Introduction
Endoscopic mucosal resection (EMR) is widely used as
an endoscopic therapy for gastric cancer (1). However, EMR is limited in resection
size and, therefore, piecemeal resection is performed in cases of
large lesions resulting in an imprecise histological evaluation and
in a high frequency of local recurrence (2). Endoscopic submucosal dissection (ESD)
is a novel endoscopic technique that enables the en bloc resection
of large superficial gastric cancers (3). In a multicenter retrospective study
of endoscopic resection for early gastric cancer (EGC) (4), the 3-year cumulative
residual/recurrence-free rate following ESD was higher compared
with that of EMR. Regarding the overall survival rate of patients
subjected to ESD, we previously demonstrated that the 5-year
overall survival rate was 97.1% in patients with lesions that
fulfilled the standard guideline criteria and 97.2% in patients
with EGC that did not meet the guideline criteria but fulfilled the
expanded inclusion criteria (5).
In addition, ESD not only reduces the surgical risk and the
recurrence rate of gastric cancer, but also improves the quality of
life (QOL) of patients (6). Even
though ESD is useful in the treatment of EGC, it has been reported
that in addition to the risk of bleeding and perforation, patients
subjected to ESD may also experience gastrointestinal (GI) symptoms
such as belching and bloating (7).
Proton pump inhibitors (PPIs) are widely used to treat ESD-induced
gastric bleeding and ulcers (8,9).
However, PPIs are not able to totally relieve ulcer symptoms and
upper abdominal discomfort when the ulcer is large (8,9).
Since the GI symptoms and gastric motor functions following ESD
have been fully elucidated, it has become possible to manage the
clinical condition of a patient after ESD. In the present study, we
initially investigated the characteristics of GI symptoms and
gastric emptying following ESD.
Rikkunshito, a traditional Japanese medicine, is
widely used to treat upper GI symptoms such as gastroesophageal
reflux (10), dyspeptic symptoms
(11) and chemotherapy-induced
nausea (12). Rikkunshito has been
reported to have a dual action on the stomach, including relaxation
of the proximal stomach (13) and
contraction of the distal stomach (14). Thus, since rikkunshito may improve
the QOL of patients subjected to ESD, we also investigated the
effects of a PPI alone (PPI monotreatment group) and a PPI combined
with rikkunshito (PPI + rikkunshito group) on GI symptoms following
ESD in the present study, .
Patients and methods
Subjects
A total of 33 patients with gastric cancer (mean age
70 years; male/female, 25/8) who had undergone ESD at Nagasaki
University Hospital (Nagasaki, Japan) between January 2010 and
September 2011 were included in the present study as primary
subjects (Step 1). The primary subjects met the following inclusion
criteria: i) patients with gastric cancer subjected to ESD, ii)
patients aged >20 and <81 years, iii) patients for whom oral
administration was possible, and iv) patients who provided written
informed consent regarding their participation in the study. The
exclusion criteria were the following: i) presence of an additional
type of cancer, ii) patients who needed chemotherapy, iii) patients
with serious complications (liver, kidney, heart, blood or
metabolic disorders), iv) patients with alcohol or drug dependence,
v) patients under treatment for a psychological disease, vi)
patients with ulcerative colitis, Crohn’s disease or irritable
bowel syndrome (IBS), vii) women who were pregnant or wished to
become pregnant during the study or the follow-up period, as well
as lactating women, viii) patients who had received traditional
Japanese medicine including the test drug within one month prior to
the administration of the test drug, ix) patients with a history of
hypersensitivity to traditional Japanese medicine including the
test drug, and x) patients who were not considered eligible for
inclusion in the present study by the chief investigator.
Study design
This study (UMIN000002302) was a prospective,
randomized, parallel, comparative study to examine the
pharmacological effects, efficacy and safety of drug therapy in
gastric cancer patients subjected to ESD. This study was conducted
based on ethical guidelines for clinical studies, taking into
consideration the human rights and privacy of the patients. The
protocol of this study was approved by the Institutional Review
Board of Nagasaki University Hospital.
Study procedures and questionnaire
The study procedures followed are shown in Fig. 1. After obtaining written informed
consent regarding participation prior to ESD, patients who
fulfilled all the inclusion criteria were included in this study.
The GI symptoms of the patients were assessed using the
Gastrointestinal Symptom Rating Scale (GSRS) 6–8 days following
ESD. Gastric emptying was evaluated using the
[13C]-labeled acetate breath test (described in detail
later) that consisted of the administration of a liquid meal
[OKUNOS-A (200 ml) containing 13C-sodium acetate (100
mg)] and the determination of the peak time of the 13C% dose-excess
curve (T-max) after the evaluation of GI symptoms. The Japanese
Ministry of Health, Labour and Welfare has approved rikkunshito for
the treatment of abdominal pain and indigestion. Thirteen patients
who scored ≥3 more than the average GSRS scores for abdominal pain
or indigestion 6–8 days after ESD were included in the Step 2
study, and were randomized to the PPI group [standard treatment
with rabeprazole monotreatment (20 mg/day), twice/day (b.i.d); n=5]
or the PPI + rikkunshito group [rabeprazole (20 mg/day, b.i.d)
combined with rikkunshito (7.5 g/day), three times/day (t.i.d);
n=8]. The respective GSRS scores of the two groups after 4 and 8
weeks of treatment were compared with baseline values.
Measurement of GI symptoms
The GI symptoms were evaluated using the GSRS, which
is an inquiry table consisting of 15 items for the evaluation of
general GI symptoms (15). Each
GSRS item is rated on a 7-point Likert scale ranging from no
discomfort to very severe discomfort. Based on a factor analysis,
the 15 GSRS items break down into the following five scales:
abdominal pain (abdominal pain, hunger pain and nausea), reflux
syndrome (heartburn and acid regurgitation), diarrhea syndrome
(diarrhea, loose stools and urgent need for defecation),
indigestion syndrome (borborygmus, abdominal distension, eructation
and increased flatus) and constipation syndrome (constipation, hard
stools and a feeling of incomplete evacuation).
In the Step 2 study, the overall GSRS score and
subscales after 4 and 8 weeks of treatment were compared against
baseline values to evaluate the potential improvement of GI
symptoms in each group.
Measurement of gastric emptying
The gastric emptying test was performed according to
the protocol of the 13C-acetate breath test standardized
by the Japan Society of Smooth Muscle Research (16). Briefly, a commercially available
diet (OKUNOS-A; Horika Foods, Tokyo, Japan) was used as the test
meal. A 200 ml portion of the diet contained 9.8 g protein, 5.2 g
fat, 28.6 g carbohydrate and 200 kcal. The test meal was enriched
with 100 mg 13C-sodium acetate (Wako Pure Chemicals
Industries, Osaka, Japan). Breath samples were collected into 300
cc packs (Otsuka Pharmaceutical Co., Tokyo, Japan). The analysis of
the 13CO2/12CO2
enrichment in the breath samples was performed using an infrared
spectrophotometer (UBiT-IR200; Otsuka Electronics Co., Tokyo,
Japan). The T-max of gastric emptying was calculated using Excel
software (Star Medical, Tokyo, Japan). The cumulative excretion of
13CO2 (as a percentage of the ingested dose)
was also calculated.
Once baseline measurements had been taken after an
overnight fast, subjects consumed the test meal within 15 min. Upon
completion of the meal (t=0 min), sequential postprandial
measurements of gastric emptying were taken. Expired breath samples
were collected at t=0 min and every 5 min for the first half hour
after the meal was consumed, and at 15-min intervals from t=30 min
until 90 min for the detection of 13CO2.
Adverse events, safety and
tolerability
Safety and tolerability were assessed by recording
all adverse events, and changes in hematological and clinical
laboratory variables were measured during the screening visit and
after the post-dose esophagogastroduodenoscopy. An adverse event
was defined as any unfavorable or unintended sign, whether or not
it was considered to be causally related to the drugs used in this
study.
Compliance
Treatment compliance was defined as the percentage
of the test drug used. A treatment compliance of ≥66.6% was
considered acceptable.
Statistical analysis
The treatment response in each group was evaluated
based on changes in the GSRS or GSRS sub-item scores prior to and
following treatment using the Wilcoxon signed-rank test.
Furthermore, the improvement rate in each subject was calculated
from these scores, and the mean values were compared between the
two groups using the Wilcoxon rank-sum test. This test was also
used to compare background factors such as age and body mass index
(BMI). The distribution of gender was compared using Fisher’s exact
test. P<0.05 was considered to indicate a statistically
significant difference. All data are expressed as the mean ±
standard deviation (SD).
Results
Patient characteristics
Patient characteristics including mean age,
male/female ratio, BMI and clinicopathological characteristics of
EGC are shown in Table I. The
standard guideline criteria and expanded criteria for ESD were
established by the Japanese Gastric Cancer Association (17,18).
The standard guideline criteria were defined as differentiated
mucosal cancer of ≤2 cm without ulcer. The expanded criteria
encompassed non-ulcerative differentiated cancer >2 cm,
ulcerative differentiated cancer <3 cm, non-ulcerative
undifferentiated cancer <2 cm and differentiated, submucosal
invading (limited to 500 μm below the lamina propria) cancer <3
cm in diameter. In 11 patients the lesion fulfilled the standard
guideline criteria, in seven patients the lesion fulfilled the
expanded criteria, and in 15 patients the lesion was an adenoma or
atypical duct hyper-plasia that did not comply with either the
standard guideline or expanded criteria. The lesions were removed
by en bloc resection in all patients. The incidence of local tumor
recurrence was 3% (complete removal:incomplete removal, 32:1).
 | Table I.Clinicopathological characteristics of
early gastric cancer patients. |
Table I.
Clinicopathological characteristics of
early gastric cancer patients.
| Characteristic | Value, n (range) |
|---|
| No. of patients | 33 |
| Age (years) | 70 (56–79) |
| Gender
(male/female) | 25/8 |
| Body mass index | 23 (17–30) |
| ESD of gastric
lesions | |
| Guideline
criteria | 11 |
| Expanded
criteria | 7 |
| Other | 15 |
| Lesion size (mm) | 27 (7–70) |
| Length of the
resected specimen (mm) | 50 (18–90) |
| En bloc
resection/piecemeal removal | 33/0 |
| Complete
removal/incomplete removal | 32/1 |
| Lesion localization
in the stomach | |
| Upper third | 6 |
| Middle third | 11 |
| Lower third | 16 |
| Lesion site in the
stomach | |
| Anterior
wall | 5 |
| Posterior
wall | 7 |
| Lesser
curvature | 13 |
| Greater
curvature | 7 |
| Other | 1 |
Gastric emptying in EGC patients who had
undergone ESD
Of the 33 patients who had undergone ESD, the
evaluation of gastric emptying was possible in 32 patients. The
reference range of T-max was derived from 63 healthy volunteers by
the Japan Society of Smooth Muscle Research; the mean ± SD of the
T-max was 43.9±10.3 min (16).
Gastric emptying following ESD in the patients with EGC in the
present study is shown in Fig. 2.
The mean T-max 6–8 days after ESD was 75.4±13.6 min which was
significantly increased compared with that in healthy subjects
(43.9±10.3 min).
Incidence of GI symptoms according to
location of the lesion
GI symptoms were evaluated in 29 of the 33 patients
subjected to ESD. Of these 29 patients, 25 were scored ≥3 (≥mild
discomfort) for at least one of the 15 GSRS items. The ratio of
patients with GI symptoms (≥mild discomfort) according to lesion
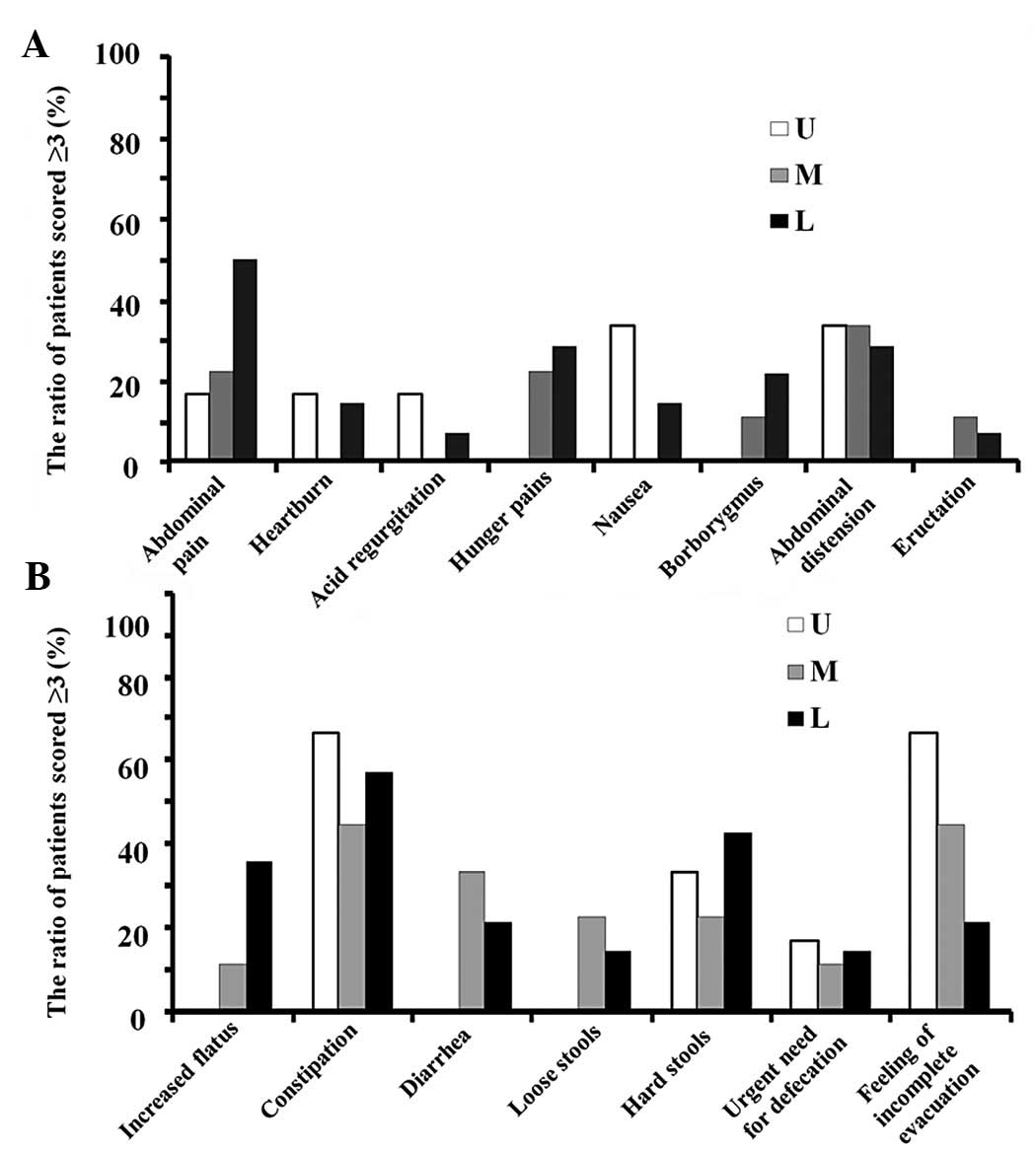
location is shown in Fig. 3. The
lesion location was defined by anatomically dividing the stomach
into three portions according to the Japanese classification of
gastric carcinoma: the upper (U), middle (M) and lower (L) parts
(17,18). Constipation (mean of U, M and L,
56%), a sense of incomplete evacuation (44%), hard stools (33%),
abdominal distension (32%) and abdominal pain (30%) were reported
in ≥30% of the patients irrespective of the lesion location. The
incidences of nausea and a sense of incomplete evacuation were
higher in the patients whose lesion was located in the U portion
(33 and 67%, respectively) compared with those in the patients
whose lesion was located in the M (0 and 44%, respectively) or L
portions (14 and 21%, respectively). The incidence of upper
abdominal pain was higher in the patients whose lesion was located
in the L portion (50%) than in those whose lesion was located in
the U (17%) or M portions (22%).
Effect of rikkunshito on GI symptoms
after ESD
Thirteen patients who scored ≥3 more than the
average scores for abdominal pain or indigestion 6–8 days after ESD
were included in the Step 2 study. The overall scores and the five
subscale scores of GSRS after 4 and 8 weeks of treatment with a PPI
alone (rabeprazole 20 mg/day, b.i.d, n=5) or a PPI (rabeprazole 20
mg/day, b.i.d, n=8) combined with rikkunshito (7.5 g/day, t.i.d)
are shown in Table II. The overall
scores and five subscale scores of GSRS did not change after 4 and
8 weeks of treatment with the PPI alone, while a significant
improvement of overall GSRS scores was observed after 4 and 8 weeks
of treatment with PPI plus rikkunshito (P=0.1313). Moreover, the
mean abdominal pain score was significantly decreased after 8 weeks
of treatment with PPI plus rikkunshito (Fig. 4), indicating the beneficial effects
of rikkunshito, particularly against abdominal and hunger pains
(Fig. 5).
 | Table II.Overall scores and the five subscale
scores of the GSRS after 4 and 8 weeks of treatment with a PPI
alone or a PPI combined with rikkunshito. |
Table II.
Overall scores and the five subscale
scores of the GSRS after 4 and 8 weeks of treatment with a PPI
alone or a PPI combined with rikkunshito.
| Subscale score | PPI group (A)
| PPI + rikkunshito
group (B)
|
|---|
| Baseline | 4 weeks | 8 weeks | Baseline | 4 weeks | 8 weeks |
|---|
| Reflux
syndrome | 1.50±0.61 | 1.40±0.65 | 1.30±0.45 | 1.63±0.64 | 1.06±0.18 | 1.13±0.35 |
| Abdominal pain | 2.20±0.51 | 1.13±0.18 | 1.27±0.37 | 2.63±1.13 | 1.42±0.50 | 1.08±0.15a |
| Indigestion
syndrome | 2.40±0.86 | 1.45±0.45 | 1.75±0.74 | 2.06±0.98 | 1.75±1.03 | 1.47±0.49 |
| Diarrhoea
syndrome | 2.40±1.21 | 1.27±0.37 | 1.25±0.50 | 1.67±0.71 | 1.38±0.58 | 1.38±0.45 |
| Constipation
syndrome | 3.27±1.40 | 2.40±1.67 | 3.08±1.60 | 2.75±1.38 | 2.67±1.55 | 2.50±1.47 |
| Overall scores | 2.35±0.79 | 1.53±0.56 | 1.73±0.66 | 2.15±0.69 | 1.65±0.58a | 1.51±0.35b |
Discussion
The mean T-max 6–8 days after ESD was 75.4±13.6 min
which was significantly longer compared with the mean T-max
previously reported in healthy subjects (43.9±10.3 min) (16). This suggests that gastric emptying
was delayed 6–8 days after ESD; however, gastric emptying beyond
this operative period remains unknown. Furthermore, we examined the
correlation between delayed gastric emptying and lesion location,
and observed that the gastric emptying tended to be more delayed in
patients whose lesion was located in the U portion [T-max (mean ±
SD), 87.5±6.1] compared with those whose lesion was located in the
M [T-max (mean ± SD), 75.0±12.2] or L portions [T-max (mean ± SD),
67.7±14.9] (data not shown). Normal gastric emptying is known to
reflect a coordinated effort of the fundus, antrum, pyloric
sphincter and duodenum (19).
Moreover, the gastric emptying of solid and liquid foods is
regulated by different mechanisms (19–21).
Liquid emptying mainly depends on the gastric-duodenal pressure
gradient derived from prolonged contraction of the fundus with less
reliance on antral peristalsis and pyloric opening. By contrast,
solids are initially retained selectively within the stomach until
particles have been triturated to a size of <2 mm, and the solid
emptying mainly depends on antral and pylorus actions. Since a
liquid meal was used in the gastric emptying test, the action of
the fundus is likely to have had a potent effect on gastric
emptying. The U portion of the stomach includes the fundus.
Therefore, gastric emptying is likely to be further delayed in
patients whose lesion is located in the U portion compared with
those whose lesion is located in the M or L portion.
GI symptoms associated with delayed gastric emptying
were observed 6–8 days after ESD. In particular, constipation, a
sense of incomplete evacuation and abdominal distension were
observed in ≥30% of the patients. It is not clear whether delayed
gastric emptying causes constipation, although voluntary
suppression of defecation is known to delay gastric emptying in
healthy subjects (22).
Furthermore, constipation-predominant IBS patients have been
reported to experience delayed gastric emptying more frequently
compared with healthy controls or diarrhea-predominant IBS patients
(23). Consequently, constipation
may further delay gastric emptying.
The present study demonstrated that the incidence of
upper abdominal pain was higher in the patients whose lesion was
located in the L portion than in those whose lesion was located in
the U or M portion. Since the L portion of the stomach includes the
antrum and the pylorus, the L portion is subject to the effects of
smooth muscle contraction/relaxation and bile acid regurgitation by
antroduodenal coordination (19).
Therefore, many patients with a lesion in the L portion appear to
experience abdominal pain.
In the present study, we evaluated the effectiveness
of rikkunshito against GI symptoms following ESD. The overall and
mean abdominal pain GSRS scores were improved following treatment
with a PPI plus rikkunshito but not after treatment with a PPI
alone. In patients with PPI-refractory gastroesophageal reflux
disease, treatment with PPI plus rikkunshito has been shown to
improve upper GI symptoms, supporting the results of the present
study (10). Since rikkunshito
does not have the gastric anti-secretory effect of an acid reducer
such as PPI 24, rikkunshito may improve abdominal pain by a
mechanism different from that of PPI. Rikkunshito has various
pharmacological actions such as a stimulatory effect on gastric
emptying (25–27), regulation of ghrelin secretion
(28–30) and protection of the gastric mucosa
(31). Rikkunshito has been shown
to improve upper GI symptoms via the stimulation of gastric
emptying in functional dyspeptic (FD) patients (25) and in patients who had undergone
pylorus-preserving gastrectomy (26). Moreover, hesperidine and
atractylodin, which are ingredients of rikkunshito, have been found
to improve delayed gastric emptying in rats following
L-NNA-administration (27,32). Ghrelin is a digestive system
hormone, originally identified in the stomach as the endogenous
ligand for the growth hormone secretagogue receptor GHS-R1a
(33). Ghrelin has a wide spectrum
of biological functions including appetite stimulation, GI motility
and gastric mucosal protection (34,35).
Recently, rikkunshito was shown to enhance ghrelin secretion and
the reactivity of its receptor (28,36).
In particular, the plasma acyl-ghrelin concentration is increased
in healthy volunteers and FD patients following treatment with
rikkunshito (29,30). Thus, the stimulatory effect of
gastric emptying and ghrelin secretion caused by rikkunshito plays
a major role in the alleviation of GI symptoms. The improvement in
abdominal pain following ESD may be due to these pharmacological
effects. The effects of rikkunshito on gastric emptying and plasma
ghrelin concentration in patients who have undergone ESD warrant
further investigation.
In conclusion, ESD affects gastric emptying and is
associated with upper GI symptoms such as abdominal pain and
indigestion. Treatment with a PPI plus rikkunshito may improve the
GI symptoms in patients who have undergone ESD when the GI symptoms
are not improved by treatment with a PPI alone.
Acknowledgements
The authors thank S. Iyoki and R.
Inami for the important advice provided. The present study was
supported by a grant from Tsumura & Co.
References
|
1.
|
Othman MO and Wallace MB: Endoscopic
mucosal resection (EMR) and endoscopic submucosal dissection (ESD)
in 2011, a Western perspective. Clin Res Hepatol Gastroenterol.
35:288–294. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Fujishiro M: Perspective on the practical
indications of endoscopic submucosal dissection of gastrointestinal
neoplasms. World J Gastroenterol. 14:4289–4295. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Kim SH, Moon JS, Youn YH, Lee KM and Lee
SJ: Management of the complications of endoscopic submucosal
dissection. World J Gastroenterol. 17:3575–3579. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Oda I, Saito D, Tada M, et al: A
multicenter retrospective study of endoscopic resection for early
gastric cancer. Gastric Cancer. 9:262–270. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Isomoto H, Shikuwa S, Yamaguchi N, et al:
Endoscopic submucosal dissection for early gastric cancer: a
large-scale feasibility study. Gut. 58:331–336. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Abe N, Takeuchi H, Ohki A, et al:
Long-term outcomes of combination of endoscopic submucosal
dissection and laparoscopic lymph node dissection without
gastrectomy for early gastric cancer patients who have a potential
risk of lymph node metastasis. Gastrointest Endosc. 74:792–797.
2011. View Article : Google Scholar
|
|
7.
|
Chung IK, Lee JH, Lee SH, et al:
Therapeutic outcomes in 1000 cases of endoscopic submucosal
dissection for early gastric neoplasms: Korean ESD Study Group
multicenter study. Gastrointest Endosc. 69:1228–1235. 2009.
View Article : Google Scholar
|
|
8.
|
Daneshmend TK, Hawkey CJ, Langman MJ,
Logan RF, Long RG and Walt RP: Omeprazole versus placebo for acute
upper gastrointestinal bleeding: randomised double blind controlled
trial. BMJ. 304:143–147. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Lee SH, Lee CK, Chung IK, et al: Optimal
duration of proton pump inhibitor in the treatment of endoscopic
submucosal dissection-induced ulcers: a retrospective analysis and
prospective validation study. Dig Dis Sci. 57:429–434. 2012.
View Article : Google Scholar
|
|
10.
|
Tominaga K, Iwakiri R, Fujimoto K, et al
GERD 4 Study Group: Rikkunshito improves symptoms in PPI-refractory
GERD patients: a prospective, randomized, multicenter trial in
Japan. J Gastroenterol. 47:284–292. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Tatsuta M and Iishi H: Effect of treatment
with liu-jun-zi-tang (TJ-43) on gastric emptying and
gastrointestinal symptoms in dyspeptic patients. Aliment Pharmacol
Ther. 7:459–462. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Seike J, Sawada T, Kawakita N, et al: A
new candidate supporting drug, rikkunshito, for the QOL in advanced
esophageal cancer patients with chemotherapy using
docetaxel/5-FU/CDDP. Int J Surg Oncol. 2011:7156232011.PubMed/NCBI
|
|
13.
|
Hayakawa T, Arakawa T, Kase Y, et al:
Liu-Jun-Zi-Tang, a kampo medicine, promotes adaptive relaxation in
isolated guinea pig stomachs. Drugs Exp Clin Res. 25:211–218.
1999.PubMed/NCBI
|
|
14.
|
Mochiki E, Yanai M, Ohno T and Kuwano H:
The effect of traditional Japanese medicine (Kampo) on
gastrointestinal function. Surg Today. 40:1105–1111.
2010.PubMed/NCBI
|
|
15.
|
Revicki DA, Wood M, Wiklund I and Crawley
J: Reliability and validity of the Gastrointestinal Symptom Rating
Scale in patients with gastroesophageal reflux disease. Qual Life
Res. 7:75–83. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Sanaka M and Nakata K: Stable isotope
breath tests for assessing gastric emptying: A comprehensive
review. J Smooth Muscle Res. 46:267–280. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Japanese Gastric Cancer Association:
Japanese classification of gastric carcinoma - 2nd English edition.
Gastric Cancer. 1:10–24. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Japanese Gastric Cancer Association:
Japanese classification of gastric carcinoma: 3rd English edition.
Gastric Cancer. 14:101–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Parkman HP and Jones MP: Tests of gastric
neuromuscular function. Gastroenterology. 136:1526–1543. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Kelly KA: Gastric emptying of liquids and
solids: roles of proximal and distal stomach. Am J Physiol.
239:G71–G76. 1980.PubMed/NCBI
|
|
21.
|
Camilleri M, Malagelada JR, Brown ML,
Becker G and Zinsmeister AR: Relation between antral motility and
gastric emptying of solids and liquids in humans. Am J Physiol.
249:G580–G585. 1985.PubMed/NCBI
|
|
22.
|
Tieerdsma HC, Smout AJ and Akkermans LM:
Voluntary suppression of defecation delays gastric emptying. Dig
Dis Sci. 38:832–836. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Lee OY: Asian motility studies in
irritable bowel syndrome. J Neurogastroenterol Motil. 16:120–130.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Hattori T: Rikkunshito and ghrelin. Int J
Pept. 2010:2835492010. View Article : Google Scholar
|
|
25.
|
Kusunoki H, Haruma K, Hata J, et al:
Efficacy of Rikkunshito, a traditional Japanese medicine (Kampo),
in treating functional dyspepsia. Intern Med. 49:2195–2202. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Takahashi T, Endo S, Nakajima K, Souma Y
and Nishida T: Effect of rikkunshito, a Chinese herbal medicine, on
stasis in patients after pylorus-preserving gastrectomy. World J
Surg. 33:296–302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Kido T, Nakai Y, Kase Y, et al: Effects of
rikkunshi-to, a traditional Japanese medicine, on the delay of
gastric emptying induced by N(G)-nitro-L-arginine. J Pharmacol Sci.
98:161–167. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Takeda H, Sadakane C, Hattori T, et al:
Rikkunshito, an herbal medicine, suppresses cisplatin-induced
anorexia in rats via 5-HT2 receptor antagonism. Gastroenterology.
134:2004–2013. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Matsumura T, Arai M, Yonemitsu Y, et al:
The traditional Japanese medicine Rikkunshito increases the plasma
level of ghrelin in humans and mice. J Gastroenterol. 45:300–307.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Arai M, Matsumura T, Tsuchiya N, et al:
Rikkunshito improves the symptoms in patients with functional
dyspepsia, accompanied by an increase in the level of plasma
ghrelin. Hepatogastroenterology. 59:62–66. 2012.PubMed/NCBI
|
|
31.
|
Arakawa T, Higuchi K, Fujiwara Y, et al:
Gastroprotection by Liu-Jun-Zi-Tang (TJ-43): possible mediation of
nitric oxide but not prostaglandins or sulfhydryls. Drugs Exp Clin
Res. 25:207–210. 1999.PubMed/NCBI
|
|
32.
|
Nakai Y, Kido T, Hashimoto K, et al:
Effect of the rhizomes of Atractylodes lancea and its
constituents on the delay of gastric emptying. J Ethnopharmacol.
84:51–55. 2003.
|
|
33.
|
Kojima M, Hosoda H, Date Y, Nakazato M,
Matsuo H and Kangawa K: Ghrelin is a growth-hormone-releasing
acylated peptide from stomach. Nature. 402:656–660. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
van der Lely AJ, Tschöp M, Heiman ML and
Ghigo E: Biological, physiological, pathophysiological, and
pharmacological aspects of ghrelin. Endocr Rev. 25:426–457.
2004.PubMed/NCBI
|
|
35.
|
Konturek PC, Brzozowski T, Pajdo R, et al:
Ghrelin - a new gastroprotective factor in gastric mucosa. J
Physiol Pharmacol. 55:325–336. 2004.PubMed/NCBI
|
|
36.
|
Fujitsuka N, Asakawa A, Uezono Y, et al:
Potentiation of ghrelin signaling attenuates cancer
anorexia-cachexia and prolongs survival. Transl Psychiatry.
26:e232011. View Article : Google Scholar : PubMed/NCBI
|