Introduction
Brown-Séquard syndrome (BSS) is a syndrome
consisting of ipsilateral upper motor neuron paralysis (hemiplegia)
and loss of proprioception with contralateral pain and temperature
sensation deficit. BSS is usually observed in association with
traumatic spinal cord injuries, extramedullary spinal cord tumors,
spinal hemorrhages, degenerative disease and infectious and
inflammatory causes, including multiple sclerosis (1). There have also been a few reports of
BSS associated with intradural spinal cord herniation or disc
herniation (2,3). Furthermore, complete BSS due to
chronic compression is rare and most patients present with an
incomplete form of this condition (4).
In the present study, a 3-dimensional finite element
method (3D-FEM) was used to analyze the stress distribution of the
spinal cord under various compression levels corresponding to five
different lengths of the transverse diameter. Three levels of
static compression corresponding to 10, 20 and 30% of the
anteroposterior (AP) diameter were used for each of these five
conditions. This model was used to investigate why the number of
cases of complete BSS resulting from chronic compression is
limited.
Materials and methods
Construction of the 3D-FEM spinal cord
model
The Abaqus 6.11 (Dassault Systèmes Simulia Corp.,
Providence, RI, USA) standard finite element package was used for
FEM simulation. The 3D-FEM spinal cord model used in this study
consisted of gray and white matter and pia mater. In order to
simplify calculation in the model, the denticulate ligament, dura
and nerve root sheaths were not included. Pia mater was included
since it has been demonstrated that spinal cord with and without
this component shows significantly different mechanical behaviors
(5). The spinal cord was assumed
to be symmetrical about the mid-sagittal plane, so that only half
the spinal cord required reconstruction and the whole model could
be integrated by mirror image. This model simulated chronic
compression of the cervical spinal cord. The vertebral canal model
consisting of lamina was established by measuring 13 cervical
computed tomographic myelographs (Fig.
1). A rigid flat plate was used as a compression factor from
the anterior surface of the spinal cord and its width was 25, 37.5,
50, 62.5 and 75% of the length of the transverse diameter of the
spinal cord (Fig. 2). The rigid
flat plate was located at the longitudinal center of the spinal
cord. The spinal cord consists of three distinct materials: the
white and gray matter and the pia mater. The mechanical properties
(Young’s modulus and Poisson’s ratio) of the gray and white matter
were determined using data obtained from the tensile stress strain
curve and stress relaxation tests under various strain rates
(6,7). The mechanical properties of the pia
mater were obtained from a previous study (8). The mechanical properties of the
lamina and flat plate were stiff enough to enable the spinal cord
to be pressed. Based on the assumption that no slippage occurs at
the interfaces of white and gray matter and pia mater, these
interfaces were glued together. Data concerning the friction
coefficient between the bone and spinal cord were not available.
The coefficient of friction between lamina and the spinal cord was
‘glue’ at the contact line and ‘frictionless’ next to the part in
contact. Similarly, the coefficient of friction between the rigid
flat plate and spinal cord was ‘glue’ at the contact interfaces and
‘frictionless’ next to the part in contact. To simulate the axial
injury model of the spinal cord, nodes at the bottom and top of the
spinal cord model were constrained in all directions. The spinal
cord and the rigid flat plate were symmetrically meshed with
20-node elements. With a FEM model of 50% of the length of the
transverse diameter of the spinal cord, the total number of
isoparametric 20-node elements was 12,535 and the total number of
nodes was 76,993.
Static compression model
For the simulation of BSS, anterior static
compression was applied to the spinal cord by the rigid flat plate
at 25, 37.5, 50, 62.5 and 75% of the length of the transverse
diameter of the spinal cord. The degrees of compression were 10, 20
and 30% of the AP diameter of the spinal cord. A 10% compression
was first applied to the spinal cord, followed by compressions of
20 and 30%. In total, 15 different compression combinations were
evaluated.
Results
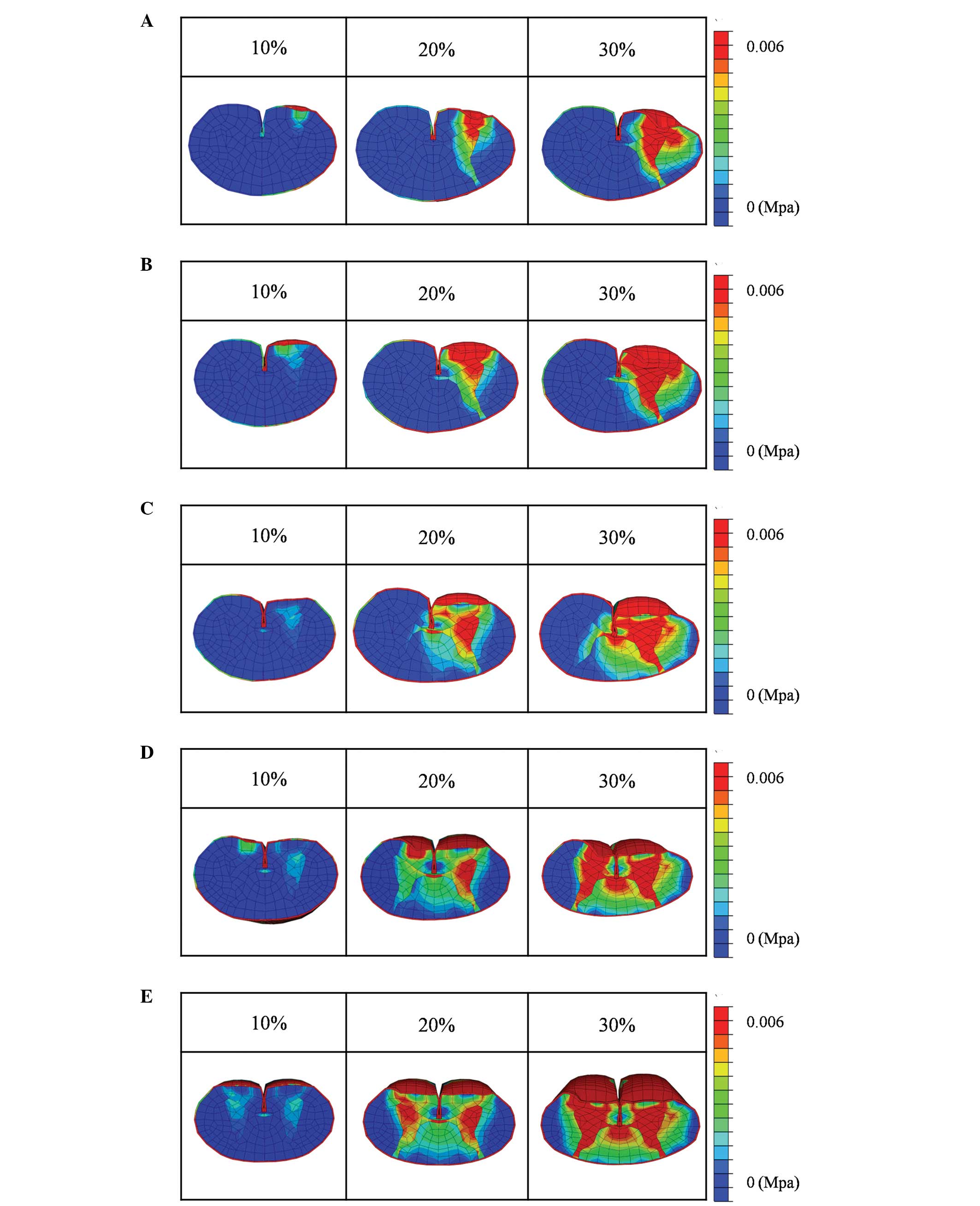
Under compression of 25% of the length of the
transverse diameter of the spinal cord with a rigid flat plate, the
stresses were extremely low when the degree of compression was 10%
of the AP diameter of the spinal cord. The stress was confined to
part of the gray matter and to the anterior funiculus. At 20%
compression, the stress on the anterior horn and part of the
anterior funiculus was slightly increased. At 30% compression, high
stresses were observed in the gray matter, anterior funiculus and
part of the lateral funiculus, but not the posterior funiculus
(Fig. 3A).
Compression of 37.5% of the length of the transverse
diameter of the spinal cord with a rigid flat plate resulted in
stresses that were very low when the compression of the AP diameter
of the spinal cord was 10%. The stresses on the gray matter and
anterior funiculus were slightly increased when 20% compression was
applied. At 30% compression, high stresses were observed in the
gray matter, anterior funiculus and lateral funiculus, while the
stress was moderately increased in part of the posterior funiculus
(Fig. 3B).
When 50% of the length of the transverse diameter of
the spinal cord was compressed with a rigid flat plate, the stress
was very low when the degree of compression was 10% of the AP
diameter of the spinal cord, and was observed only in the gray
matter and part of the anterior funiculus. At 20% compression, the
stress on the gray matter and anterior funiculus was slightly
increased. At 30% compression, the stress on the spinal cord was
increased and high stresses were observed in the gray matter and
the anterior, lateral and posterior funiculi. However, the stress
was not increased on the posterior funiculus of the non-compressed
side (Fig. 3C).
A compression of 62.5% of the length of the
transverse diameter of the spinal cord with a rigid flat plate
resulted in very low stresses when the degree of compression was
10% of the AP diameter of the spinal cord; the stress was only
slightly increased in both anterior funiculi. At 20% compression,
the stress increased in the gray matter and the anterior funiculus
on the compressed side. At 30% compression, the stress on the
spinal cord was increased and high stresses were observed in the
gray matter and anterior funiculi of both sides, the lateral
funiculus of the compressed side and both posterior funiculi. The
stress was not increased in the lateral funiculus of the
non-compressed side (Fig. 3D).
Under compression of 75% of the length of the
transverse diameter of the spinal cord with a rigid flat plate, the
stress on the spinal cord was very low at 10% compression of the AP
diameter of the spinal cord. At 20% compression, the stress
increased in the gray matter and anterior funiculi of both sides.
At 30% compression, the stress on the spinal cord was increased and
high levels of stress were observed in the gray matter of both
sides, as well as in the anterior, lateral and posterior funiculi
of both sides (Fig. 3E).
Discussion
BSS was first described by Brown-Séquard in a study
of a patient who was suffering from a knife injury and who
presented with hemicord syndrome (9). BSS involves ipsilateral loss of motor
function resulting from corticospinal tract interruption, combined
with a contralateral loss of pain and temperature sensation as a
result of spinothalamic tract dysfunction. BSS is most often
observed in association with traumatic injuries to the spinal cord
(10–12). The incidence of complete BSS due to
chronic compression is very low. In one study, complete BSS was
observed in 4.6% of 600 cases reported as BSS (4) and these comprised trauma (1.0%),
tumoral compression (0.8%) and non-tumoral compression or
non-compressive lesions (2.8%). The contribution of cervical disc
herniation to BSS has been estimated by Jomin et al
(13) to be 2.6%, but no further
details were provided in this study. Choi et al (14) reported that only five of the 2,350
cases (0.21%) in their series were retrospectively evaluated as
complete BSS caused by cervical disc herniation. Nine percent of
the patients who presented with symptoms of thoracic disc
herniation were diagnosed with BSS (15), but this study did not classify
cases into complete or incomplete BSS. BSS may also constitute the
initial symptom for idiopathic spinal cord herniation (ISCH), a
rare cause of progressive myelopathy (16–19).
ISCH is characterized by spontaneous herniation of the spinal cord
through an anterior or antero-lateral dural defect. A review of the
literature relevant to ISCH showed that 73/100 reported cases (73%)
presented with BSS, 19 (19%) with spasticity and eight (8%) with
numbness or leg pain (20)
However, this study did not provide details concerning whether the
BSS was complete or incomplete.
Based on these previous findings, we hypothesized
that pressure within a limited range of compression causes complete
BSS with static compression. To test this hypothesis, we
investigated three different degrees of static compression under
five different compressions of the transverse diameter of the
spinal cord. We calculated the stress distributions inside the
spinal cord and simulated complete and incomplete BSS using a
3D-FEM model.
The aim of the present study was to develop a 3D-FEM
spinal cord model that simulates the clinical situation. In a
similar manner to previous studies by Kato et al (21–23),
Li et al (24,25) and Nishida et al (26,27),
bovine spinal cord or magnetic resonance imaging (MRI) was used in
the model for the present analysis since it was not possible to
obtain fresh human spinal cord. The mechanical properties of the
spinal cord used in the present study were similar to those
reported in earlier studies (6–8). Li
and Dai (24) noted that it was
reasonable to make use of the mechanical properties of bovine
spinal cord since the brains and spinal cords of cattle and humans
exhibit similar changes when injured. In the present study, we also
assumed that the mechanical properties of the spinal cords from the
two species were similar. Persson et al (5) described the division of spinal cord
into pia mater and white and gray matter, and demonstrated that the
presence of pia mater had a significant effect on spinal cord
deformation; thus, pia mater is required to simulate the clinical
situation effectively.
The present study was limited to the investigation
of stress distribution caused by compression. Additional casual
factors that may contribute to cervical spondylotic myelopathy
(CSM) include ischemia, congestion and spinal cord stretch injury
(28). Blood flow was not analyzed
in this FEM analysis and only one movement (static compression) was
investigated for potential association with BSS. Long-term
compression and apoptotic factors were not considered in this FEM
analysis. Moreover, the FEM model used in the present study was
simplified in order to facilitate the calculations. Analysis errors
were reduced by using a FEM mesh, assuming the spinal cord was
symmetric, not including the denticulate ligament, dura and nerve
root sheaths, and setting a close distance between the spinal cord
and lamina, spinal cord and anterior compression of the spinal
cord.
When the rigid-flat-plate compression was applied to
<37.5% of the length of the transverse diameter of spinal cord,
the stress on the gray matter, anterior funiculus and lateral
funiculus was increased, but not the stress on the posterior
funiculus. Consequently, contralateral loss of pain, temperature
sensation and ipsilateral loss of motor function are likely to
occur, but not ipsilateral disorders of vibration and position
sense. This may correspond to cases of incomplete BSS.
When the rigid-flat-plate compression was applied to
>62.5% of the length of the transverse diameter of the spinal
cord, the stress increased on the gray matter, the anterior
funiculi and the posterior funiculi on both sides and on the
lateral funiculus on the compression side, but not the lateral
funiculus on the non-compression side. Based on these results, the
loss of pain and temperature sensation, as well as ipsilateral loss
of motor function on the compression side are expected to occur.
Disorders of vibration and position sense are also likely to occur
and, thus, this situation may correspond to cases with incomplete
BSS.
Under a rigid-flat-plate compression of 50% of the
length of the transverse diameter of the spinal cord and
compression of 10% of the AP diameter of the spinal cord, the
stress levels in the gray matter were very low. At 20% compression,
the stress levels were slightly increased in the gray matter and
anterior funiculus, while at 30% compression, the levels of stress
were increased in the gray matter, as well as in the anterior,
lateral and posterior funiculi. This may result in the
contralateral loss of pain and temperature sensation due to
anterior funiculus compression, ipsilateral loss of motor function
due to lateral funiculus compression, and ipsilateral disorders of
vibration and position sense due to posterior funiculus
compression. However, the distribution of stress to the posterior
funiculus of the non-compressed side and was not observed; thus,
this may correspond to cases of complete BSS.
The simulation model used in the present study
showed that only at a compression of 50% of the length of the
transverse diameter of the spinal cord did the stress distribution
lead to complete BSS. However, compression within such a limited
range is an infrequent clinical event and, thus, this may explain
why the number of cases of complete BSS associated with chronic
compression is rare.
References
|
1.
|
Kraus JA, Stuper BK and Berlit P: Multiple
sclerosis presenting with Brown-Séquard syndrome. J Neurol Sci.
156:112–113. 1998.
|
|
2.
|
Fisher RG: Protrusions of thoracic disc.
The factor of herniation through the dura matter. J Neurosurg.
22:591–593. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Love JG and Schorn VG: Thoracic-disc
protrusions. JAMA. 191:627–631. 1965. View Article : Google Scholar
|
|
4.
|
Koehler PJ and Endtz LJ: The Brown-Séquard
syndrome. True or false? Arch Neurol. 43:921–924. 1986.
|
|
5.
|
Persson C, Summers J and Hall RM: The
importance of fluid-structure interaction in spinal trauma models.
J Neurotrauma. 28:113–125. 2011. View Article : Google Scholar
|
|
6.
|
Ichihara K, Taguchi T, Shimada Y,
Sakuramoto I, Kawano S and Kawai S: Gray matter of the bovine
cervical spinal cord is mechanically more rigid and fragile than
the white matter. J Neurotrauma. 18:361–367. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Ichihara K, Taguchi T, Sakuramoto I,
Kawano S and Kawai S: Mechanism of the spinal cord injury and the
cervical spondylotic myelopathy: new approach based on the
mechanical features of the spinal cord white and gray matter. J
Neurosurg. 99(Suppl 3): 278–285. 2003.PubMed/NCBI
|
|
8.
|
Tunturi AR: Elasticity of the spinal cord,
pia, and denticulate ligament in the dog. J Neurosurg. 48:975–979.
1978. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Brown Sequard CE: De la transmission des
impressions sensitives par la moelle epiniere. CR Soc Biol.
1:192–194. 1849.
|
|
10.
|
Kobayashi N, Asamoto S, Doi H and Sugiyama
H: Brown-Séquard syndrome produced by cervical disc herniation:
report of two cases and review of the literature. Spine J.
23:530–533. 2003.
|
|
11.
|
Kohno M, Takahashi H, Yamakawa K, Ide K
and Segawa H: Postoperative prognosis of Brown-Séquard-type
myelopathy in patients with cervical lesions. Surg Neurol.
51:241–246. 1999.
|
|
12.
|
Mastronardi and Ruggeri A: Cervical disc
herniation producing Brown-Séquard syndrome: case report. Spine
(Phila Pa 1976). 29:E28–E31. 2004.PubMed/NCBI
|
|
13.
|
Jomin M, Lesoin F, Lozes G, et al:
Herniated cervical discs. Analysis of a series of 230 cases. Acta
Neurochir (Wien). 79:107–113. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Choi KB, Lee CD, Chung DJ and Lee SH:
Cervical disc herniation as a cause of Brown-Séquard syndrome. J
Korean Neurosurg Soc. 46:505–510. 2009.
|
|
15.
|
Arce CA and Dohrmann GJ: Herniated
thoracic disks. Neurol Clin. 3:383–392. 1985.
|
|
16.
|
Massicotte EM, Montanera WR, Ross Fleming
JF, Tucker WS, Willinsky R, TerBrugge K and Fehlings MG: Idiopathic
spinal cord herniation: report of eight cases and review of the
literature. Spine (Phila Pa 1976). 27:E233–E241. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Miyaguchi M, Nakamura H, Shakudo M, Inoue
Y and Yamano Y: Idiopathic spinal cord herniation associated with
intervertebral disc extrusion: a case report and review of the
literature. Spine (Phila Pa 1976). 26:1090–1094. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Wada E, Yonebu K and Kang J: Idiopathic
spinal cord herniation: report of three cases and review of the
literature. Spine (Phila Pa 1976). 25:1984–1988. 2000. View Article : Google Scholar
|
|
19.
|
White BD and Tsegaye M: Idiopathic
anterior spinal cord hernia: under-recognized cause of thoracic
myelopathy. Br J Neurosurg. 18:246–249. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Sasari M, Ozer AF, Vural M and Sarioglu
AC: Idiopathic spinal cord herniation: case report and review of
the literature. J Spinal Cord Med. 32:86–94. 2009.PubMed/NCBI
|
|
21.
|
Kato Y, Kataoka H, Ichihara K, et al:
Biomechanical study of cervical flexion myelopathy using a
three-dimensional finite element method. J Neurosurg Spine.
8:436–441. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Kato Y, Kanchiku T, Imajo Y, et al:
Flexion model simulating spinal cord injury without radiographic
abnormality in patients with ossification of the longitudinal
ligament: the influence of flexion speed on the cervical spine. J
Spinal Cord Med. 32:555–559. 2009.
|
|
23.
|
Kato Y, Kanchiku T, Imajo Y, et al:
Biomechanical study of the effect of the degree of static
compression of the spinal cord in ossification of the posterior
longitudinal ligament. J Neurosurg Spine. 12:301–305. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Li XF and Dai LY: Three-dimensional finite
element model of the cervical spinal cord: preliminary results of
injury mechanism analysis. Spine (Phila Pa 1976). 34:1140–1147.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Li XF and Dai LY: Acute central cord
syndrome: injury mechanisms and stress features. Spine (Phila Pa
1976). 35:E955–E964. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Nishida N, Kato Y, Imajo Y, Kawano S and
Taguchi T: Biomechanical study of the spinal cord in thoracic
ossification of the posterior longitudinal ligament. J Spinal Cord
Med. 34:518–522. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Nishida N, Kato Y, Imajo Y, Kawano S and
Taguchi T: Biomechanical analysis of cervical spondylotic
myelopathy: the influence of dynamic factors and morphometry of the
spinal cord. J Spinal Cord Med. 35:256–261. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Henderson FC, Geddes JF, Vaccaro AR,
Woodard E, Berry KJ and Benzel EC: Stretch-associated injury in
cervical spondylotic myelopathy: new concept and review.
Neurosurgery. 56:1101–1113. 2005.PubMed/NCBI
|