226–46232. 2002. View Article : Google Scholar
|
|
10.
|
Crowe DL and Chandraratna RA: A retinoid X
receptor (RXR)-selective retinoid reveals that RXR-alpha is
potentially a therapeutic target in breast cancer cell lines, and
that it potentiates antiproliferative and apoptotic responses to
peroxisome proliferator-activated receptor ligands. Breast Cancer
Res. 6:R546–R555. 2004. View
Article : Google Scholar
|
|
11.
|
Yadav S, Anbalagan M, Shi Y, Wang F and
Wang H: Arsenic inhibits the adipogenic differentiation of
mesenchymal stem cells by down-regulating peroxisome
proliferator-activated receptor gamma and CCAAT enhancer-binding
proteins. Toxicol In Vitro. 27:211–219. 2013. View Article : Google Scholar
|
|
12.
|
Lo Furno D, Graziano AC, Caggia S, et al:
Decrease of apoptosis markers during adipogenic differentiation of
mesenchymal stem cells from human adipose tissue. Apoptosis.
18:578–588. 2013.PubMed/NCBI
|
|
13.
|
Dong YW, Wang XP and Wu K: Suppression of
pancreatic carcinoma growth by activating peroxisome
proliferator-activated receptor gamma involves angiogenesis
inhibition. World J Gastroenterol. 15:441–448. 2009. View Article : Google Scholar
|
|
14.
|
Fenner MH and Elstner E: Peroxisome
proliferator-activated receptor-gamma ligands for the treatment of
breast cancer. Expert Opin Investig Drugs. 14:557–568. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Pellikainen JM and Kosma VM: Activator
protein-2 in carcino-genesis with a special reference to breast
cancer - a mini review. Int J Cancer. 120:2061–2067. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Zuk PA, Zhu M, Mizuno H, et al:
Multilineage cells from human adipose tissue: implications for
cell-based therapies. Tissue Eng. 7:211–228. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Wieczorek E, Reszka E, Gromadzinska J and
Wasowicz W: Genetic polymorphism of matrix metalloproteinases in
breast cancer. Neoplasma. 59:237–247. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Velinov N, Poptodorov G, Gabrovski N and
Gabrovski S: The role of matrix metalloproteinases in the tumor
growth and metastasis. Khirurgiia (Sofiia). 1:44–49. 2010.(In
Bulgarian).
|
|
19.
|
Kunigal S, Lakka SS, Gondi CS, Estes N and
Rao JS: RNAi-mediated downregulation of urokinase plasminogen
activator receptor and matrix metalloprotease-9 in human breast
cancer cells results in decreased tumor invasion, angiogenesis and
growth. Int J Cancer. 121:2307–2316. 2007. View Article : Google Scholar
|
|
20.
|
Lakka SS, Gondi CS, Dinh DH, et al:
Specific interference of urokinase-type plasminogen activator
receptor and matrix metal-loproteinase-9 gene expression induced by
double-stranded RNA results in decreased invasion, tumor growth,
and angiogenesis in gliomas. J Biol Chem. 280:21882–21892. 2005.
View Article : Google Scholar
|
|
21.
|
Tang L and Han X: The urokinase
plasminogen activator system in breast cancer invasion and
metastasis. Biomed Pharmacother. 67:179–182. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Holst-Hansen C, Johannessen B,
Hoyer-Hansen G, Romer J, Ellis V and Brunner N: Urokinase-type
plasminogen activation in three human breast cancer cell lines
correlates with their in vitro invasiveness. Clin Exp Metastasis.
14:297–307. 1996.PubMed/NCBI
|
|
23.
|
Wang XF, Zhou QM, Du J, Zhang H, Lu YY and
Su SB: Baicalin suppresses migration, invasion and metastasis of
breast cancer via p38MAPK signaling pathway. Anticancer Agents Med
Chem. 13:923–931. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Jezierska A and Motyl T: Matrix
metalloproteinase-2 involvement in breast cancer progression: a
mini-review. Med Sci Monit. 15:RA32–RA40. 2009.PubMed/NCBI
|
|
25.
|
Figueira RC, Gomes LR, Neto JS, Silva FC,
Silva ID and Sogayar C: Correlation between MMPs and their
inhibitors in breast cancer tumor tissue specimens and in cell
lines with different metastatic potential. BMC Cancer. 9:202009.
View Article : Google Scholar
|
|
26.
|
Perera RJ, Marcusson EG, Koo S, et al:
Identification of novel PPARgamma target genes in primary human
adipocytes. Gene. 369:90–99. 2006. View Article : Google Scholar : PubMed/NCBI
|