Introduction
Prostate cancer is the most common types of
noncutaneous cancer with a high mortality rate in American males
(1). Despite the significant
progress that has been made in the treatment of the disease,
therapeutic options for advanced and metastatic prostate cancer
remain unsatisfactory (2,3). The absence of effective therapies for
prostate cancer has entailed an intensive search for novel
anticancer strategies. In recent years, rapid progress has
increased the understanding of prostate cancer immunotherapy.
Prostate stem cell antigen (PSCA), first described
by Reiter et al (4), is a
surface glycoprotein that is upregulated in androgen-dependent and
-independent prostate cancer xenografts and downregulated in the
normal prostate (4). The PSCA gene
encodes a 123-amino acid protein that is a
glycosylphosphatidylinositol (GPI)-anchored cell surface antigen
associated with the Thy-1/Ly-6 family. Binding to cellular
membranes with covalent linkages, which may be degraded by
phosphatase (5,6). In addition, there is a direct
correlation between the expression level of PSCA and the tumor
stage and grade and the bone metastases (7). To date, numerous studies have
indicated that a vaccination based on PSCA enhances the cytotoxic T
lymphocyte (CTL) response and inhibits PSCA+ tumor
growth in mice (8–10).
Animal models are important tools to facilitate an
enhanced understanding of cancer biology and may be used to
evaluate the activities of investigational agents. Xenografts of
human tumor cell lines inoculated subcutaneously into mice have
been used to investigate cancer treatment since the late 1950s
(11). However, traditional animal
models typically require the sacrifice of the animal. Furthermore,
it is not possible to visualize the growth and metastasis of the
tumor and there is a lack of sensitivity (12). Therefore, novel sensitive methods
of detecting and monitoring in vivo tumor growth and
metastatic disease are required, with less invasive approaches.
Whole-body fluorescence and bioluminescence imaging
have transformed the study of gene expression and protein function
by enabling external visualization using sensitive detection
systems (13–15). Cancer cell lines stably transfected
either with the firefly luciferase (Luc) or green fluorescent
protein have been used to monitor local tumor growth and metastasis
in living mice (16).
In the present study, we have investigated for the
first time, to the best of our knowledge, the feasibility of
whole-body bioluminescent reporter imaging for the visualization of
the in vivo development of local tumor growth following the
inoculation of Luc and PSCA co-transfected RM-1 cells
(RM-PSCA/Luc), a prostate cancer cell line. The results showed that
it was possible to monitor tumor growth with noninvasive, sensitive
and quantitative localization in vivo using whole-body
bioluminescent reporter imaging.
Material and methods
Mice and cell lines
Male C57BL/6 mice (4–6 weeks old) were purchased
from the Center for Laboratory Animals (Beijing, China). The mouse
prostate tumor cell line RM-1, syngeneic to C57BL/6, was purchased
from the Shanghai Cell Institute (Shanghai, China). This study was
carried out in strict accordance with the recommendations in the
Guide for the Care and Use of Laboratory Animals of the Academy of
Military Medical Sciences (Beijing, China). The protocol was
approved by the Committee on the Ethics of Animal Experiments of
the Academy of Military Medical Sciences.
Plasmid DNA constructs
All constructs were cloned into the pcDNA3.1(+)
vector (Invitrogen Life Technologies, Carlsbad, CA, USA). The human
PSCA gene was amplified from the vector pMD-PSCA with the following
primers: 5′-CCC AAG CTT ACC ATG AAG GCT GTG CTG CTT-3′ and 5′-CCC
GGA TCC CTA TAG CTG GCC GGG TCC-3′, and cloned into the
HindIII and BamHI sites of pcDNA3.1 to generate
pcDNA-PSCA. To generate pcDNA-Luc, the Luc gene was amplified from
the vector pGL3 with the following primers: 5′-CCG GCT AGC ATG GAA
GAC GCC AAA AAC-3′ and 5′-CCG AAG CTT TTA CAC GGC GAT CTT TCC-3′,
prior to being cloned into the HindIII and NheI sites
of pcDNA3.1. PSCA amplification was performed for 3 min at 94°C,
immediately followed by 30 sec at 94°C, 30 sec at 55°C and 30 sec
at 72°C for 30 cycles. The reaction mixture of Luc was incubated
for 3 min at 94°C, followed by 30 sec at 94°C, 30 sec at 55°C and
90 sec at 72°C for 30 cycles. An additional extension step was
performed for 10 min at 72°C for Luc and PSCA, respectively. DNA
sequencing was performed to confirm that all constructs had the
desired sequence and open reading frame. Following this, pcDNA-PSCA
or pcDNA-Luc was transformed into DH5α-competent Escherichia
coli. Plasmid DNA copies were amplified in liquid culture and
purified using a Plasmid Mini kit (Promega, Madison, WI, USA).
Construction of stable transfectants
expressing the Luc and PSCA reporter genes
To generate a Luc and human PSCA-expressing cell
population, RM-PSCA/Luc, RM-1 was transfected with pcDNA-PSCA and
pcDNA-Luc plasmids, followed by a Geneticin® (G418)
selection (Invitrogen Life Technologies). Subsequently,
luminometry, reverse transcription-polymerase chain reaction
(RT-PCR) and flow cytometry were used to detect the validity of
these constructs. The expression of Luc was detected by
luminometry. Following selection using Geneticin (G418), tumor
cells were treated with cell culture lysis buffer. Having been
mixed with luciferin at a ratio of 1:5, the tumor cells were then
assessed for Luc expression. Tumor cells with luciferase activity
>1 were reserved for the analysis of PSCA expression. For RT-PCR
analysis, the following primers: 5′-TAA TAC GAC TCA CTA T-3′ and
5′-CTT GCC CAC GTA GTA G-3′ were used to amplify PSCA, while 5′-ACC
ACA GTC CAT GCC ATC AC-3′ and 5′-TCC ACC ACC CTG TTG CTG TA-3′ were
used for β-actin. Expression of PSCA on the cell surface was
detected by staining the cell with anti-PSCA antibody (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA USA) and fluorescein
isothiocyanate (FITC)-conjugated goat anti-rabbit immunoglobulin
(Ig) G antibody (Santa Cruz Biotechnology, Inc.), followed by flow
cytometric analysis.
Murine model of human prostate
cancer
Five 4 to 6-week-old male C57BL/6 mice were
inoculated subcutaneously at the right flank with 1×106
RM-PSCA/Luc cells. According to the result of our preliminary
experiment, the expression of Luc was detectable using the
luminometer when the RM-PSCA/Luc tumor cell population was
1×106. Mice were imaged using whole-body bioluminescent
reporter imaging for the first time one week subsequent to the
inoculation of the cells, and this was followed by weekly
imaging.
Bioluminescent reporter imaging
The tumor growth was monitored using an imaging unit
(IVIS Imaging System 50; Xenogen Corp., Alameda, CA, USA). The mice
were anesthetized via an intraperitoneal injection of ketamine
hydrochloride (0.66 mg/kg body weight) and xylazine (0.13 mg/kg
body weight) in phosphate-buffered saline (PBS). Following this, an
aqueous solution of luciferin was injected intraperitoneally. The
mice were then placed in a light-obstructing chamber and the
Luc-expressing cells were detected. The bioluminescent signal was
quantified by measuring the number of highlighted pixels in the
area shaped around each site of photon emission, with the aid of
the imaging unit software.
Tumor volume and survival time of
mice
Following inoculation with RM-PSCA/Luc cells, the
mice were monitored twice a week when the tumor was palpable. The
tumor size was measured using vernier calipers and the tumor volume
(V) was calculated according to the formula: V = 0.5a ×
b2, where a and b are the long and short diameters of
the tumor, respectively. In addition, the survival time of the mice
was recorded.
Immunohistochemical examination
Tumor tissues were fixed overnight in 4%
paraformaldehyde and the tissues were then transferred to 1:1
formaldehyde/ethanol for 1 h prior to being transferred to 70%
ethanol until processing. Tissues were dehydrated using a graded
ethanol series and embedded in paraffin wax at 58–60°C. The frozen
tissue sections (4–6 μm) were then washed for 10 min in
dimethylbenzene twice for deparaffinization, 30 sec in 99% ethanol,
30 sec in 95% ethanol and 5 min in PBS. Following this, the
sections were maintained at room temperature for 10 min with the
addition of H2O2 and endogenous peroxidase
activity was quenched. The tissue sections were then incubated for
24 h at 4°C with anti-PSCA polyclonal antibody (Santa Cruz
Biotechnology, Inc.). Subsequent to being washed three times in PBS
for 5 min, respectively, the sections were incubated for 20 min at
37°C with FITC-conjugated goat anti-rabbit IgG antibody (Santa Cruz
Biotechnology, Inc.). This was followed by coloration with
3,3′-diaminobenzidine (DAB) using a DAB kit (Zhongshan Biotech Co.,
Beijing, China), in accordance with the provided instructions.
Results
Generation of the Luc and PSCA genes
The Luc and PSCA genes were amplified using PCR from
the vectors pGL3 and pMD-PSCA, respectively. The sequencing of the
Luc and PSCA genes was in accordance with the previous publications
in GenBank (Luc cDNA, GenBank original accession no. AM295157; PSCA
cDNA, GenBank original accession no. AF043498; http://www.ncbi.nlm.nih.gov/genbank/).
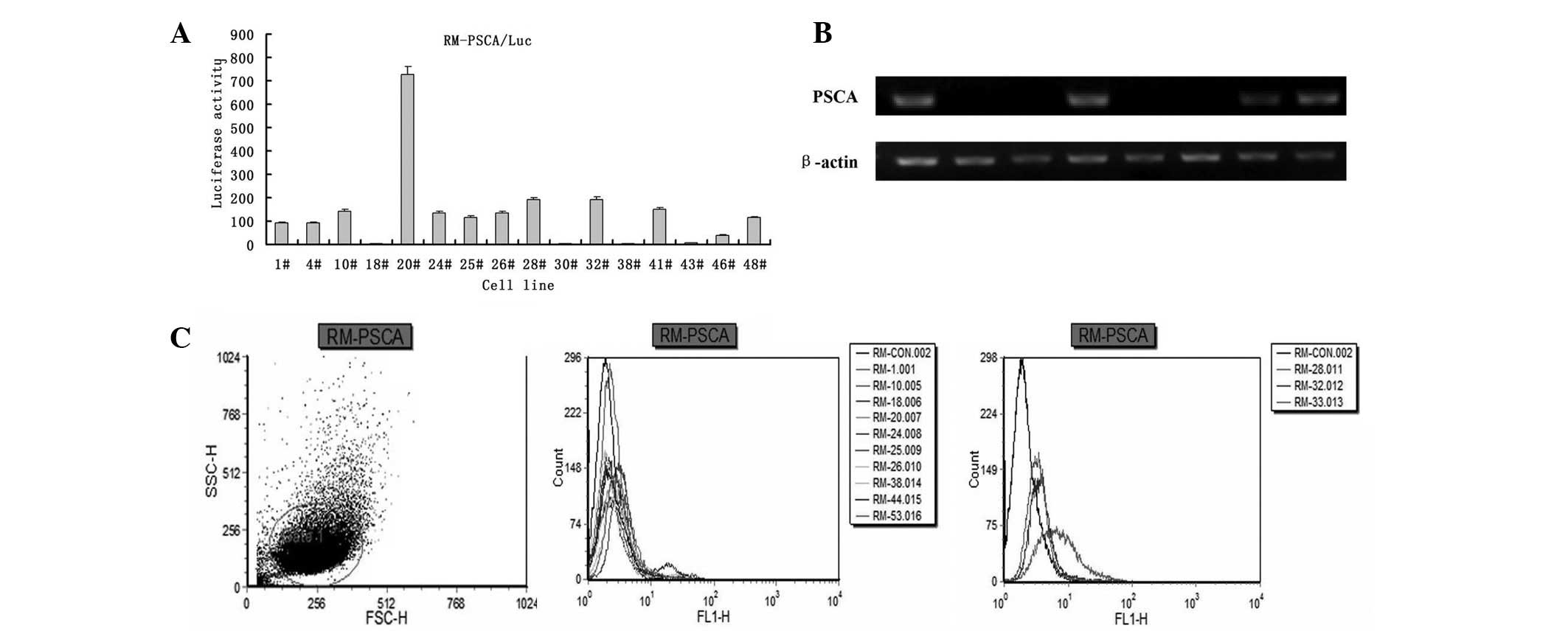
Detection of stable transfectants
expressing Luc and PSCA
Following treatment with cell culture lysis buffer,
RM-PSCA/Luc tumor cells were mixed with luciferin and then detected
using a luminometer (Fig. 1A). The
tumor cells with luciferase activity >1 subsequently underwent
the test for PSCA expression using RT-PCR (Fig. 1B), prior to the expression of PSCA
on the cell surface being detected using flow cytometry (Fig. 1C).
Detection of the luciferase activity of
RM-PSCA/Luc tumor cells
RM PSCA/Luc tumor cells were prepared with serial
2-fold dilution from 2×106 to 5×105. The
photon emission of Luc was detectable using a luminometer when the
cell population of RM-PSCA/Luc was 1×106 (Fig. 2).
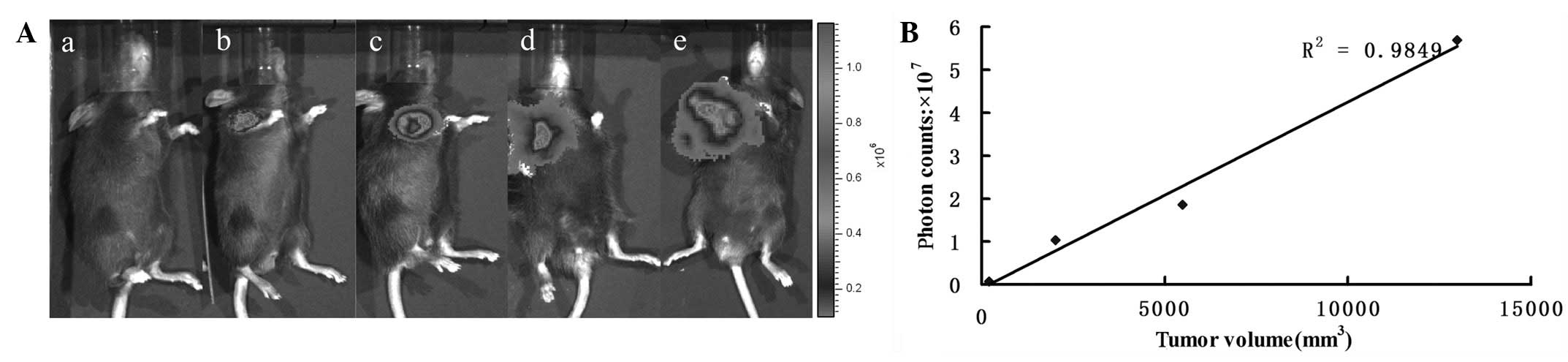
In vivo bioluminescence imaging of the
prostate tumor
All the mice inoculated with RM-PSCA/Luc cells
exhibited a detectable tumor within two weeks, as assessed using
bioluminescent reporter imaging. The bioluminescent emission
registered at day 7 increased substantially from first appearance
until day 34 (Fig. 3A).
Quantification of the Luc signal was used for the in vivo
monitoring of tumor growth. The correlation between the tumor size
and photon counts was evaluated externally in the living mice.
There was good correlation (R2=0.9849) between the
photon counts and tumor volume (Fig.
3B); therefore, an in vivo imaging system may be used as
a quantitative tool to monitor tumor growth. The bioluminescent
signal was positively correlated with the tumor burden.
Development of the tumor and the survival
time of the mice
The tumor progressed quickly (Fig. 4) and the mean survival time of the
mice was 38.4±3.05 days (34, 37, 39, 40 and 42 days).
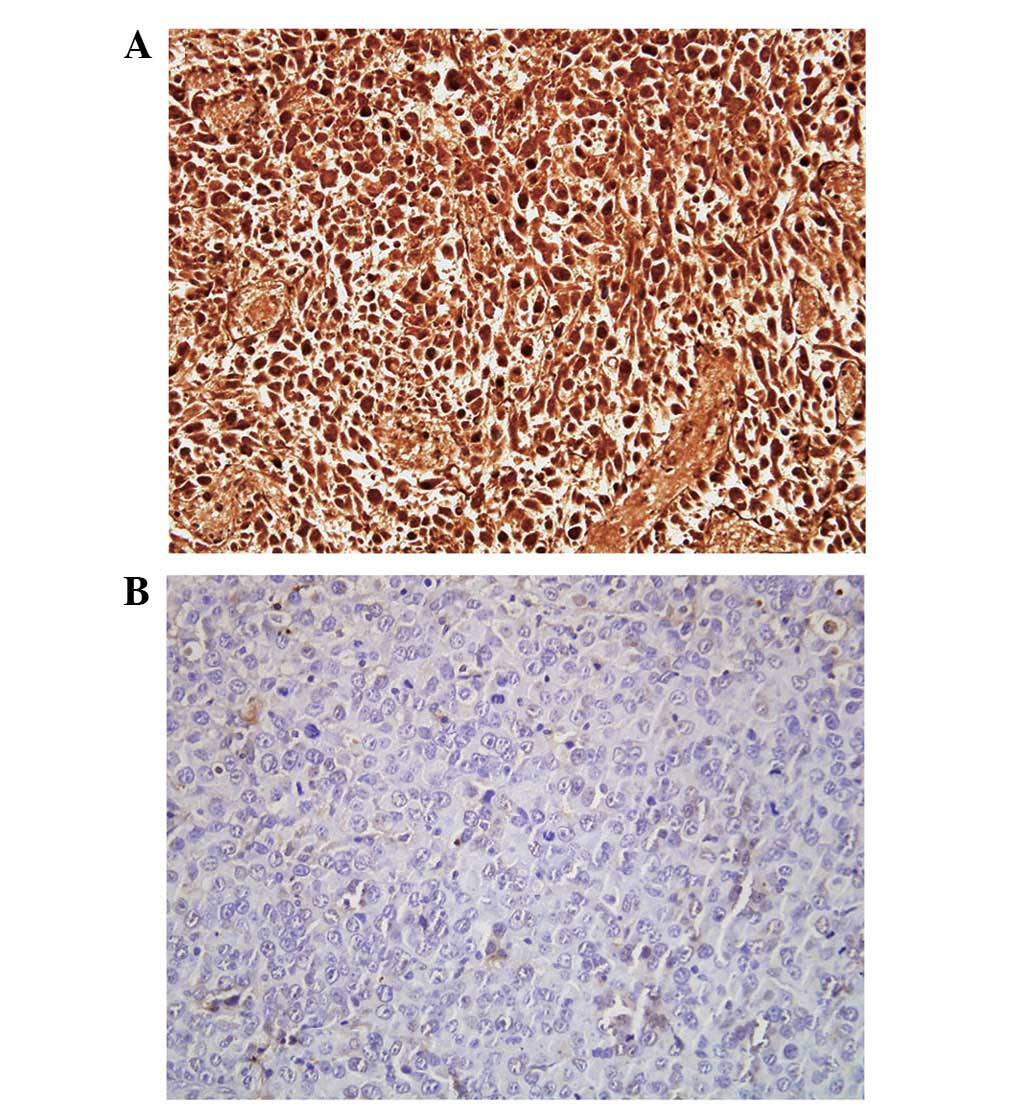
Immunohistochemical analysis
The cell line RM-PSCA/Luc was examined using
immunohistochemical analysis. Tumor tissues with or without
expression of PSCA were stained buffy (Fig. 5A) or blue (Fig. 5B), respectively. Analysis of the
immunohistochemistry confirmed the presence of cancer cells in
tumor tissues to be the sites of bioluminescent emission, detected
using bioluminescent reporter imaging.
Discussion
To the best of our knowledge, this study has, for
the first time, presented a new model to enable the monitoring of
prostate tumor growth using subcutaneous inoculation with Luc and
PSCA-expressing RM-1 cells. The injection of RM-PSCA/Luc cells,
combined with bioluminescent reporter imaging, may facilitate the
early detection, continuous monitoring and quantitative
localization of tumor growth in vivo in a noninvasive and
sensitive manner.
To study the biological function of human PSCA and
to evaluate the activities of anticancer drugs or vaccines for
prostate cancer, we have established a traditional prostate tumor
animal model with RM-PSCA cells and successfully evaluated the
effect of a DNA vaccine based on PSCA and an HSP70 adjuvant
(8,17). Traditional tumor animal models have
a number of limitations, as follows: (i) Tumor growth and
metastasis are not able to be visualized; (ii) there is a
requirement for animals to be sacrificed at different time-points
during the experiment, in order to obtain temporal information
without consecutive study; (iii) the reflection of the time and
space-expression of cells and genes is difficult. However, compared
with the traditional tumor animal model, bioluminescent reporter
imaging presents numerous advantages. It is time-saving and results
in the generation of more data per experimental series, which leads
to statistically sound results that are obtained more rapidly.
Furthermore, it reduces the individual variation and the number of
animals required (18). The
orthotopic and allotopic tumor progression may be easily detected
to monitor tumor growth and metastasis, without invasive
procedures. Due to the predominance of Luc, bioluminescent reporter
imaging for the detection of cells frequently reveals biological
phenomena, including the progression process of definite gene
expression, infectious diseases, tumor escape mechanisms and
patterns of metastasis, and has been already generally applied in
infection, gene therapy, organ transplantation, autoimmune disease,
pharma projects, tumor immunity and treatment (19,20).
In this study, the RM-PSCA/Luc cell line with stable
expression of PSCA and Luc was successfully constructed and was
capable of establishing tumor growth in vivo. Compared with
the athymic mouse, the C57BL/6 mouse has a normal immune system,
which is favorable for evaluating the activities of anticancer
drugs or vaccines.
In the present study, it was observed that RM-1
cells were transfected with PSCA and Luc genes without altering the
efficacy of tumor establishment and growth. Following the
subcutaneous inoculation of RM-PSCA/Luc cells, the subcutaneous
tumor was able to be visualized using bioluminescent reporter
imaging. However, tumor metastasis was not observed in this study.
This may be due to the cell line or the fact that the tumor grew so
quickly that the mice died prior to the occurrence of
metastasis.
In conclusion, we have established a new model that
utilizes the subcutaneous injection of Luc-reporter-positive
transfected cancer cells. This RM-PSCA/Luc model, coupled with
bioluminescent reporter imaging, provides a valuable experimental
tool for the preclinical evaluation of the in vivo antitumor
activity of investigational agents in the same animal. This model
is likely to facilitate studies of the molecular mechanisms
involved in the early stages of tumor progression and the
development of new anticancer therapeutic strategies.
References
|
1.
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar
|
|
2.
|
Hillman GG, Triest JA, Cher ML, Kocheril
SV and Talati BR: Prospects of immunotherapy for the treatment of
prostate carcinoma - a review. Cancer Detect Prev. 23:333–342.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Crawford ED, Rosenblum M, Ziada AM and
Lange PH: Hormone refractory prostate cancer. Urology. 54(Suppl):
1–7. 1999. View Article : Google Scholar
|
|
4.
|
Reiter RE, Gu z, Watabe T, Thomas G,
Szigeti K, Davis E, Wahl M, Nisitani S, Yamashiro J, Le Beau MM,
Loda M and Witte ON: Prostate stem cell antigen: a cell surface
maker over-expressed in prostate cancer. Natl Acad Sci USA.
95:1735–1740. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Antica M, Wu L and Scollay R: Stem cell
antigen 2 expression in adult and developing mice. Immunol Lett.
55:47–51. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Nakamura K, Yasunaga Y, Ko D, Xu LL, Moul
JW, Peehl DM, Srivastava S and Rhim JS: Cadmium-induced neoplastic
transformation of human prostate epithelial cells. Int J Oncol.
20:543–547. 2002.PubMed/NCBI
|
|
7.
|
Gu Z, Thomas G, Yamashiro J, Shintaku IP,
Dorey F, Raitano A, Witte ON, Said JW, Loda M and Reiter RE:
Prostate stem cell antigen (PSCA) expression increases with high
Gleason score, advanced stage and bone metastasis in prostate
cancer. Oncogene. 19:1288–1296. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Zhang X, Yu C, Zhao J, Fu L, Yi S, Liu S,
Yu T and Chen W: Vaccination with a DNA vaccine based on human PSCA
and HSP70 adjuvant enhances the antigen-specific CD8+
T-cell response and inhibits the PSCA+ tumors growth in
mice. J Gene Med. 9:715–726. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Garcia-Hernandez Mde L, Gray A, Hubby B,
Klinger OJ and Kast WM: Prostate stem cell antigen vaccination
induces a long-term protective immune response against prostate
cancer in the absence of autoimmunity. Cancer Res. 68:861–869.
2008.
|
|
10.
|
Ahmad S, Casey G, Cronin M, Rajendran S,
Sweeney P, Tangney M and O’Sullivan GC: Induction of effective
antitumor response after mucosal bacterial vector mediated DNA
vaccinationwith endogenous prostate cancer specific antigen. J
Urol. 186:687–693. 2011. View Article : Google Scholar
|
|
11.
|
Kalra J, Anantha M, Warburton C,
Waterhouse D, Yan H, Yang YJ, Strut D, Osooly M, Masin D and Bally
MB: Validating the use of a luciferase labeled breast cancer cell
line, MDA435LCC6, as a means to monitor tumor progression and to
assess the therapeutic activity of an established anticancer drug,
docetaxel (Dt) alone or in combination with the ILK inhibitor,
QLT0267. Cancer Biol Ther. 11:826–838. 2011.
|
|
12.
|
Dithmar S, Rusciano D and Grossniklaus HE:
A new technique for implantation of tissue culture melanoma cells
in a murine model of metastatic ocular melanoma. Melanoma Res.
10:2–8. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Ntziachristos V, Ripoll J, Wang LV and
Weissleder R: Looking and listening to light: the evolution of
whole-body photonic imaging. Nat Biotechnol. 23:313–320. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Herschman HR: Molecular imaging: looking
at problems, seeing solutions. Science. 302:605–608. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Weissleder R and Ntziachristos V: Shedding
light onto live molecular targets. Nat Med. 9:123–128. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Edinger M, Hoffmann P, Contag CH and
Negrin RS: Evaluation of effector cell fate and function by in vivo
bioluminescence imaging. Methods. 31:172–179. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Dong L, Zhang X, Yi S, Mao Y, Yu T, Hou L,
Fu L, Yu C and Chen W: Establishment of a mouse model of human
PSCA-expressing prostate cancer. Acta Lab Anim Sci Sin. 17:428–431.
2009.(In Chinese).
|
|
18.
|
Edinger M, Sweeny TJ, Tucker AA, Olomu AB,
Negrin RS and Contag CH: Noninvasive assessment of tumor cell
proliferation in animal models. Neoplasia. 1:303–310. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Wang X, Rosol M, Ge S, Peterson D,
McNamara G, Pollack H, Kohn DB, Nelson MD and Crooks GM: Dynamic
tracking of human hematopoietic stem cell engraftment using in vivo
bioluminescence imaging. Blood. 102:3478–3482. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Zhang H, Li Y, Wang Z and Zhang B:
Establishment of xenograft mouse models to study human lung cancer
by using in vivo imaging system. Sheng Wu Gong Cheng Xue Bao.
25:1204–1210. 2009.(In Chinese).
|