Introduction
Nasopharyngeal carcinoma (NPC) is a relatively
uncommon condition globally, with an incidence of less than 1 per
100,000 population (1). However,
the disease occurs with much greater frequency in southern China,
particularly in the province of Guangdong, where the incidence
rises to 20–30 per 100,000 (1).
Radiotherapy is the predominant treatment modality for this type of
cancer. With the development of radiation technology and
chemoradiotherapy, the 5-year overall and 5-year disease-free
survival rates of patients with NPC have been reported to be 74.5
and 76.7%, respectively (2).
Local-regional relapse and distant metastases remain the main
causes of treatment failure in patients with NPC (2–4).
These challenges make it necessary to explore new treatment
modalities for NPC.
Radiotherapy is the radical treatment for patients
with NPC. The use of chemotherapy drugs as radiotherapy sensitizers
has been studied extensively in patients with NPC, including the
use of fluorouracil (5-FU), cisplatin and taxanes (5,6).
However, these drugs are limited in their clinical use due to
severe acute toxicities, such as leukopenia and mucositis (5). In recent years, new molecular
targeted therapies, including epidermal growth factor receptor
(EGFR)-targeted therapy, have been widely recognized, and this
recognition has been accompanied by significant breakthroughs in
basic research and translational studies.
The EGFR is located primarily on cells of epithelial
origin and is a transmembrane glycoprotein that belongs to the
tyro-sine kinase factor family. The EGFR is overexpressed in the
majority of human carcinomas, including breast, non-small cell
lung, ovarian, bladder and head and neck cancer (7–10).
Our previous study demonstrated that the EGFR was expressed in all
patients with NPC, and it has been suggested that the
over-expression of EGFR in NPC is correlated with an aggressive
malignant progression and poor survival rates (11,12).
These observations make NPC an appealing type of tumor in which to
assay the effects of blocking the EGFR signaling pathway.
Tyrosine kinase inhibitors targeted against the
EGFR, which block tyrosine kinase phosphorylation, have been shown
to inhibit the EGFR-mediated proliferation of EGFR-rich cancer
cells. Erlotinib is a small, reversible tyrosine kinase inhibitor
that has been used in the treatment of several types of cancers.
Erlotinib was designed to bind to the ATP pocket of the
intracellular tyrosine kinase domain of the EGFR, inhibiting
phosphorylation and thereby blocking the initiation of the
intracellular cascade of transduction signals (13,14).
Erlotinib has been shown to induce apoptosis and inhibit growth in
several tumor cell lines in vitro, with the effects being
associated with the induction of p27kip1 expression and blockade in
the G1 phase of the cell cycle (13). In addition, erlotinib has been
demonstrated to exert a substantial effect on the tumor growth of
human HN5 xenografts in athymic mice and on pancreas-derived
xenografts; the inhibitory effect was identified to be correlated
with a reduction in the phosphorylation of
extracellular-signal-regulated kinase (ERK), but not of Akt
(14,15). In vitro, erlotinib has been
shown to inhibit the proliferation of numerous types of cancer
cells and enhance the antitumor effects of radiation (16).
The aim of this study was to investigate whether
erlotinib is able to enhance the radiosensitivity of NPC and to
explore its effects on tumor cell proliferation, apoptosis and the
cell cycle in NPC cell lines.
Materials and methods
Cell culture and reagents
Human NPC cell lines (CNE1 and CNE2) were cultured
in RPMI-1640 (Invitrogen Life Technologies, Carlsbad, CA, USA)
supplemented with 10% fetal bovine serum (Hyclone, Logan, UT, USA)
at 37°C in 5% CO2. Erlotinib was obtained from Roche
(Basel, Switzerland). The apoptosis detection and cell cycle kits
were purchased from Keygen Biotech Co., Ltd. (Nanjing, China). All
other reagents were obtained from Sigma (St. Louis, MO, USA).
Radiation technique
An X-radiometer was purchased from Rad Source
Technologies, Inc. (Suwanee, GA, USA). Deep X-ray irradiation, with
160 kV voltage, 25 mA current, 0.3 mm copper filter and a dose rate
of 623 cGy/min was performed. Six-well culture plates or 25 ml
culture flasks were arranged in the center position of the
apparatus.
MTS assay
Exponentially growing NPC cells were seeded into
96-well plates at a density of 2,000 cells/well, incubated
overnight at 37°C in 5% CO2 and treated with erlotinib
at different concentrations for 72 h. Following the addition of 20
μl of 5 mg/ml MTS to each well, the cells were incubated for
2 h at 37°C. The absorbance was read using a microplate reader
(BioTek Instruments, Inc., Winooski, VT, USA) at a wavelength of
490 nm. Each experiment was performed in triplicate. The data were
calculated as the mean values of three different experiments.
Radiation cell survival assay
Exponentially growing NPC cells were plated in
six-well plates, treated with 150 mmol erlotinib and incubated
overnight at 37°C. The cells were then irradiated using X-rays at a
dose rate of 623 cGy/min and were returned to the incubator for
colony formation. After treating with erlotinib for 72 h, the cells
were transferred to culture media without erlotinib. Following a
period of 10–14 days, the clones were fixed in −20°C ethanol and
stained with 1% crystal violet. Those clones that contained >50
cells were counted. Plating efficiency (PE) was calculated as the
fraction of colonies counted divided by the number of cells plated
without either erlotinib or ionizing radiation. The survival
fraction (SF) was then calculated as the average number of colonies
counted divided by the number of cells seeded multiplied by the PE.
Using Sigmaplot™ 10.0 software (Systat Software, Inc., Chicago, IL,
USA), the cell survival curves were fitted according to the
survival data using single hit multi-target (SHMT) radiobiological
models.
Apoptosis and cell cycle analysis
NPC cells were treated with radiation, erlotinib
(150 mmol/l) or the two in combination for different time periods.
The cells were harvested and washed with ice-cold
phosphate-buffered saline (PBS), fixed in 95% ethanol and stored at
4°C overnight. Following rehydration in PBS for 30 min at 4°C,
cells were treated with 1% RNAase for 30 min at 37°C and stained
with propidium iodide for 5 min. Cells were filtered through a
nylon mesh with a pore size of 95 μm and analyzed using a
flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA).
Animal experiments
Animal care and treatment was performed at the
Animal Center of Guangzhou Medical College (Guangzhou, China). A
total of 32 (16 males and 16 females) 6–7-week-old SCID mice were
used in the study. Briefly, exponentially growing CNE2 cells
(5×105) were injected subcutaneously (s.c.) into the
left hind flank of the mice on day 0. Eight days subsequent to the
inoculation, the tumors reached a volume of 100–200 mm3.
According to tumor volume, the animals were randomized into four
groups, erlotinib (1.6 mg/day) alone, radiation (8 Gy) alone and
erlotinib plus radiation. Erlotinib was administered by oral gavage
once daily from day 8 to day 22. Radiation treatment was delivered
once at a dose of 8 Gy using a custom lead block designed to expose
only the tumor bed to radiation. Calipers were used to measure the
length (L) and width (W) of the subcutaneous tumors. The tumor
volume (TV) was calculated as: TV = (L×W2)/2. Mice were
sacrificed one week subsequent to the end of the treatment and
excised tumors were fixed in paraffin for immunohistochemical
analysis. All animal studies were approved by the animal research
ethics committee of Guangzhou Medical College (Guangzhou,
China).
Statistical analysis
SPSS version 12.0 statistical software (SPSS Inc.,
Chicago, IL, USA) was used for statistical analysis. The data were
collected and calculated as the mean ± standard error (SE). Using
one-way analysis of variance, the differences in the effect of each
treatment alone and in combination were evaluated. P<0.05 was
considered to indicate a statistically significant difference.
Statistical significance was established by a post hoc least
significant difference (LSD) pairwise comparison.
Results
Erlotinib inhibits cell proliferation of
the NPC CNE2 cell line
The inhibition of NPC cell proliferation in the
presence of erlotinib is shown in Fig.
1. The proliferation of the CNE2 cell line was inhibited by
erlotinib but this was not concentration-dependent. However, the
inhibition was not particularly effective in CNE2 cells, with a
maximum inhibition rate of 9.74% at a concentration of 150 mmol.
Similarly, the proliferation of the CNE1 cells was not inhibited by
erlotinib.
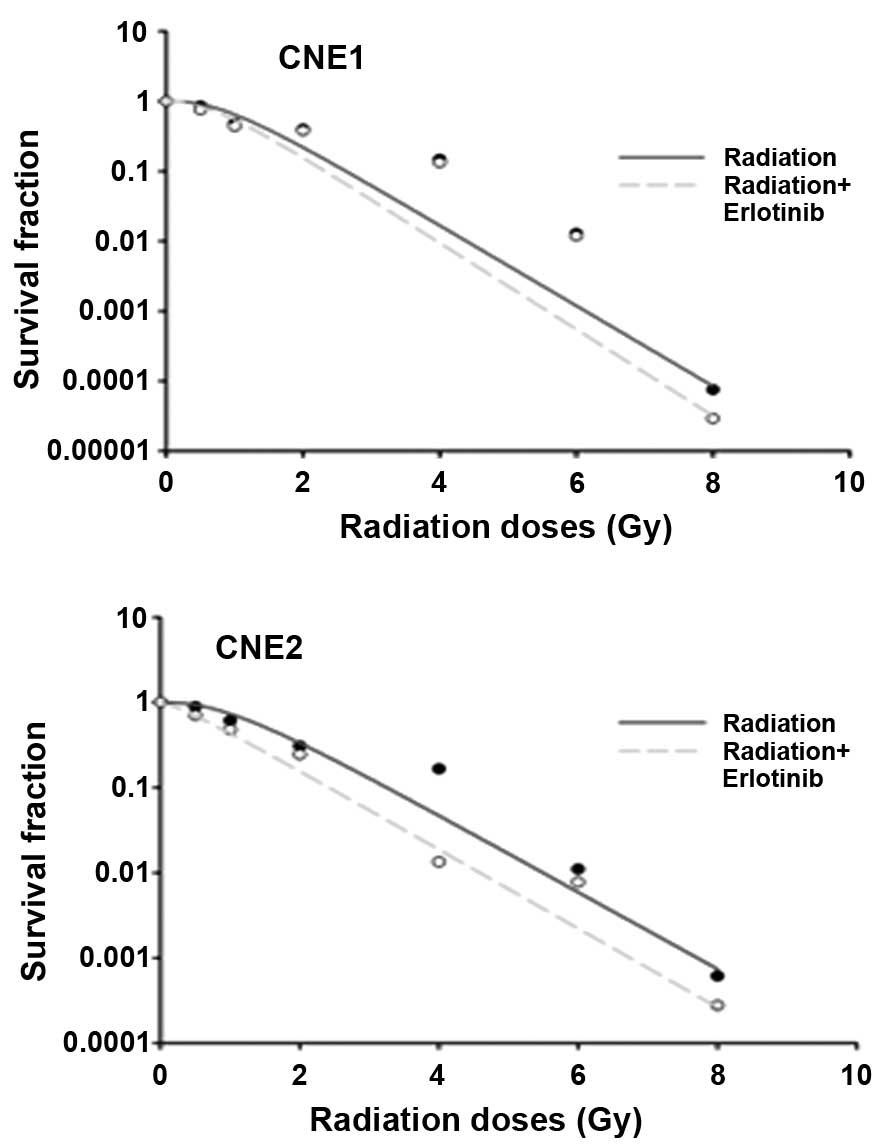
Erlotinib enhances radiosensitivity
To better understand the interaction of erlotinib
and radiation in combination, a gold standard assessment of
radiosensitivity was undertaken utilizing an in vitro colony
formation assay. Fig. 2 depicts
the radiation-survival curves for the two NPC cell lines, in which
cells were exposed to 150 mmol erlotinib following radiation
exposure at 0, 0.5, 1, 2, 4, 6 or 8 Gy. It was demonstrated that
the survival fractions at 2 Gy (SF2) were 30.21 and
15.48% in the CNE2 cells treated with radiation alone and with the
combination of erlotinib and radiation, respectively. Similarly,
the data demonstrated a reduction in SF2 of 6.43% (from
21.90 to 15.47%) in the CNE1 cells following exposure to erlotinib
and radiation. According to the single-hit multi-target model, this
indicated that erlotinib enhanced the radiosensitivity of NPC cells
(for the CNE1 and CNE2 cell lines), and the sensitization
enhancement ratios (SERs) were 1.076 and 1.109, respectively.
Erlotinib enhances radiation-induced
apoptosis
In order to examine whether erlotinib induced an
apoptotic response in NPC cells, NPC cells were exposed to
erlotinib for 24 and 48 h in the presence or absence of radiation
and flow cytometry using propidium iodide was performed to assess
apoptosis. The results demonstrated that apoptosis was not induced
in the CNE1 and CNE2 cells treated with erlotinib alone either for
24 or 48 h (Fig. 3). In addition,
the effect of erlotinib on radiation-induced apoptosis was
investigated. Statistically, the combined treatment of erlotinib
with radiation significantly enhanced apoptosis in the CNE2 cells
at 24 h (P=0.047). However, erlotinib combined with radiation did
not enhance apoptosis in the CNE1 cells (P>0.05).
Erlotinib induces G2/M cell cycle
arrest
The capacity of erlotinib to inhibit cell cycle
progression was evaluated using flow cytometric analyses (Fig. 4). Following exposure to erlotinib
for 24 or 48 h, the accumulation of cells in the G2/M phase was not
significantly different from the control in either the CNE1 or CNE2
cell lines. However, in the CNE2 cells treated with erlotinib for
48 h combined with radiation, the accumulation of cells in the G2/M
phase (83.53%) was significantly higher than that of CNE2 cells
treated with radiation alone (70.57%; P<0.05). Similarly,
treatment with erlotinib combined with radiation in the CNE1 cells
also led to a more marked G2/M phase arrest compared with treatment
with radiation alone (P<0.05).
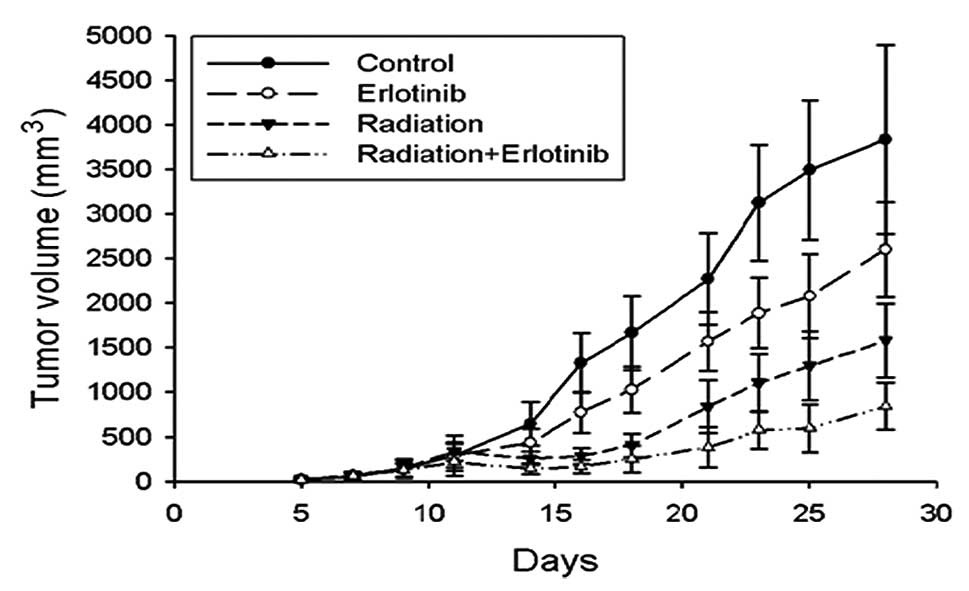
Erlotinib augments the in vivo tumor
response of NPC xenografts to radiation
Human NPC (CNE2) cells were injected s.c. into
athymic nude mice and allowed to grow for 8 days, prior to
randomization of the mice into four groups. Eight days was the time
interval required for the xenografts to reach 100∼200
mm3 in volume. As shown in Fig. 5, treatment with radiation alone or
erlotinib alone produced a modest inhibition of tumor growth in the
CNE2 xenografts. When combined with radiation, erlotinib enhanced
the tumor growth inhibition profile over the 28-day observation
period. Statistical analysis confirmed that the combination
treatment resulted in a synergistic inhibitory effect on tumor
growth in the CNE2 xenografts (P<0.05).
Discussion
EGFR is a transmembrane tyrosine kinase growth
factor receptor, whose molecular weight is 170 kD. It is divided
into an extracellular amino terminal, a transmembrane segment and
an intracellular carboxyl end. The intracellular region exhibits
tyrosine kinase activity. A variety of tumors overexpress EGFR; in
NPC tissue the expression rates have been shown to be 70.9–100%
(11,17). High expression levels of EGFR in
patients with NPC are correlated with a poor prognosis (17). Therefore, EGFR inhibitors may be of
significance in the treatment of NPC. Erlotinib is an oral EGFR
tyrosine kinase inhibitor and is currently one of the most
extensively studied molecularly targeted agents. A clinical trial
demonstrated that erlotinib enhanced the sensitivity to radiation
therapy and improved survival rates in head and neck squamous cell
carcinoma (18). A follow-up of
this study performed in 2010 also demonstrated prolonged survival
rates with minimal side effects (19). Several previous studies have
demonstrated that erlotinib helps disrupt cell cycle pathways, as
well as enhancing the sensitivity of cells to radiation (20). Tortora et al hypothesized
that radiation therapy may enhance the effectiveness of erlotinib
by creating a hypoxic environment at the tumor site (21).
The present study demonstrated that treatment of NPC
cells with erlotinib alone had no significant effect on tumor cell
proliferation. However, it was observed that erlotinib enhanced the
radiosensitivity of the NPC cell lines. The CNE1 and CNE2 cells
treated with erlotinib were shown to have SERs of 1.076 and 1.109,
respectively, which were significantly higher than those of the
cells treated with radiation therapy alone. One of the mechanisms
by which erlotinib enhances the radio-sensitivity of NPC may be the
induction of apoptosis of the tumor cells. Bai et al
indicated that erlotinib induced apoptosis of A549 cells, a lung
adenocarcinoma cell line, by regulating apoptosis-related genes
(23). To confirm this hypothesis,
we performed a cell cycle analysis of irradiated NPC cells that
were exposed to erlotinib. It was observed that erlotinib alone was
not able to induce apoptosis of tumor cells. However, the
combination therapy of NPC cells with erlotinib and radiation led
to CNE2 cell apoptosis (P=0.047). Based on in vitro studies
in other types of cancer, we hypothesized that erlotinib enhanced
radiation-induced cell cycle arrest in NPC cells (20). Earlier studies using lung cancer
cell lines demonstrated that erlotinib induced cell cycle arrest at
the G0/G1 phase (23,24). Erlotinib combined with radiotherapy
induced cycle cell arrest at the G1 and G2/M phase, with a marked
reduction in the S phase (24).
However, it was observed in the present study that erlotinib alone
had no significant effect on the cell cycle in NPC cells.
Interestingly, erlotinib combined with ionizing radiation induced a
significantly higher G2/M arrest in CNE1 and CNE2 cells compared
with radiation alone.
An earlier study using H226 and UM-SCC6 tumor
xenograft models demonstrated that erlotinib combined with RT
dramatically inhibited tumor growth (24). Sarkaria et al showed that
erlotinib and higher-dose radiation therapy resulted in an additive
antitumor effect in a xenograft model of glioblastoma multiforme
(25). In the present study a
similar effect was observed in an NPC xenograft model using
NOD-SCID mice. Erlotinib in combination with a single dose of
irradiation led to a significant reduction in tumor volume compared
with radiation alone.
In conclusion, the present study demonstrated that
the EGFR tyrosine kinase inhibitor, erlotinib, combined with
ionizing radiation induced cell cycle arrest at the G2/M phase and
reduced tumor volume in a xenograft model. These results suggested
that this may be a mechanism by which erlotinib enhances the
sensitivity to radiation therapy in NPC. Further studies are
required to elucidate other modes of action utilized by
erlotinib.
Acknowledgements
The authors would like to thank
professor Zhi-Ming He at The Tumor Hospital of Guangzhou Medical
College who provided the technical support in this study. The work
was supported by The Guangzhou Science and Technology Bureau (No.
2009Z1-E281) and the special fund of Tumor Tumor Hospital of
Guangzhou Medical College (2011-yz-09).
References
|
1.
|
Parkin DM, Whelan SL, Ferlay J, Raymond L
and Young J: Cancer Incidence in Five Continents. IARC Scientific
Publications. 143:814–815. 1997.
|
|
2.
|
Xiao WW, Huang SM, Han F, et al: Local
control, survival, and late toxicities of locally advanced
nasopharyngeal carcinoma treated by simultaneous modulated
accelerated radiotherapy combined with cisplatin concurrent
chemotherapy: long-term results of a phase 2 study. Cancer.
117:1874–1883. 2011. View Article : Google Scholar
|
|
3.
|
Ng WT, Lee MC, Hung WM, et al: Clinical
outcomes and patterns of failure after intensity-modulated
radiotherapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol
Phys. 79:420–428. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Song CH, Wu HG, Heo DS, Kim KH, Sung MW
and Park CI: Treatment outcomes for radiotherapy alone are
comparable with neoadjuvant chemotherapy followed by radiotherapy
in early-stage nasopharyngeal carcinoma. Laryngoscope. 118:663–670.
2008. View Article : Google Scholar
|
|
5.
|
Lee AW, Lau WH, Tung SY, Chua DT, Chappell
R, et al: Preliminary results of a randomized study on therapeutic
gain by concurrent chemotherapy for regionally-advanced
nasopharyngeal carcinoma: NPC-9901 Trial by the Hong Kong
Nasopharyngeal Cancer Study Group. J Clin Oncol. 23:6966–6975.
2005. View Article : Google Scholar
|
|
6.
|
Wee J, Tan EH, Tai BC, et al: Randomized
trial of radiotherapy versus concurrent chemoradiotherapy followed
by adjuvant chemotherapy in patients with American Joint Committee
on Cancer/International Union against cancer stage III and IV
nasopharyngeal cancer of the endemic variety. J Clin Oncol.
23:6730–6738. 2005. View Article : Google Scholar
|
|
7.
|
Herbst RS and Langer CJ: Epidermal growth
factor receptors as a target for cancer treatment: the emerging
role of IMC-C225 in the treatment of lung and head and neck
cancers. Semin Oncol. 29(Suppl 4): S27–S36. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Meche A, Cimpean AM and Raica M:
Immunohistochemical expression and significance of epidermal growth
factor receptor (EGFR) in breast cancer. Rom J Morphol Embryol.
50:217–221. 2009.PubMed/NCBI
|
|
9.
|
Hirsch FR, Varella-Garcia M and Cappuzzo
F: Predictive value of EGFR and HER2 overexpression in advanced
non-small-cell lung cancer. Oncogene. 28(Suppl 1): S32–S37. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Leong JL, Loh KS, Putti TC, Goh BC and Tan
LK: Epidermal growth factor receptor in undifferentiated carcinoma
of the nasopharynx. Laryngoscope. 114:153–157. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Yuan TZ, Li XX, Cao Y, Qian CN, Zeng MS
and Guo X: Correlation of epidermal growth factor receptor
activation to metastasis-free survival of nasopharyngeal carcinoma
patients. Ai Zheng. 27:449–454. 2008.(In Chinese).
|
|
12.
|
Yuan Y, Zhou X, Song J, et al: Expression
and clinical significance of epidermal growth factor receptor and
type 1 insulin-like growth factor receptor in nasopharyngeal
carcinoma. Ann Otol Rhinol Laryngol. 117:192–200. 2008.PubMed/NCBI
|
|
13.
|
Moyer JD, Barbacci EG, Iwata KK, et al:
Induction of apoptosis and cell cycle arrest by CP-358,774, an
inhibitor of epidermal growth factor receptor tyrosine kinase.
Cancer Res. 57:4838–4848. 1997.PubMed/NCBI
|
|
14.
|
Pollack VA, Savage DM, Baker DA, et al:
Inhibition of epidermal growth factor receptor-associated tyrosine
phosphorylation in human carcinomas with CP-358,774: dynamics of
receptor inhibition in situ and antitumor effects in athymic mice.
J Pharmacol Exp Ther. 291:739–748. 1999.
|
|
15.
|
Ng SS, Tsao MS, Nicklee T and Hedley DW:
Effects of the epidermal growth factor receptor inhibitor OSI-774,
Tarceva, on downstream signaling pathways and apoptosis in human
pancreatic adenocarcinoma. Mol Cancer Ther. 1:777–783.
2002.PubMed/NCBI
|
|
16.
|
Chinnaiyan P, Huang S, Vallabhaneni G, et
al: Mechanisms of enhanced radiation response following epidermal
growth factor receptor signaling inhibition by erlotinib (Tarceva).
Cancer Res. 65:3328–3335. 2005.
|
|
17.
|
Ma BB, Poon TC, To KF, et al: Prognostic
significance of tumor angiogenesis, Ki 67, p53 oncoprotein,
epidermal growth factor receptor and HER2 receptor protein
expression in undifferentiated nasopharyngeal carcinoma - a
prospective study. Head Neck. 25:864–872. 2003. View Article : Google Scholar
|
|
18.
|
Bonner JA, Harari PM, Giralt J, et al:
Radiotherapy plus cetuximab for squamous-cell carcinoma of the head
and neck. N Engl J Med. 354:567–578. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Bonner JA, Harari PM, Giralt J, et al:
Radiotherapy plus cetuximab for locoregionally advanced head and
neck cancer: 5-year survival data from a phase 3 randomized trial,
and relation between cetuximab induced rash and survival. Lancet
Oncol. 11:21–28. 2010.PubMed/NCBI
|
|
20.
|
Nyati MK, Morgan MA, Feng FY and Lawrence
TS: Integration of EGFR inhibitors with radiochemotherapy. Nat Rev
Cancer. 6:876–885. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Tortora G, Gelardi T, Ciardiello F and
Bianco R: The rationale for the combination of selective EGFR
inhibitors with cytotoxic drugs and radiotherapy. Int J Biol
Markers. 22(Suppl 4): S47–S52. 2007.PubMed/NCBI
|
|
22.
|
Bai XX, Mou XX, Jiang SJ, et al: Effects
of Erlotinib on apoptosis in human pulmonary adenocarcinoma.
Chinese Journal of Gerontology. 30:1073–1076. 2010.(In
Chinese).
|
|
23.
|
Xiong X, Liu H, Fu L, et al: Antitumor
activity of a new N-substituted thiourea derivative, an EGFR
signaling-targeted inhibitor against a panel of human lung cancer
cell lines. Chemotherapy. 54:463–474. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Huang S, Armstrong EA, Benavente S,
Chinnaiyan P and Harari PM: Dual-agent molecular targeting of the
epidermal growth factor receptor (EGFR): combining anti-EGFR
antibody with tyrosine kinase inhibitor. Cancer Res. 64:5355–5362.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Sarkaria JN, Carlson BL, Schroeder MA, et
al: Use of an orthotopic xenograft model for assessing the effect
of epidermal growth factor receptor amplification on glioblastoma
radiation response. Clin Cancer Res. 12:2264–2271. 2006. View Article : Google Scholar : PubMed/NCBI
|