Introduction
The iliopectineal bursa, which is a physiological
structure of the iliofemoral joint in humans, reportedly causes
pelvic or inguinal masses in rare cases (1). An enlargement of the iliopectineal
bursa is often associated with rheumatoid arthritis (RA),
osteoarthritis of the hip and pigmented villonodular synovitis.
RA is a chronic polyarthritis of unknown etiology,
affecting ∼1% of the population globally (2). The clinical features of RA typically
include polyarthritis with joint swelling of the hands and feet,
although any of the large joints, such as the hips, knees,
shoulders, elbows and ankles, may also become involved. Persistent
synovitis results in bone destruction and various deformities of
the joints (2). A variety of
disease-modifying antirheumatic drugs (DMARDs) are available for
the purpose of preventing joint destruction and improving the
quality of life of patients with RA. Among them, methotrexate (MTX)
has been the global standard DMARD, used either as a monotherapy or
in combination therapy (3). MTX
has been shown to improve the symptoms of RA and slow the
radiographic progression of joint destruction (4).
Tacrolimus, another DMARD, targets T cells and
causes the selective immunosuppression of T cells, the
tacrolimus/tacrolimus-binding protein complex further binds to
calcineurin to inhibit the translocation of cytoplasmic nuclear
factors into the nucleus, thereby inhibiting the expression of
cytokines, such as interleukin (IL)-2, IL-3, IL-4, interferon-γ and
tumor necrosis factor (TNF)-α (5,6). It
has been suggested that, in elderly patients with an insufficient
response to DMARD therapy, tacrolimus is safe and well-tolerated
and thus provides some clinical benefit (7). Another study indicated that
tacrolimus may be successfully used as part of combination RA
therapy with MTX (8). In addition,
for a patient with a history of RA and myelodysplastic syndrome
(MDS), combination therapy with tacrolimus and prednisolone
improved the pancytopenia and the polyarthritis (9). Recently, certain biological agents
have been shown to have significant efficacy in the treatment of RA
(10), and novel biological agents
continue to be developed.
Joint replacement is indicated when there is severe
joint damage and an unsatisfactory control of symptoms with
conservative treatment, such as medication or rehabilitation. The
long-term outcomes of joint replacement are good, with only 4 to
13% of large joint replacements requiring revision within 10 years
(11). The present study describes
a case of leg lymphedema due to iliopectineal bursitis associated
with RA, which was successfully controlled by surgical resection
and combination therapy with MTX and tacrolimus. An ethics
committee in Komaki City Hospital (Komaki, Japan) approved this
study. Informed consent was obtained from the patient.
Case report
A 68-year-old male with a six-year history of RA and
a 20-year history of MDS was treated at Komaki City Hospital. To
treat the patient’s MDS, metenolone was administered from 2005 to
2006, and for anemia, blood transfusions were performed as
required. For the treatment of the patient’s RA, the patient first
received bucillamine in 2006, prior to the bucillamine being
replaced by salazosulfapyridine in January 2008. In October 2008,
the patient complained of right hip joint pain following a fall. In
March 2009, the patient became aware of a right inguinal soft
tissue mass. The mass gradually increased in size and swelling was
present in the right lower extremity. At that time, the patient was
submitted to hospital with gradually increasing right hip pain and
leg edema.
Upon physical examination, the patient was measured
to be 150 cm tall and 44 kg in weight, with a body temperature of
36.6°C. The inguinal mass was easily palpable, but localized heat
was not apparent around the hip. The range of motion (ROM) of the
right hip was extremely limited. The ROM was 50° in flexion, 0° in
extension, 20° in abduction, 20° in adduction, 20° in external
rotation and 0° in internal rotation. The right leg of the patient
was shorter than the left by 2 cm, and a diffuse swelling of the
lower extremity was observed. A colorless transparent lymph fluid
leaked from the patient’s leg, and leg lymphedema was thus
diagnosed.
Hematological examination revealed a white blood
cell (WBC) count of 6,800/μl, a C-reactive protein (CRP)
level of 11.0 mg/dl, a matrix metalloproteinase-3 (MMP-3) level of
209 ng/ml and a rheumatoid factor (RF) level of 106 IU/ml (Table I).
 | Table I.Laboratory data. |
Table I.
Laboratory data.
| Parameters | At the time of
hospitalization | Eighteen months after
surgery | Normal range |
|---|
| Complete blood
counts | | | |
| WBC
(/μl) | 6800 | 6200 | 3500–9000 |
| Segs (%) | 85.0 | 91.0 | |
| Stabs (%) | 0.0 | 0.0 | |
| Lymphocytes
(%) | 11.0 | 7.0 | |
| Monocytes (%) | 3.0 | 2.0 | |
| Eosinophils
(%) | 0.0 | 0.0 | |
| Basophils (%) | 1.0 | 0.0 | |
| Blast (%) | 0.0 | 0.0 | |
| RBC
(/μl) |
410×104 |
394×104 |
410–530×104 |
| Hb (g/dl) | 13.2 | 13.1 | 12.4–17.2 |
| Hct (%) | 39.6 | 39.3 | 38.0–54.0 |
| Plt
(/μl) |
23.8×104 |
21.3×104 |
14.0–35.0×104 |
| Blood Chemistry | | | |
| Total protein
(g/dl) | 6.4 | 6.0 | 6.7–8.3 |
| Albumin (g/dl) | 3.3 | 3.7 | 4.0–5.0 |
| AST (IU/l) | 34.1 | 31.5 | 13.0–33.0 |
| ALT (IU/l) | 30.2 | 29.5 | 6.0–30.0 |
| Urinary nitrogen
(mg/dl) | 22.1 | 11.6 | 8.0–22.0 |
| Creatinine
(mg/dl) | 0.67 | 0.87 | 0.60–1.10 |
| Serum sodium
(mEq/l) | 139.0 | 140.6 | 138.0–146.0 |
| Serum potassium
(mEq/l) | 4.0 | 3.9 | 3.6–4.9 |
| Serum chloride
(mEq/l) | 104.0 | 107.0 | 99.0–109.0 |
| Immunology | | | |
| CRP (mg/dl) | 11.0 | 1.7 | 0.0–0.3 |
| RF (IU/ml) | 106.4 | 22.7 | 0.0–15.0 |
| MMP-3 (ng/ml) | 208.8 | 234.9 | 36.9–121.0 |
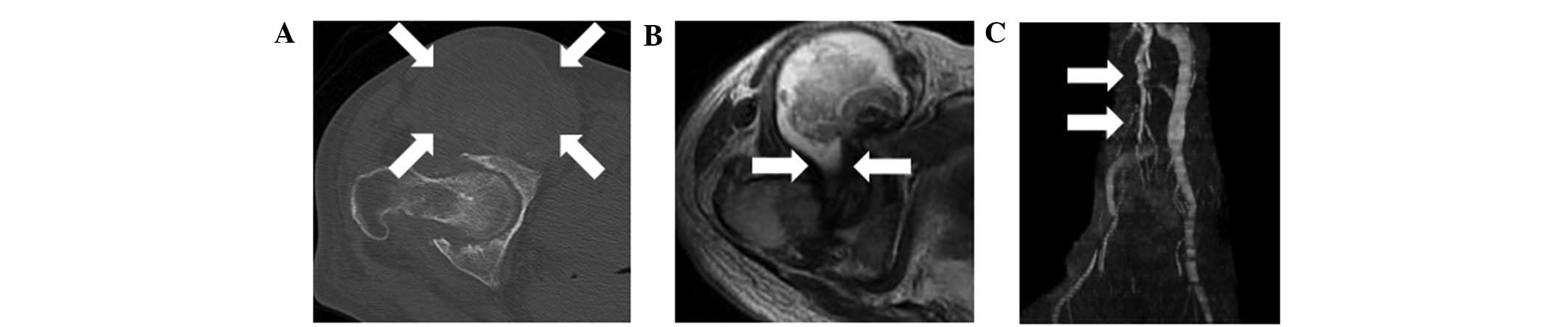
Plain radiographs showed destruction of the right
hip and collapse of the right femoral head. Computed tomography
(CT) showed joint space narrowing and an enlarged mass anterior to
the right hip joint (Fig. 1A).
Magnetic resonance imaging (MRI) showed an enlarged mass anterior
to the right hip joint. The mass displaced the iliopsoas muscle
laterally, and was shown to connect with the joint space of the
right hip (Fig. 1B). The signal
intensity of the lesion was abnormal on T1- and T2-weighted images.
An MRI venography showed that the femoral vein was displaced
medially by the mass (Fig. 1C).
Needle aspiration yielded 110 ml of black-brown fluid. The cytology
and culture results were negative. The diagnosis of iliopectineal
bursitis associated with destruction of a rheumatoid hip joint was
made on the basis of these findings, and surgery was thus
performed.
Surgical excision was carried out via an anterior
approach. The cystic black-brown fluid with the fibrinoid necrotic
tissue had erupted. The contents of the cyst were resected, and
partial bursal excision was performed. Following the partial bursal
excision, total hip arthroplasty (THA) was performed using the
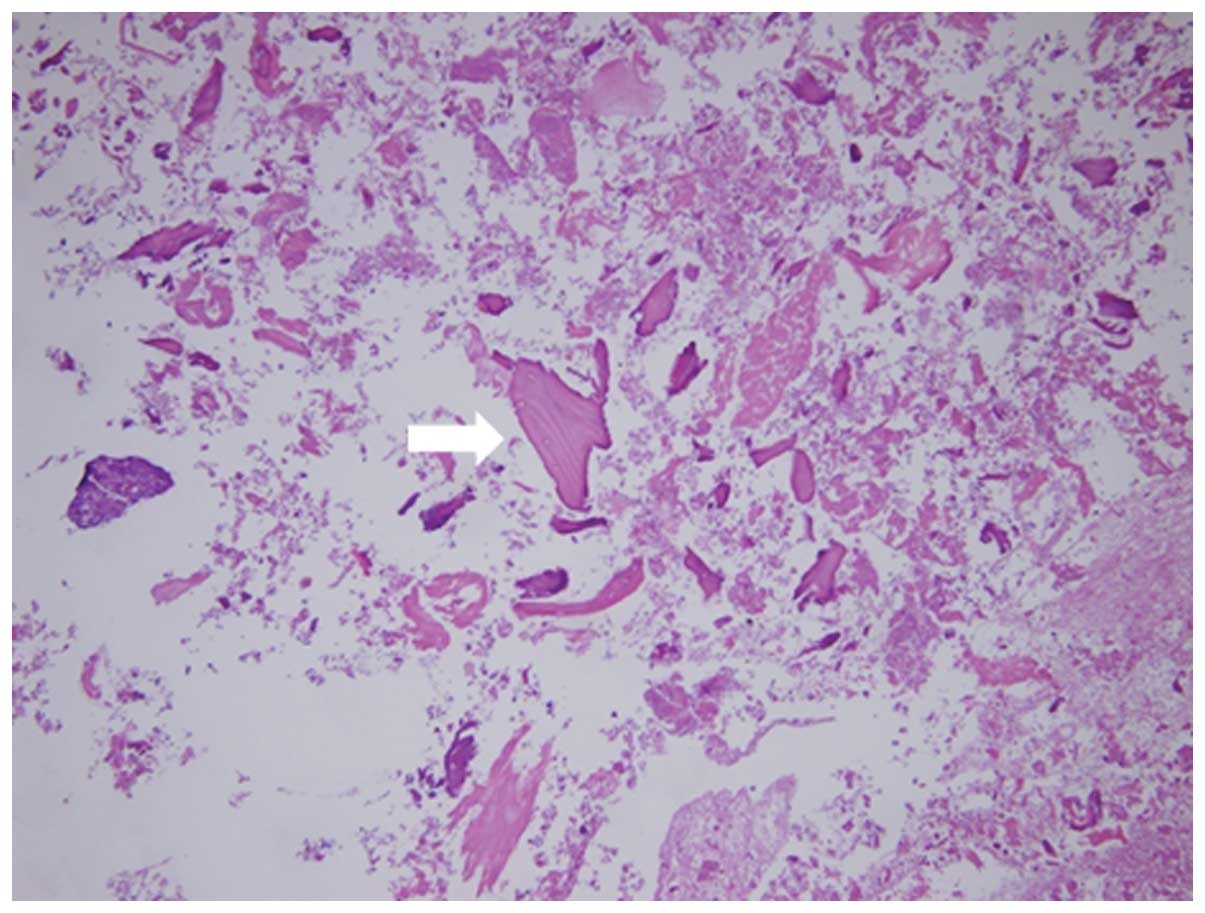
Hardinge approach. The contents of the bursa were stained with
hematoxylin and eosin. The uptake of bone cartilage debris and
fibrinoid necrosis deposition was apparent (Fig. 2). This content of the bursa has the
same structure as the synovial tissue of the hip joint.
THA was performed with a desirable surgical result,
and the RA disease activity was suppressed by the use of MTX at one
month subsequent to the surgery. MTX was initiated at a dose of 4
mg postoperatively, and the dose subsequently remained unchanged.
To control the RA more strictly, tacrolimus was added to the MTX
six months subsequent to the surgery. Tacrolimus was initiated at a
dose of 1.0 mg. The RA was well controlled, without any increases
in the levels of inflammatory markers, such as CRP and MMP-3, being
observed (Table I). The MDS
control did not change postoperatively. The patient’s leg
lymphedema disappeared rapidly following the surgery and the
iliopectineal bursa did not become re-enlarged. The patient was
able to walk normally without complaint one year subsequent to the
surgery.
Discussion
The iliopectineal bursa is the largest bursa in the
human body (1). It lies posterior
to the iliopsoas tendon, lateral to the femoral vessels and
overlies the hip joint capsule. The size of the bursa normally
ranges between 5 and 7 cm in length and 2 and 4 cm in width
(1,12). Communication existing between the
iliopectineal bursa and the hip joint has been demonstrated in ∼14%
of cadavers (13,14). In the present case, the size of the
bursa was 6 cm in length and 7 cm in width. An enlargement of the
iliopectineal bursa was first described in 1834 by Fricke (15). An enlargement of the iliopectineal
bursa is often associated with RA, osteoarthritis of the hip and
pigmented villonodular synovitis. In addition, iliopectineal
bursitis has been associated with acute destruction of the hip
joint and rapid resorption of the femoral head in patients with RA
(1,12,16,17,18).
Coventry et al discussed three possible
mechanisms responsible for the occurrence of synovial cysts in
patients with RA (19). Firstly,
the overproduction of synovial fluid in a rheumatoid joint may
increase the intra-articular pressure and distend the capsule in
the joint. A second theory is that the involvement of the
iliopectineal bursa in the rheumatoid process may lead to the
formation of excessive quantities of fluid, enlargement of the
bursa and hypertrophic and villous proliferation of the bursal
lining. The third theory is that necrosis of a subcutaneous
periarticular rheumatoid nodule may result in the formation of a
juxta-articular cyst simulating the appearance of a synovial
cyst.
In the present case, the overproduction of synovial
fluid in the arthritic joint may have led to increased
intra-articular pressure and protrusion of the synovial membranes
into the potential space of the iliopectineal bursa, via
communication between the bursa and the hip joint. The elevated
pressure, due to fluid overproduction in the bursa, may have
irritated the femoral vessels and exacerbated the leg lymphedema.
When the iliopectineal bursa is enlarged, it compresses adjacent
structures, such as the femoral vessels, the femoral nerve, the
urinary tract and the bladder, and may cause a variety of symptoms
(20). However, the leakage of a
colorless transparent lymph fluid from the leg and lymphedema of
the leg have, to the best of our knowledge, not been reported
previously as complications of iliopectineal bursitis.
Matsumoto et al reported that they had not
identified any incidences of iliopectineal bursitis recurring
following THA, regardless of whether the patient had previously
undergone a bursal excision (21).
In the present case, we performed a partial bursal excision, due to
the fact that the patient had a history of MDS, and extensive or
total resection of the bursa was considered to be too invasive. The
iliopectineal bursitis resolved following the THA, without complete
excision of the intrapelvic bursa. If the RA is well controlled
postoperatively, we propose that synovitis of the hip joint may be
prevented, and that the production of synovial fluid in the hip
joint, which is the likely cause of bursitis, may also be
suppressed. Moreover, since communication between the hip joint and
the bursa is one-way, due to a valve mechanism, we considered that
the synovial fluid in the hip joint was not likely to spread to the
bursa following THA.
In conclusion, we report the case of a 68-year-old
male with RA who developed leg lymphedema due to an enlarged
iliopectineal bursa associated with destruction of the hip joint.
The iliopectineal bursitis was resolved following THA without
complete excision of the intrapelvic bursa. The patient’s leg
lymphedema disappeared quickly, and the iliopectineal bursa has not
re-enlarged since the surgery. MTX and tacrolimus treatments were
initiated following the surgery to provide RA control. Therefore,
the present results strongly suggest that the iliopectineal
bursitis was resolved following THA, without complete excision of
the intrapelvic bursa, and that strict RA control led to a good
clinical course without recurrent inflammation of the bursa.
References
|
1.
|
Tokita A, Ikari K, Tsukahara S, et al:
Iliopsoas bursitis-associated femoral neuropathy exacerbated after
internal fixation of an intertrochanteric hip fracture in
rheumatoid arthritis: a case report. Mod Rheumatol. 18:394–398.
2008. View Article : Google Scholar
|
|
2.
|
Olsen NJ and Stein CM: New drugs for
rheumatoid arthritis. N Engl J Med. 350:2167–2179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Kremer JM: Rational use of new and
existing disease-modifying agents in rheumatoid arthritis. Ann
Intern Med. 134:695–706. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Weinblatt ME, Maier AL, Fraser PA and
Coblyn JS: Longterm prospective study of methotrexate in rheumatoid
arthritis: conclusion after 132 months of therapy. J Rheumatol.
25:238–242. 1998.PubMed/NCBI
|
|
5.
|
Kino T, Hatanaka H, Miyata S, et al:
FK-506, a novel immunosuppressant isolated from a
Streptomyces. II. Immunosuppressive effect of FK-506 in
vitro. J Antibiot. 40:1256–1265. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Kelly PA, Burckart GJ and Venkataramanan
R: Tacrolimus: a new immunosuppressive agent. Am J Health Syst
Pharm. 52:1569–1571. 1987.
|
|
7.
|
Kawai S and Yamamoto K: Safety of
tacrolimus, an immunosuppressive agent, in the treatment of
rheumatoid arthritis in elderly patients. Rheumatology (Oxford).
45:441–444. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Dutta S and Ahmad Y: The efficacy and
safety of tacrolimus in rheumatoid arthritis. Ther Adv
Musculoskelet Dis. 3:283–291. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Nozaki Y, Nagare Y, Kinoshita K, Urase F
and Funauchi M: Successful treatment using tacrolimus plus
corticosteroid in a patient with RA associated with MDS. Rheumatol
Int. 28:487–490. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Upchurch KS and Kay J: Evolution of
treatment for rheumatoid arthritis. Rheumatology (Oxford). 51(Suppl
6): vi28–vi36. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Wolfe F and Zwillich SH: The long-term
outcomes of rheu matoid arthritis: a 23-year prospective,
longitudinal study of total joint replacement and its predictors in
1,600 patients with rheumatoid arthritis. Arthritis Rheum.
41:1072–1082. 1988.PubMed/NCBI
|
|
12.
|
Tatsumura M, Mishima H, Shiina I, et al:
Femoral nerve palsy caused by a huge iliopectineal synovitis
extracting to the iliac fossa in a rheumatoid arthritis case. Mod
Rheumatol. 18:81–85. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Chandler SB: The iliopsoas bursa in man.
Anat Rec. 58:235–240. 1934. View Article : Google Scholar
|
|
14.
|
Yoshioka T, Tachihara A, Koyama T, et al:
Rapid destruction of the hip joint associated with enlarged
iliopsoas bursa in a patient with refractory rheumatoid arthritis.
J Nippon Med Sch. 75:233–238. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Fricke JI: Uber die bursa mucosa iliaca
und deren communikation mit dem hueftgelenke. J Chir
Augenheilkunde. 21:2231834.(In German).
|
|
16.
|
Yoshino K, Momohara S, Ikari K, et al:
Acute destruction of the hip joints and rapid resorption of femoral
head in patients with rheumatoid arthritis. Mod Rheumatol.
16:395–400. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Jones PB, Economou G, Adams J, et al:
Iliopsoas bursa presenting as deep vein thrombosis in rheumatoid
arthritis. British J Rheumatology. 32:832–834. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Kataoka M, Torisu T, Nakamura M and Uchida
K: Iliopsoas bursa of rheumatoid hip joint. A case report and
review of the literature. Clin Rheumatol. 14:358–364. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Coventry MB, Polley HF and Weiner AD:
Rheumatoid synovial cyst of hip; report of three cases. J Bone
Joint Surg Am. 41A:721–730. 1954.
|
|
20.
|
McLaughlin GE: Sudden death in rheumatoid
arthritis. J Clin Rheumatol. 8:208–211. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Matsumoto T, Juji T and Mori T: Enlarged
psoas muscle and iliopsoas bursitis associated with a rapidly
destructive hip in a patient with rheumatoid arthritis. Mod
Rheumatol. 16:52–54. 2006. View Article : Google Scholar : PubMed/NCBI
|