Introduction
Multiple myeloma (MM) is a type of malignant plasma
cell proliferative disease (1). MM
was first reported in 1848 and is characterized by the
proliferation of malignant plasma cells and subsequent enrichment
of monoclonal paraproteins. In patients with MM, antibody-forming
cells, such as plasma cells, are malignant and may potentially
result in unusual manifestations. The proliferation of plasma cells
in patients with MM may affect the normal production of blood
cells, resulting in leukopenia, anemia and various diseases, as the
aberrant antibodies impair humoral immunity. The survival rate is
higher in young patients with MM and lower in elderly patients.
MM may cause diseases ranging from those that are
asymptomatic to those that are severely symptomatic with
complications requiring treatment (2). Although treatments may benefit
patients by resulting in a longer life, less pain and fewer
complications, currently no effective cure for MM exists. However,
advances in therapeutic treatments have helped to reduce the
occurrence and severity of the adverse effects of MM.
The complete remission (CR) rate of traditional
chemotherapy is <5% and the median survival time is 3 years
(3). Autologous hematopoietic stem
cell transplantation (ASCT) following high-dose chemotherapy
significantly improves the CR rate and extends the progression-free
survival (PFS) and overall survival (OS) times in patients with MM.
Therefore, it is the first-line standard treatment program for
patients <65 years old (4).
The clinical application of novel targeted agents,
including thalidomide, bortezomib and lenalidomide, may alter the
induction therapy and conditioning regimen prior to
transplantation, maintenance and consolidation therapies following
transplantation. Therefore, the present study reviewed the clinical
data of 27 patients with MM who had been treated by ASCT. Through
the comparison between patients with very good partial remission
(VGPR) following concurrent introduction therapy and patients with
VGPR following consolidation chemotherapy, the efficacy of ASCT
treatment in patients with MM, was examined. In addition, the
importance of combining ASCT and novel agents for the treatment of
MM was explored.
Materials and methods
Patient information
Twenty-seven patients with MM who were treated by
ASCT at The First Affiliated Hospital of Soochow University
(Suzhou, China) from May 2004 to August 2011 were studied. Of the
27 patients, two presented with POEMS syndrome. Twenty-eight
patients with MM, who had received induction chemotherapy at The
First Affiliated Hospital of Soochow University and achieved at
least a VGPR without ASCT treatment in the same period, served as
the control group. All patients met the diagnostic criteria of the
2012 NCCN guidelines (5). The
basic clinical patient information is shown in Table I and there were no significant
differences between the two treatment groups in terms of gender,
age, genotype and staging. The study was approved by the ethics
committee of Soochow University (Suzhou, China). Prior written and
informed consent was obtained from every patient.
 | Table I.Comparison of general clinical
information from the two groups of patients with MM. |
Table I.
Comparison of general clinical
information from the two groups of patients with MM.
| Groups | Cases | Age (years) | Gender (case) | Genotype (case) | ISS stage | DS stage |
|---|
|
|
|
|
|---|
| Male | Female | IgG | IgA | IgD | Light | I | II | III | I | II | III |
|---|
| Transplantation | 27 | 51 (36–64) | 18 | 9 | 13 | 12 | 1 | 1 | 2 | 13 | 12 | 2 | 2 | 23 |
|
Non-transplantation | 28 | 54 (43–69) | 15 | 13 | 17 | 5 | 1 | 5 | 1 | 12 | 15 | 2 | 5 | 21 |
Treatment plan
ASCT group
Induction chemotherapy
Eleven patients received traditional chemotherapy,
which consisted of vincristine + doxorubicin/liposomal doxorubicin
+ dexamethasone (VAD/DVD); vincristine, melphalan, cyclophosphamide
and prednisone (VMCP); and vincristine, BCNU, melphalan,
cyclophosphamide and prednisone (M2). The other 16 patients
received bortezomib + dexamethasone ± doxorubicin/liposomal
doxorubicin (VD/PAD) as chemotherapy. The median number of
chemotherapy treatments was four (range, 1–16). Following the
induction therapy, there were eight cases of CR, 12 cases of non-CR
(nCR), six cases of VGPR and one case of partial remission
(PR).
Mobilization chemotherapy
Fourteen patients received mobilization chemotherapy
and were treated with 3–4 g/m2 high-dose
cyclophosphamide (HDCTX) and 5–10 g/kg/day granulocyte
colony-stimulating factor (G-CSF). Moreover, 12 patients received
G-CSF only and one patient switched to plerixafor (AMD3100) with
G-CSF for one to three treatments following G-CSF mobilization
failure. Of these, one patient demonstrated a concentration of
CD34+ cells of <1.0×106/kg in the
peripheral blood stem cell collection and the autologous bone
marrow stem cells were collected. The collected median
CD34+ cell count was 2.89×106/kg
(range, 1.14−6.54×106/kg).
Conditioning regimen
Eleven patients were treated with a single high-dose
of melphalan (200 mg/m2) and 15 patients received a
single 200 mg/m2 dose of melphalan combined with 1.3
mg/m2 velcade (6 and 3 days before and 1 and 4 days
after).
Maintenance therapy
Nineteen patients received maintenance and
consolidation therapy following ASCT. Of these, 13 were treated
with 100–200 mg/day thalidomide, one received 25 mg/day
lenalidomide, one had 3 million units/day interferon, three
received treatment with 1.3 mg/m2 bortezomib (1, 4, 8
and 11 days following ACST) and one received progressive disease
(PD) chemotherapy. However, the PD patient was unstable before
ASCT. A further eight patients terminated their treatment due
thalidomide intolerance, which manifested as neurotoxicity.
Non-ASCT group
Induction chemotherapy
Ten patients received conventional chemotherapy and
18 patients were treated with bortezomib. The median number of
chemotherapy treatments was two (range, 1–10). Following the
induction therapy, there were nine cases of CR, 15 cases of nCR and
four cases of VGPR.
Consolidation chemotherapy
regimens
When all patients had received induction
chemotherapy and achieved at least VGPR, they continued to receive
consolidation chemotherapy, including 13 cases of conventional
chemotherapy and 15 of bortezomib treatment. The median number of
consolidation chemotherapy treatments was two (range, 1–8).
Maintenance chemotherapy
Following consolidation chemotherapy, 16 patients
received 100–200 mg/day thalidomide as the maintenance therapy.
Another 12 cases terminated their treatment, but once the disease
recurred, they received chemotherapy again. There were no
significant differences between the two groups of patients
regarding treatment efficacy, and induction and maintenance
therapy.
Follow-up and efficacy
All patients were followed up until October 30, 2011
to determine PFS and OS times. The PFS time was defined as the time
interval between transplantation and disease progression or
recurrence in the ASCT group, and the time from the first maximum
effect following induction chemotherapy in the non-transplant
group. The OS time was defined as the time interval from definitive
diagnosis to mortality or termination during follow-up. The
efficacy was defined as disclosed in the 2012 NCCN guidelines and
was divided into CR, nCR, VGPR, PR, no change (NC), plateau, PD and
CR recurrence (5). The total
therapy (TT) was defined as bortezomib-based induction therapy
prior to transplantation, two to four administrations of
consolidation therapy once remission was achieved, autologous
transplantation and administration of thalidomide as the
maintenance therapy following the transplantation.
Statistical analysis
SPSS statistical software, version 16.0 (SPSS, Inc.,
Chicago, IL, USA) was used for statistical analysis. Measurement
data was not normally distributed and presented as the median
compared with the Mann-Whitney U test. Categorical data were
compared with Fisher’s exact test and the survival curve was drawn
by the Kaplan-Meier method. The log-rank test was used for
univariate analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Impact of ASCT on treatment
efficacy
All patients demonstrated recovered hematopoietic
function and no transplant-related mortality occurred. Three months
following transplantation, 19 patients achieved CR, which included
nine cases of nCR and three of PR prior to transplantation.
Additionally, five cases reached nCR, two cases reached PR and one
had PD 3 months following transplantation. The efficacy in the 27
patients prior to transplantation was 92.6%, and the rates of CR,
CR + nCR and PR were 25.9, 70.4 and 22.2%, respectively. The
efficacy in the 27 patients 3 months after ACST, was 96.3%, and the
rates of CR, CR + nCR and PR were 70.4, 88.9 and 7.4%,
respectively. The CR incidence rate was significantly higher 3
months following ACST than that prior to transplantation
(P<0.01; Table II).
 | Table II.Treatment efficacy comparison before
and 3 months following transplantation (cases). |
Table II.
Treatment efficacy comparison before
and 3 months following transplantation (cases).
| Transplantation
groups | CR | nCR | PR | SD | PD | Relapse |
|---|
| Before | 7 | 12 | 6 | 1 | 0 | 1 |
| After | 19a | 5 | 2 | 0 | 1 | 0 |
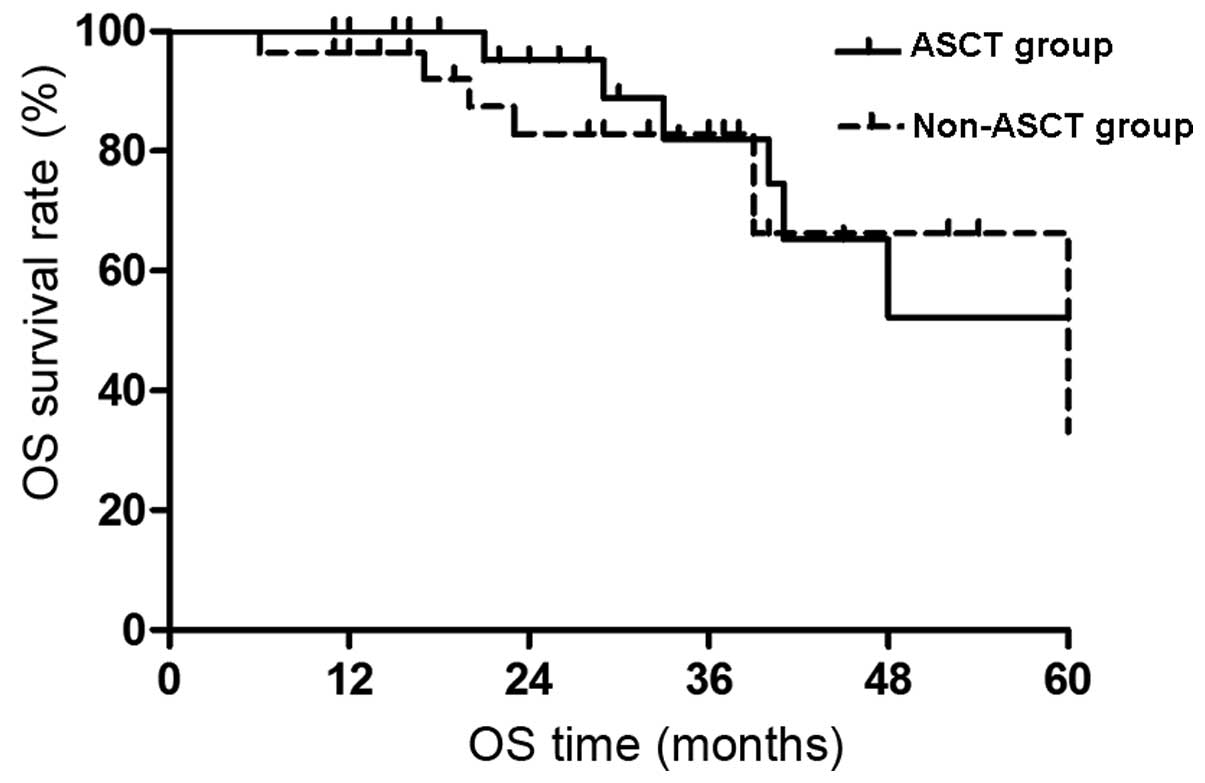
Impact of ASCT on OS
The median follow-up time for the 55 patients was 37
months (range, 11–99 months). In the ASCT group of 27 patients,
there were five mortalities due to recurrence and a further
mortality due to a severe lung infection 22 months following
transplantation. In the non-ASCT group, there were six mortalities
due to recurrence and one mortality from treatment-related causes
out of the 28 patients. The ASCT group did not reach the median OS
time at the final follow-up examination and the expected 5-year OS
rate was 52.2±15.7%. The median OS time of the non-ASCT group was
60 months and the expected 5-year OS rate was 33.1±24.2%. The
difference was not significant (P>0.05; Fig. 1).
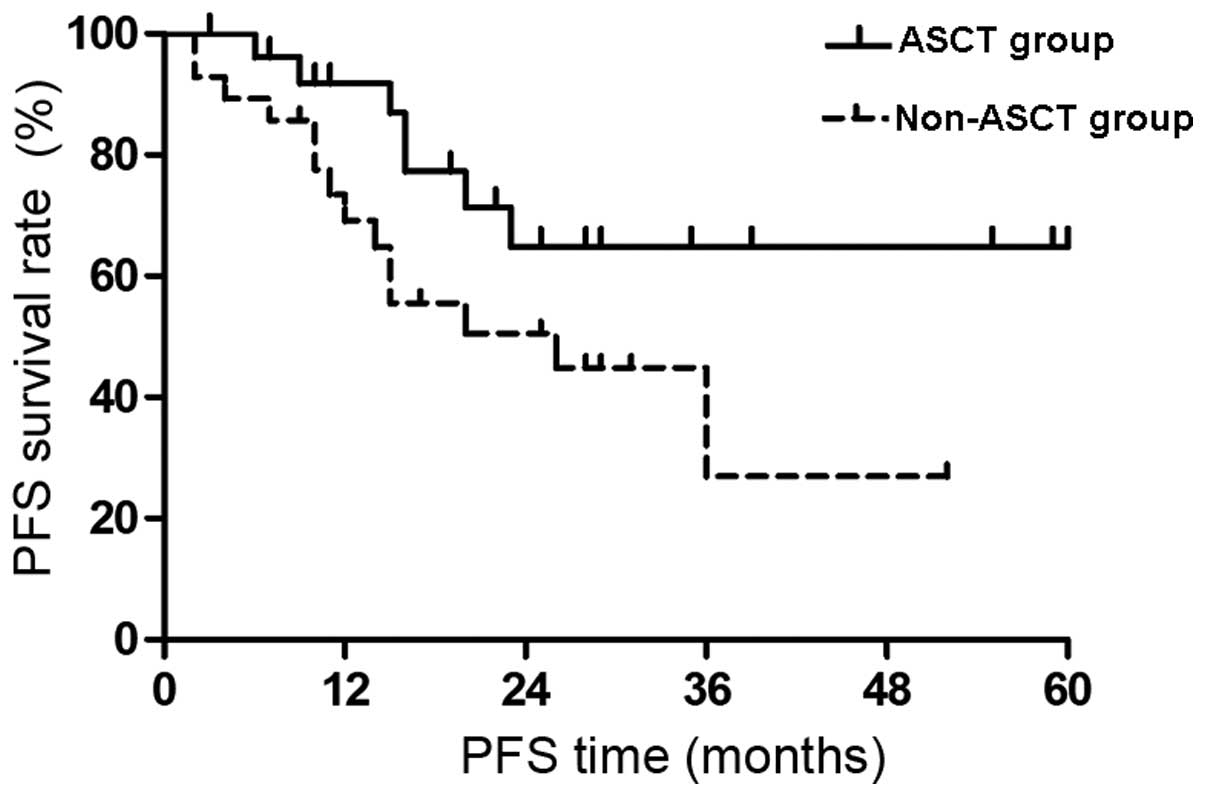
Impact of ASCT on PFS
The PFS survival curves of the two groups are shown
in Fig. 2. There was no median PFS
time in the ASCT group at the end of the follow-up period and the
expected 4-year PFS was 64.9±11.1%. The median PFS time in the
non-ASCT group was 20 months and the expected 4-year PFS was
26.9±11.7%. The PFS time was significantly extended in the ASCT
group compared with that of the non-ASCT group (P<0.05).
Prognostic factors of the ASCT
group
Univariate analysis of the prognostic factors for
PFS and OS in the ASCT group are shown in Table III. The prognostic factors of PFS
are as follows: i) PFS time was extended in patients receiving
maintenance therapy following transplantation (P=0.01); ii) PFS
time was significantly increased in patients with TT at the final
follow-up examination (P=0.01); and iii) PFS time was increased in
patients who were treated with bortezomib as the induction therapy,
but without statistical significance. The prognostic factors of OS
are as follows: i) OS time was extended in patients who received
maintenance therapy following transplantation (P=0.008); ii) all
patients undergoing TT survived; and iii) patients treated with
bortezomib in the conditioning regimen showed an extended OS time,
but without statistical significance. There were no clear
relationships between the clinical indicators, age, gender,
chromosome, disease type and staging with the PFS and OS times.
 | Table III.Univariate analysis of the
prognostic factors
for PFS and OS. |
Table III.
Univariate analysis of the
prognostic factors
for PFS and OS.
| Prognostic
factors | Cases | PFS | OS |
|---|
| Gender | | | |
| Male | 18 | | |
| Female | 9 | 0.566 | 0.320 |
| Age (years) | | | |
| ≥55 | 9 | | |
| <55 | 18 | 0.325 | 0.167 |
| Genotype | | | |
| IgA+IgD | 13 | | |
| Other | 14 | 0.885 | 0.183 |
| β2-MG (mg/l) | | | |
| ≥5.5 | 13 | | |
| <5.5 | 14 | 0.105 | 0.211 |
| Chromosome | | | |
| Abnormal | 5 | | |
| Normal | 22 | 0.801 | 0.652 |
| Induction
chemotherapy | | | |
| Traditional | 12 | | |
| Bortezomib | 15 | 0.626 | 0.524 |
| Before
transplantation | | | |
| Without CR | 20 | | |
| With CR | 7 | 0.476 | 0.460 |
| Refractory or
relapsed | | | |
| Yes | 7 | | |
| No | 20 | 0.174 | 0.138 |
| Time span of
transplantation and diagnosis (months) | | | |
| >6 | 11 | | |
| <6 | 16 | 0.563 | 0.743 |
| Conditioning
regimen | | | |
| HDM | 11 | | |
|
Bortezomib+HDM | 16 | 0.471 | 0.219 |
| CD34+
count (/kg) | | | |
|
<3×106 | 15 | | |
|
≥3×106 | 12 | 0.767 | 0.586 |
| Efficacy after
transplantation | | | |
| Without CR | 8 | | |
| With CR | 20 | 0.157 | 0.289 |
| Maintenance
treatment | | | |
| No | 8 | | |
| Yes | 19 | 0.010 | 0.008 |
| Overall
treatment | | | |
| No | 13 | | |
| Yes | 14 | 0.010 | 0.106 |
Analysis of the prognostic factors in all
patients
Univariate analysis of the prognostic factors of PFS
and OS for all patients is shown in Table IV. Prognostic factors for PFS are
as follows: i) PFS time was extended in patients <55 years old
(P=0.008); ii) PFS time was extended in patients who underwent ASCT
treatment (P=0.048); iii) PFS time was extended in patients
receiving maintenance treatment, but without statistical
significance; and iv) PFS time was increased in patients receiving
TT (P=0.01). Furthermore, the prognostic factors for OS are as
follows: i) OS time was extended in patients <55 years old
(P=0.025); ii) OS time was extended in patients receiving ASCT
treatment or TT, but without statistical significance; and iii) OS
time was increased in patients receiving maintenance treatment
(P=0.012).
 | Table IV.Univariate analysis of the prognostic
factors for PFS and OS. |
Table IV.
Univariate analysis of the prognostic
factors for PFS and OS.
| Prognostic
factors | Cases | PFS | OS |
|---|
| Gender | | | |
| Male | 33 | | |
| Female | 22 | 0.293 | 0.613 |
| Age (years) | | | |
| ≥55 | 31 | | |
| <55 | 24 | 0.008 | 0.025 |
| Genotype | | | |
| IgA+IgD | 19 | | |
| Other | 36 | 0.250 | 0.574 |
| Bone destruction
(sites) | | | |
| ≥3 | 30 | | |
| <3 | 25 | 0.180 | 0.804 |
| Hb (g/l) | | | |
| ≤85 | 30 | | |
| >85 | 25 | 0.215 | 0.354 |
| ALB (g/l) | | | |
| ≤30 | 24 | | |
| >30 | 31 | 0.058 | 0.345 |
| Cr
(μmol/l) | | | |
| ≥177 | 12 | | |
| <177 | 43 | 0.366 | 0.702 |
| Ca (mmol/l) | | | |
| ≥2.17 | 30 | | |
| <2.17 | 25 | 0.534 | 0.675 |
| LDH (U/l) | | | |
| ≥200 | 28 | | |
| <200 | 27 | 0.434 | 0.435 |
| β2-MG (mg/l) | | | |
| ≥5 | 30 | | |
| <5 | 25 | 0.116 | 0.167 |
| ISS stage | | | |
| III | 27 | | |
| I+II | 28 | 0.121 | 0.247 |
| DS stage | | | |
| III | 44 | | |
| I+III | 11 | 0.437 | 0.294 |
| Proportion of
plasma cells (%) | | | |
| ≥30 | 28 | | |
| <30 | 27 | 0.797 | 0.942 |
| Chromosome | | | |
| Abnormal | 8 | | |
| Normal | 47 | 0.318 | 0.736 |
| Induction
chemotherapy | | | |
| Traditional | 21 | | |
| Bortezomib | 34 | 0.136 | 0.578 |
| Efficacy of
induction chemotherapy | | | |
| Without CR | 37 | | |
| With CR | 18 | 0.087 | 0.262 |
| ASCT | 27 | | |
| Efficacy of
induction chemotherapy | | | |
| Non-ASCT | 28 | 0.048 | 0.373 |
| Maintenance
treatment | | | |
| No | 20 | | |
| Yes | 35 | 0.143 | 0.012 |
| Overall
treatment | | | |
| No | 41 | | |
| Yes | 14 | 0.010 | 0.106 |
Discussion
MM accounts for 10% of malignant blood diseases and
there has been significant progress in its treatment in recent
years. High-dose chemotherapy with ASCT and novel targeted agents,
such as thalidomide, bortezomib and lenalidomide, have been
clinically applied, resulting in a significantly increased median
survival time, particularly in young patients (6). However, thus far, MM remains
incurable, the recurrence rate is high and it is difficult to
obtain long-term survival. The present study examined the effects
of ACST with novel targeted agents in patients with MM.
This study retrospectively analyzed the clinical
data of 27 patients with ASCT from The First Affiliated Hospital of
Soochow University. The majority of patients showed sensitivity to
induction chemotherapy. The total effective rate was 92.6% and the
CR rate was 25.9% prior to transplantation. Following
transplantation, the efficacy was 96.3% and the CR rate increased
to 70.4%. Therefore, ASCT further improved the response rate and
remission quality, which is consistent with a previous study
(7). The impact of ASCT on the
prognosis of patients who achieved at least VGPR following
induction chemotherapy, excluding the efficacy differences, showed
that ASCT significantly extended the PFS time (P<0.05) and
increased the median OS time (P>0.05). This may be due to the
relatively short-term follow-up period (median, 37 months) and so
the survival benefit may not have been presented. Barlogie et
al were the first to demonstrate that ASCT with thalidomide
increased the CR rate and PFS time, without increasing the OS time.
When the median follow-up time reached 72 months, there was a
correlation between CR rate and OS time, which was particularly
significant in patients with cytogenetic abnormalities (8,9).
Although there was no significant correlation between ASCT and OS
time, the extension of PFS time is significantly important in
improving patient quality of life (10).
A meta-analysis identified that in patients with MM
treated by ASCT, the maximum efficacy of induction chemo-therapy
was positively correlated with PFS and OS times (11). Harousseau et al compared the
PD plan (bortezomib and dexamethasone) with that of VAD
(vincristine, doxorubicin and dexamethasone) with regard to the
efficacy and safety of induction therapy prior to transplantation.
It was determined that before and after transplantation, the CR +
nCR rate in the PD group was significantly increased compared with
that of the VAD group (12).
Various studies have also shown that induction therapy of novel
agents, particularly a triple-drug combination, significantly
improved the CR rate in patients with MM before and after
transplantation (13–15). Furthermore, a phase II clinical
study by Intergroupe Francophone du Myelome identified that the
conditioning therapy of bortezomib in combination with high-dose
melphalan, may further improve the CR rate following ASCT (16). In addition, studies have shown that
for untreated or relapsed patients with MM, the maximum efficacy
following treatment is the critical prognostic factor. A
significant correlation has been identified between CR rate and PFS
time, and the OS time has been observed to be significantly
increased in patients with CR (17–19).
In the present study, there was no improvement in the prognosis of
patients who had received induction therapy and conditioning
regimen with bortezomib, which may have been due to the short-term
follow-up period. However, different results were observed in
patients receiving TT. The TT plan is an integrated treatment that
includes conditioning, transplantation, consolidation and
maintenance therapies. TT1–TT3 data has demonstrated that as the
intensity of the treatment increases, particularly in consolidation
and maintenance therapies, the CR and OS rates significantly
improve (20). Furthermore, in the
present study, factors affecting the survival rate of patients with
MM following hematopoietic stem cell transplantation were analyzed.
No progress was identified in the PFS time in patients who received
bortezomib; however, there was a positive correlation between PFS
and OS times. This demonstrates the survival advantages of
bortezomib and requires further study.
As there were no plateaus in the PFS and OS curves
in patients with MM following transplantation, the residual myeloma
cells will eventually result in relapse. Therefore, consolidation
and maintenance therapies are recommended in order to prolong the
release time. Thalidomide, used as maintenance therapy, aids in the
inhibition of residual myeloma cells, thus improving the CR rate
and extending the PFS and OS times (8,21,22).
The present study analyzed the factors affecting survival and
identified that the OS time in patients receiving maintenance
therapy was significantly prolonged. In addition, PFS time was
significantly increased in patients who had received transplant
consolidation or maintenance therapy. Therefore, it is currently
considered that combination chemotherapy using three types of novel
agents, high-dose melphalan combined with intensive ASCT treatment,
with similar induction therapy as consolidation chemotherapy and
subsequent immunomodulatory agents as maintenance therapy, may be
the optimal strategy for treating MM (23).
In conclusion, the present study indicated that ASCT
improves the CR rate, PFS time, quality of life and potentially
increases the OS rate following induction chemotherapy, in patients
with MM. Novel therapeutic agents, such as bortezomib, further
optimized ASCT treatment in induction and consolidation or
maintenance therapies, subsequently benefiting patients with
MM.
Acknowledgements
This study was supported by the Talent
Foundation of Health Department of Jiangsu Province (grant no.
RC2007074) and the Technology Development Plan of Suzhou City
(grant no. YJS0914).
References
|
1.
|
Kyle RA and Rajkumar SV: Multiple myeloma.
New Engl J Med. 351:1860–1873. 2004. View Article : Google Scholar
|
|
2.
|
Talamo G, Farooq U, Zangari M, et al:
Beyond the CRAB symptoms: a study of presenting clinical
manifestations of multiple myeloma. Clin Lymphoma Myeloma Leuk.
10:464–468. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Myeloma Trialists’ Collaborative group:
Combination chemotherapy versus melphalan plus prednisone as
treatment for multiple myeloma: An overview of 6,633 patients from
27 randomized trials. J Clin Oncol. 16:3832–3842. 1998.PubMed/NCBI
|
|
4.
|
Bladé J, Rosiñol L, Cibeira MT, Rovira M
and Carreras E: Hematopoietic stem cell transplantation for
multiple myeloma beyond 2010. Blood. 115:3655–3663. 2010.PubMed/NCBI
|
|
5.
|
Anderson KC, Alsina M, Bensinger W, et al:
Multiple myeloma. J Natl Compr Canc Netw. 9:1146–1183. 2011.
|
|
6.
|
Brenner H, Gondos A and Pulte D: Recent
major improvement in long-term survival of younger patients with
multiple myeloma. Blood. 111:2521–2526. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Lahuerta JJ, Mateos MV, Martínez-López J,
et al: Influence of pre- and post-transplantation responses on
outcome of patients with multiple myeloma: sequential improvement
of response and achievement of complete response are associated
with longer survival. J Clin Oncol. 26:5775–5782. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Barlogie B, Tricot G, Anaissie E, et al:
Thalidomide and hematopoietic-cell transplantation for multiple
myeloma. N Engl J Med. 354:1021–1030. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Barlogie B, Pineda-Roman M, van Rhee F, et
al: Thalidomide arm of Total Therapy 2 improves complete remission
duration and survival in myeloma patients with metaphase
cytogenetic abnormalities. Blood. 112:3115–3121. 2008. View Article : Google Scholar
|
|
10.
|
Harousseau JL and Moreau P: Autologous
hematopoietic stem-cell transplantation for multiple myeloma. N
Engl J Med. 360:2645–2654. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
van de Velde HJ, Liu X, Chen G, Cakana A,
Deraedt W and Bayssas M: Complete response correlates with
long-term survival and progression-free survival in high-dose
therapy in multiple myeloma. Haematologica. 92:1399–1406.
2007.PubMed/NCBI
|
|
12.
|
Harousseau JL, Attal M, Avet-Loiseau H, et
al: Bortezomib plus dexamethasone is superior to vincristine plus
doxorubicin plus dexamethasone as induction treatment prior to
autologous stem-cell transplantation in newly diagnosed multiple
myeloma: Results of the IFM 2005-01 phase III trial. J Clin Oncol.
28:4621–4629. 2010. View Article : Google Scholar
|
|
13.
|
Cavo M, Zamagni E, Tosi P, et al:
Superiority of thalidomide and dexamethasone over
vincristine-doxorubicindexamethasone (VAD) as primary therapy in
preparation for autologous transplantation for multiple myeloma.
Blood. 106:35–39. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Moreau P, Facon T, Attal M, et al:
Comparison of reduced-dose bortezomib plus thalidomide plus
dexamethasone (vTD) to bortezomib plus dexamethasone (VD) as
induction treatment prior to ASCT in de novo multiple myeloma (MM):
Results of IFM2007-02 study. J Clin Oncol. 28(Suppl 15):
A80142010.
|
|
15.
|
Richardson PG, Weller E, Lonial S, et al:
Lenalidomide, bortezomib, and dexamethasone combination therapy in
patients with newly diagnosed multiple myeloma. Blood. 116:679–686.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Roussel M, Moreau P, Huynh A, et al:
Bortezomib and high-dose melphalan as conditioning regimen before
autologous stem cell transplantation in patients with de novo
multiple myeloma: A phase 2 study of the Intergroupe Francophone du
Myelome (IFM). Blood. 115:32–37. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Harousseau JL, Attal M and Avet-Loiseau H:
The role of complete response in multiple myeloma. Blood.
114:3139–3146. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Niesvizky R, Richardson PG, Rajkumar SV,
et al: The relationship between quality of response and clinical
benefit for patients treated on the bortezomib arm of the
international, randomized, phase 3 APEX trial in relapsed multiple
myeloma. Br J Haematol. 143:46–53. 2008. View Article : Google Scholar
|
|
19.
|
Harousseau JL, Weber D, Dimopoulos M, et
al: Relapsed /refractory multiple myeloma treated with
lenalidomide/dexamethasone who achieve a complete or near complete
response have longer overall survival and time to progression
compared with patients achieving a partial response. Blood.
110:35982007.
|
|
20.
|
Pineda-Roman M, Zangari M, Haessler J, et
al: Sustained complete remissions in multiple myeloma linked to
bortezomib in total therapy 3: comparison with total therapy 2. Br
J Haematol. 140:625–634. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Spencer A, Prince HM, Roberts AW, et al:
Consolidation therapy with low-dose thalidomide and prednisolone
prolongs the survival of multiple myeloma patients undergoing a
single autologous stem-cell transplantation procedure. J Clin
Oncol. 27:1788–1793. 2009. View Article : Google Scholar
|
|
22.
|
Attal M, Harousseau JL, Leyvraz S, et al:
Maintenance therapy with thalidomide improves survival in patients
with multiple myeloma. Blood. 108:3289–3294. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Moreau P, Avet-Loiseau H, Harousseau JL
and Attal M: Current trends in autologous stem-cell transplantation
for myeloma in the era of novel therapies. J Clin Oncol.
29:1898–1906. 2011. View Article : Google Scholar : PubMed/NCBI
|