Introduction
Pulmonary hypertension (PH) is defined as a mean
pulmonary arterial pressure (mPAP) >25 mmHg at rest or as
pulmonary vascular resistance (PVR) >3 Wood units (1). PH comprises apparently heterogeneous
conditions that may have comparable clinical and hemodynamic
characteristics, and is characterized by progressive obliteration
of pulmonary arterioles, leading to the increase of PVR,
right-heart failure and mortality. Over the past years, numerous
advances in researching and understanding the pulmonary vascular
biology have been revealed. The evaluation of the severity of PH is
important in the diagnostic process and therapeutic
decision-making.
The clinical history, physical examination and
biochemical markers of a patient may suggest PH and right
ventricular dysfunction. Currently, right-heart catheterization
enables the direct measurement of hemodynamics and remains the
reference standard for the evaluation of PH (2,3), and
it is yet to be replaced by another approach. However, invasiveness
and surgical skill requirements are the major weaknesses of heart
catheterization. Echocardiography is able to evaluate pulmonary
pressure and right ventricular function in a noninvasive manner.
However, occasionally echocardiography underestimates the mild
increased pulmonary pressure and is unable to supply information
concerning emboli of the distal pulmonary artery. B-type
natriuretic peptide (BNP) and its prohormone N-terminal pro-B-type
natriuretic peptide (NT-proBNP) are peptides released from the
myocardium, and studies suggest an abnormal increase in the
NT-proBNP response to ventricular wall stress and dysfunction. In
patients with PH, increasing evidence suggests that NT-proBNP is a
useful marker for right ventricular dysfunction and predicts the
outcome of PH (4–7).
Recently, computed tomographic pulmonary angiography
(CTPA) has been used to provide much more important information,
including establishing the diagnosis, defining its cause,
quantifying its hemodynamic parameters, and aiding therapeutic
planning and monitoring in pulmonary vascular disease. CTPA is
already established as a first line test in acute pulmonary
embolism (APE) (8–11). The retrospective
electrocardiography (ECG)-gating technique in CT angiography now
enables more comprehensive evaluation of cardiac vessels and
function. Previously, studies have reported that certain
cardiovascular parameters from CTPA are able to assess the extent
of pulmonary artery obstruction and right ventricular function
(12–15). An angle in transversal images of
CTPA between the connecting line from the midpoint of the sternum
to the thoracic vertebral spinous process and interventricular
septum, named as Septal angle by us firstly, was reported to be
correlated with PVR in patients with chronic thromboembolic
pulmonary hypertension (CTEPH) (16,17).
For further clarification of the role of the Septal angle in
assessment severity of PH and its correlation with right
ventricular function, we performed a retrospective study to explore
the association between the CTPA-derived Septal angle and
hemodynamics, and the level of NT-proBNP in patients with PH.
Materials and methods
Patients
Between January 2007 and March 2011, 106 consecutive
patients with confirmed PH including 76 CTEPH patients and 30
pulmonary artery hypertension (PAH) patients in Peking University
Shougang Hospital and Chaoyang Hospital of Capital Medical
University (Beijing, China) were included in this retrospective
study. PH was diagnosed according to the European Society of
Cardiology (ESC)/European Respiratory Society (ERS) Guidelines
(2). The medical record of each
patient was reviewed by one doctor. Patients who underwent CTPA,
right-heart catheterization and NT-proBNP tests were included.
Exclusion criteria included the following: i) patients that were
not subject to CTPA or CTPA with inferior image quality; ii)
patients without right-heart catheterization; and iii) patients
without NT-proBNP testing. The control group was composed of 106
age- and gender-matched control subjects without PH and pulmonary
embolism. The study protocol was approved by the institutional
ethics review committees of Peking University and Captial Medical
University. All patients provided written informed consent.
CTPA acquisition and image analysis
A 64-row multidetector CT scanner (LightSpeed VCT;
GE Healthcare, Milwaukee, WI, USA) using the retrospective
ECG-gated mode was used for CTPA scanning. The whole chest was
scanned from the lung apex to the diaphragm with a single
breath-hold. Scan parameters included the following: current of
300–550 mA modulated by personal body mass index (BMI) dose; tube
voltage of 100–140 kV and collimation of 0.625 mm, gantry rotation
time of 0.8 sec, table speed of 39.37 mm/sec and reconstruction
increment of 1 mm. A mechanical injector was used for intravenous
bolus injection of iopromide (370 mg/ml, Ultravist; Bayer Schering
Pharma, Berlin, Germany) at a flow rate of 4.5–5.0 ml/sec. The
automatic bolus-tracking technique had the region of interest
positioned at the level of the main pulmonary artery with a
pre-defined threshold of 100 HU, and a fixed delay of 5 sec was
employed for data acquisition.
Images were transferred to the electronic picture
archiving and communication systems (GE Centricity 3000 RA1000; GE
Healthcare) and reviewed by two radiologists together blinded to
clinical information. Fig. 1 shows
that the Septal angle was measured in diastole in the transverse
CTPA image. According our previously described methods (15–17),
other parameters including the diameter of main pulmonary artery
(MPAd), diameter of ascending aorta (AAd), transverse diameter of
right atrium (RAd), transverse diameter of right ventricle and left
ventricle (RVd and LVd), interventricular septal thickness (IVST),
and right and left ventricular area (RVa and LVa) were all measured
on the transverse images. The ratio of MPAa and AAd (MPAa/AAd), the
ratio of RVd and LVd (RVd/LVd) and the ratio of RVa and LVa
(RVa/LVa) were measured.
Right-heart catheterization
Right-heart catheterization was used at 3–5 days
after CTPA. By using the Seldinger technique, an 8F Swan-Ganz
catheter (Baxter Healthcare, Irvine, CA, USA) was introduced
through a right internal jugular vein and positioned under
fluoroscopic guidance in a pulmonary artery. After a 10-min rest
for stabilization, hemodynamic parameters including right atrial
pressure (RAP), pulmonary arterial systolic pressure (sPAP),
pulmonary artery diastolic pressure (dPAP), pulmonary capillary
wedge pressure (PCWP) and cardiac output (CO) were obtained at
end-expiration, then the mPAP and PVR were calculated. The PVR was
calculated as described by Tramarin et al(18). CO was determined by the
thermodilution method (19), with
the exception of two cases of congenital heart disease-associated
PAH where the Fick method (20)
was used to determine CO, as the mean of three consecutive
measurements not varying by >10%.
Plasma level of NT-proBNP
When we performed pulmonary angiography and
right-heart catheterization, a blood sample was drawn from the
pulmonary artery and immediately transferred into
ethylenediaminetetraacetic acid-glass tubes and centrifuged at
3,000 rpm for 15 min at 4°C. The level of NT-proBNP was determined
by an enzyme-linked sandwich immunoassay (Elecsys NT-proBNP; Roche
Diagnostics, Indianapolis, IN, USA).
Statistical analysis
All data are expressed as mean ± standard deviation
(SD), unless otherwise specified. All analyses were performed with
a statistical package (SPSS 13; SPSS, Inc., Chicago, IL, USA). A
Mann-Whitney U test was used to compare the Septal angle in the PH
and normal control groups. The association of Septal angle with
clinical parameters was calculated by univariate analysis. The
correlations of Septal angle with NT-proBNP and hemodynamics were
analyzed with the Spearman's correlation. Stepwise linear
regression analysis was used to evaluate the predictive power of
independent CTPA variables for PVR. The receiver-operating
characteristic (ROC) method was used to analyze the cut-off value
of Septal angle and NT-proBNP for assessing PVR ≥1,000
(dyn.sec/cm5). All P-values were for 2-sided tests.
P<0.05 was considered to indicate a statistically significant
result.
Results
Characteristics of the study
population
The clinical characteristics of the PH group (age,
14–84 years; median, 51 years) are shown in Table I. The average Septal angle in the
PH group was 65.33±11.93° with a range of 40.00–97.30° (Fig. 1A) and all patients had clear signs
of PAH as revealed in Table I. In
the control group, the average Septal angle was 40.47±6.11° with
range of 25.50–54.50° and a median of 40.25° (Fig. 1B). As shown in Fig. 2, the Septal angle in the PH group
was significantly higher than that in the control group
(Mann-Whitney U test, U=280.5, P=0.000).
 | Table IClinical and hemodynamic
characteristics of patients with PH. |
Table I
Clinical and hemodynamic
characteristics of patients with PH.
| Characteristics | Values |
|---|
| Baseline parameters
(mean ± SD) |
| Age (years) | 50.86±13.40 |
| Gender
(male/female) | 53/53 |
| Body mass index
(kg/m2) | 23.44±3.12 |
| Body surface area
(m2) | 1.73±0.18 |
| NT-proBNP
(pg/ml) | 1716.09±1498.30 |
| Septal angle
(degree) | 65.32±11.93 |
| Clinical
classification of PH (n) |
| CTEPH | 76 |
| Idiopathic
PAH | 24 |
| Connective tissue
diseases associated |
| PAH | 2 |
| Heritable PAH | 2 |
| PAH associated
with congenital heart disease | 2 |
| Class of PAP
(n) |
| Mild (25–39
mmHg) | 17 |
| Moderate (40–69
mmHg) | 70 |
| Severe (≥70
mmHg) | 19 |
| NYHA classification
(n) |
| I | 6 |
| II | 44 |
| III | 46 |
| IV | 10 |
| Hemodynamics (mean
± SD) |
| RAP (mmHg) | 7.78±6.11 |
| sPAP (mmHg) | 86.14±23.70 |
| dPAP (mmHg) | 33.11±12.63 |
| mPAP (mmHg) | 51.58±15.65 |
| CO (l/min) | 3.47±1.36 |
| PCWP (mmHg) | 9.13±3.72 |
| PVR
(dynes.sec.mm−5) | 1121.09±582.37 |
No correlation was observed between the Septal angle
and age (r=0.101, P=0.307), BMI (r=−0.120, P=0.221) or body surface
area (r=−0.150, P=0.127). The Septal angle in males and females was
65.86±13.38 and 64.78±10.36°, respectively, which has no
significant difference (Mann-Whitney U test; U=1370.0, P=0.959).
The Septal angle in CTEPH and PAH groups was 65.76±12.25 and
64.19±11.19°, respectively, and there was no difference in Septal
angle between the two groups (Mann-Whitney U test; U=1053.5,
P=0.728). The Septal angle was weakly correlated with the class of
PAP (r=0.278, P=0.004; Fig. 3A)
and New York Heart Association (NYHA) classification (r=0.255,
P=0.009; Fig. 3B). No difference
in hemodynamic parameters was identified between CTEPH and PAH, as
shown in Table II.
 | Table IIComparison of Septal angle and
hemodynamics in CTEPH and PAH. |
Table II
Comparison of Septal angle and
hemodynamics in CTEPH and PAH.
| Parameters | CTEPH | PAH | Ua | P-value |
|---|
| Septal angle
(°) | 65.76±12.25 | 64.19±11.19 | 1053.5 | 0.728 |
| RAP (mmHg) | 8.04±5.90 | 7.13±6.68 | 993.5 | 0.303 |
| sPAP (mmHg) | 85.62±20.61 | 91.00±30.24 | 1030.0 | 0.440 |
| dPAP (mmHg) | 31.71±9.97 | 36.67±17.40 | 921.0 | 0.124 |
| mPAP (mmHg) | 50.00±12.73 | 55.56±21.08 | 939.0 | 0.158 |
| PCWP (mmHg) | 8.79±2.97 | 9.96±5.12 | 1054.0 | 0.612 |
| CO (l/min) | 3.42±1.22 | 3.62±1.36 | 1033.0 | 0.453 |
| PVR
(dynes.sec.mm−5) | 1113.66±564.96 | 1139.93±673.60 | 1106.0 | 0.812 |
Correlation of the Septal angle with
hemodynamics and NT-proBNP in PH
Correlations between the Septal angle and the
hemodynamic parameters evaluated by right-heart catheterization
(Table III) showed that the
Septal angle strongly correlated with PVR (r=0.642, P=0.000),
moderately correlated with CO (r=−0.535, P=0.000) and cardiac index
(CI; r=−0.534, P=0.000) and weakly correlated with RAP (r=0.255,
P=0.009), sPAP (r=0.258, P=0.008), dPAP (r=0.275, P=0.005) and mPAP
(r=0.294, P=0.002), but did not correlate with pulse oxygen
saturation (SPO2; r=0.015, P=0.885) or PCWP (r=−0.025,
P=0.803). Correlations between the CTPA variables and PVR (Table IV) suggest that PVR strongly
correlated with the Septal angle and moderately correlated with
RVa/LVa (r=0.537, P=0.000) and RVd/LVd (r=0.479, P=0.000).
 | Table IIICorrelations between Septal angle and
hemodynamic parameters in PH and its subgroups. |
Table III
Correlations between Septal angle and
hemodynamic parameters in PH and its subgroups.
| Spearman's
correlation analysis (r, P-value) |
|---|
|
|
|---|
| Hemodynamics | PH (n=106) | CTEPH (n=76) | PAH (n=30) |
|---|
| RAP | 0.255, 0.009 | 0.301, 0.008 | 0.105, 0.587 |
| sPAP | 0.258, 0.008 | 0.209, 0.070 | 0.304, 0.030 |
| dPAP | 0.275, 0.005 | 0.251, 0.029 | 0.387, 0.038 |
| mPAP | 0.294, 0.002 | 0.256, 0.026 | 0.343, 0.016 |
| PCWP | −0.025, 0.803 | −0.053, 0.655 | −0.221, 0.249 |
| CO | −0.535, 0.000 | −0.586, 0.000 | −0.553, 0.000 |
| CI | −0.534, 0.000 | −0.595, 0.000 | −0.561, 0.000 |
| PVR | 0.642, 0.000 | 0.676, 0.000 | 0.623, 0.000 |
|
SPO2 | 0.015, 0.885 | 0.022, 0.853 | 0.119, 0.540 |
 | Table IVCorrelations between CTPA variables
and PVR in patients with PH. |
Table IV
Correlations between CTPA variables
and PVR in patients with PH.
| CTPA
parameters | Correlation
coefficienta | P-value |
|---|
| Septal angle | 0.642 | 0.000 |
| RVa/LVa | 0.537 | 0.000 |
| RVd/LVd | 0.479 | 0.000 |
| RAd | 0.321 | 0.010 |
| RVa | 0.248 | 0.013 |
| RVd | 0.240 | 0.016 |
| MPAd/AAd | 0.217 | 0.027 |
| IVST | −0.139 | 0.163 |
When all CTPA variables were analyzed in a stepwise
forward regression analysis, the Septal angle and RVa/LVa were
entered into the final equation for predicting PVR, as shown in
Table V, leading to the following
two equations: i) PVR = 28.256 × Septal angle - 728.72; and ii) PVR
= 23.005 × Septal angle + 207.992 × RVa/LVa - 718.69. The level of
NT-proBNP was 1,716.09±1,498.30 pg/ml with a range of
19.79–5,909.00 pg/ml. Correlations between CTPA variables and
NT-proBNP (Fig. 4) demonstrated
that NT-proBNP strongly correlated with the Septal angle (r=0.693,
P=0.000) and moderately correlated with RVa/LVa (r=0.520, P=0.000),
RAd (r=0.447, P=0.000) and RVd/LVd (r=0.395, P=0.000).
 | Figure 4Correlation of computed tomographic
pulmonary angiography (CTPA) variables with the level of NT-proBNP.
(A) Correlation between Septal angle and NT-proBNP (r=0693,
P=0.000); (B) correlation between RVa/LVa and NT-proBNP (r=0.520,
P=0.000); (C) correlation between RAd and NT-proBNP (r=0.477,
P=0.000); (D) correlation between RVd/LVd and NT-proBNP (r=0.395,
P=0.000). NT-proBNP, N-terminal pro-B-type natriuretic peptide;
RVa, right ventricular area; LVa, left ventricular area; RAd,
transverse diameter of right atrium; RVd, transverse diameter of
right ventricle; LVd, transverse diameter of left ventricle. |
 | Table VStepwise linear regression analysis
of the relation between CTPA predictions and PVR. |
Table V
Stepwise linear regression analysis
of the relation between CTPA predictions and PVR.
| Modela | R | Predictorsb | F-value | B | t | Significance |
|---|
| Model 1 | 0.588 | Septal angle | 52.351 | 28.256 | 7.235 | 0.000 |
| | (constant) | | −728.740 | −2.811 | 0.000 |
| Model 2 | 0.631 | Septal angle | 32.363 | 23.005 | 5.504 | 0.000 |
| | RVa/LVa | | 207.992 | 2.905 | 0.005 |
| | (constant) | | −718.690 | −2.784 | 0.005 |
Association of Septal angle and
hemodynamics, NT-proBNP in CTEPH
In the CTEPH group, Spearman's correlations between
the Septal angle and hemodynamic data evaluated by right-heart
catheterization (Table III)
showed that the Septal angle had a strong correlation with PVR
(r=0.676, P=0.000), a moderate correlation with CO (r=−0.586,
P=0.000) and CI (r=−0.595, P=0.000), a weak correlation with RAP
(r=0.301, P=0.008), dPAP (r=0.251, P=0.029) and mPAP (r=0.256,
P=0.026), but had no correlation with SPO2 (r=0.022,
P=0.853), sPAP (r=0.209, r=0.070) or PCWP (r=−0.053, P=0.655). The
level of plasma NT-proBNP in CTEPH was 1,809.52±1,532.16 pg/ml with
a range of 19.79–5,909.00 pg/ml, and the Septal angle had a strong
correlation with NT-proBNP (r=0.668, P=0.000).
Correlation of Septal angle and
hemodynamics, NT-proBNP in PAH
In the PAH group, Spearman's correlations between
Septal angle and hemodynamic data evaluated by right-heart
catheterization (Table III)
showed that the Septal angle strongly correlated with PVR (r=0.623,
P=0.000), moderately correlated with CO (r=−0.553, P=0.000) and CI
(r=−0.561, P=0.000), weakly correlated with sPAP (r=0.304,
P=0.030), dPAP (r=0.387, P=0.038) and mPAP (r=0.343, P=0.016), but
did not correlate with SPO2 (r=0.119, P=0.540), RAP
(r=0.105, P=0.587) or PCWP (r=−0.221, P=0.249). The level of plasma
NT-proBNP in PAH was 1,582.43±1,387.30 pg/ml with a range of
83.10–4,453.00 pg/ml and the Septal angle had a strong correlation
with the level of NT-proBNP (r=0.616, P=0.003).
ROC analysis
In PH, as shown in ROC analysis (Fig. 5), a Septal angle cut-off point of
≥67.55° had a 77.1% sensitivity and a 87.5% specificity for
predicting PVR ≥1,000 (dynes.sec.mm−5); its area under
the curve (AUC) was 0.850±0.040. An NT-proBNP cut-off point ≥1,443
pg/ml had a 75.0% sensitivity and a 75.0% specificity for
predicting PVR ≥1,000 (dynes.sec.mm−5); its AUC was
0.808±0.046, comparable or even inferior to the AUC of the Septal
angle for predicting PVR ≥1,000 (dynes.sec.mm−5).
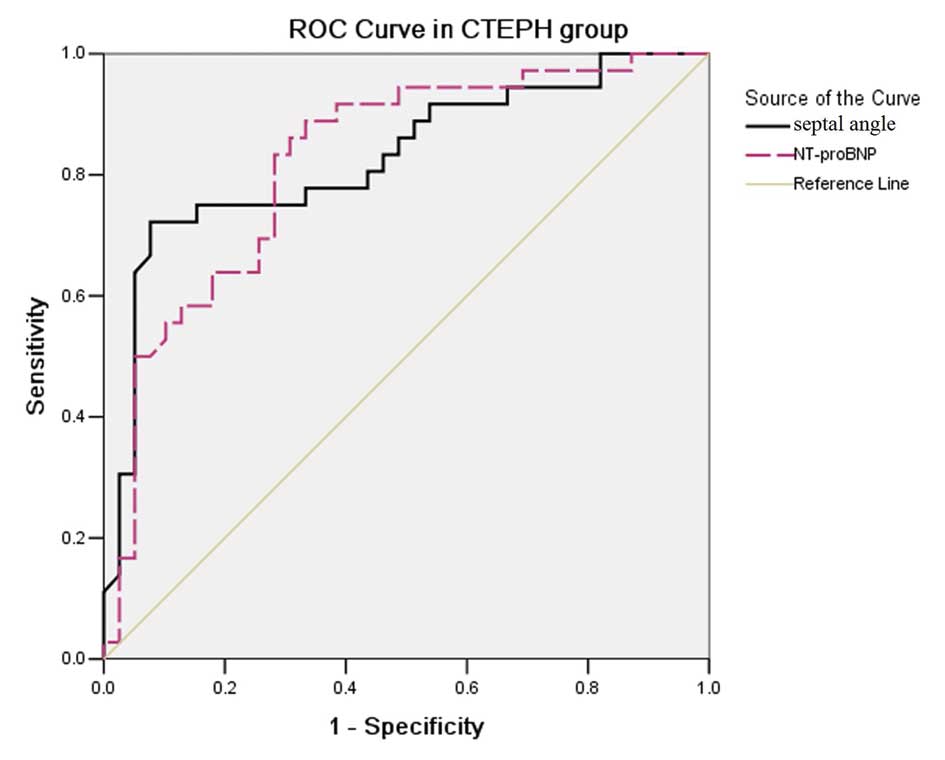
In the CTEPH group, ROC analysis (Fig. 6) demonstrated that a Septal angle
cut-off point of ≥67.55° had a 75.0% sensitivity and a 84.6%
specificity for predicting PVR ≥1,000 (dynes.sec.mm−5),
and its AUC was 0.827±0.050. An NT-proBNP cut-off point of ≥1,443
pg/ml had a 83.3% sensitivity and a 71.8% specificity for
predicting PVR ≥1,000 (dynes.sec.mm−5), and its AUC was
0.822±0.049, comparable to the AUC of the Septal angle for
predicting PVR ≥1,000 (dynes.sec.mm−5).
Discussion
Our prior studies suggest that CTPA clearly
describes the obstructed pulmonary artery (15) and the cardiovascular parameters of
CTPA may be used to evaluate the hemodynamics in CTEPH (16,17).
In the present study, we analyzed the correlation of Septal angle
with the hemodynamics and the level of NT-proBNP in patients with
PH and its two subgroups, CTEPH and PAH. We demonstrated that: i)
the Septal angle in PH patients is larger than that in the normal
control, but weakly correlated with PAP. ii) the Septal angle is
strongly correlated with PVR and NT-proBNP in PH and its two
subgroups (CTEPH and PAH). iii) In CTEPH, a Septal angle cut-off
value of 67.55 has a sensitivity of 77% and a specificity of 81% in
predicting PVR ≥1,000 (dyn.sec/cm5), comparable to the
level of NT-proBNP. iv) In PH, a Septal angle cut-off value of
67.55° is comparable to the NT-proBNP cut-off value of 1,443 pg/ml
in predicting PVR ≥1,000 (dyn.sec/cm5).
Septal angle is a CTPA-derived parameter that is an
angle between the connection line from the midpoint of the sternum
to thoracic vertebral spinous process and interventricular septum.
This angle represents the balance of left and right ventricular
load. Our results revealed that in the control group, the right
ventricular (RV) pressure is lower than the left ventricular (LV)
pressure; the mean Septal angle is 40.47° (range 25.50–54.50°) and
the Septal angle in the PH group is significantly higher than that
in the control group, but there is no difference in the CTEPH and
PAH groups. This suggests the increased Septal angle is a predictor
of PH, and is not affected by the etiology of PH. We observed that
Septal angle only weakly increased with the extent of PAP. This
suggests PAP does not directly impact on the Septal angle. Although
the Septal angle in PH was not affected by age, BMI or body surface
area, there was an overlap of Septal angle between PH and the
control group, so the normal value of the Septal angle requires
validation in a large population. During the progress of PH, the
marked increase in PVR limits the rate at which the right ventricle
is able to pump blood through the lungs, leading to RV overload,
hypertrophy and dilatation (21–24).
RV overload causes flattening and leftward displacement of the
interventricular septum, and cardiac clockwise rotation, so we
suggest that the enlarged septal angle is a sign of the right
ventricular overload in patients with PH (23,24).
Resting hemodynamics measured by right-heart
catheterization may assess the severity and predict the prognosis
of PH (2,3,25).
PVR is one of the most important parameters in this, since the
increased PVR leads to right ventricular overload and dysfunction.
Previous studies demonstrated that parameters such as RVd/LVd
correlated with hemodynamic data (16,17).
There is no data available so far associating the Septal angle with
the resting hemodynamics of patients with PH. We observed a strong
correlation of Septal angle and a moderate correlation of RVd/LVd
with PVR in PH patients. By a stepwise regression analysis, the
Septal angle was entered into a final equation for predicting PVR.
This suggests the Septal angle may be a superior indicator for
evaluating PVR than RVd/LVd. NT-proBNP is released from myocytes
and may be used as an indicator of the severity of PH (26). The very high serum NT-proBNP
concentrations observed in the patients with PH who were studied
most likely result from an increased RV wall stretch and marked
hypertrophy of the RV walls (27–29).
Our data indicated that the Septal angle strongly correlated with
NT-proBNP and demonstrated that the Septal angle may also be a
superior predictor of severity and RV dysfunction in patients with
PH.
PVR was critical in the assessment of CTEPH for its
importance in the prediction of potential candidates for pulmonary
endarterectomy and postoperative outcome (30) and NT-proBNP has been used as a
noninvasive marker of the severity of right ventricular dysfunction
in CTEPH (31). Although we did
not analyze the threshold of the Septal angle in predicting the
increased PVR and NT-proBNP in a large population, our results
showed that the Septal angle strongly correlated with PVR and
NT-proBNP in the CTEPH group. Jamieson et al(32) reported that patients with a
preoperative PVR >1,000 (dynes.sec.mm−5) had a
significantly higher mortality rate than those with a preoperative
PVR <1,000 (dynes.sec.mm−5), so we selected 1,000
(dynes.sec.mm−5) as the threshold of PVR. We assessed
the value of the Septal angle in predicting PVR ≥1,000
(dynes.sec.mm−5). Using ROC curve analysis, Septal angle
>67.55° demonstrated superior sensitivity and specificity with a
higher AUC to identify PVR ≥1,000 (dynes.sec.mm−5) and
there was no difference in the AUC between the Septal angle and
NT-proBNP level, suggesting that Septal angle and NT-proBNP have
similar value in predicting a PVR ≥1,000
(dynes.sec.mm−5). These findings may be of practical
importance for patients with CTEPH, CTPA not only demonstrates the
distribution of clots but also supplies the information of the
operability of pulmonary endarterectomy and predicts the survival
of patients.
Hemodynamic data and biomarkers aid the diagnosis
and assessment of PAH (33).
Mukerjee et al(34) showed
that NT-proBNP levels correlated significantly with PVR in
scleroderma patients with PAH. Souza et al(35) showed NT-proBNP levels had a high
correlation with hemodynamic parameters, particularly PVR in
patients with idiopathic pulmonary artery hypertension (IPAH). Our
study also showed that the Septal angle was strongly correlated
with CO, PVR and NT-proBNP in PAH patients, suggesting that the
severity of PAH or right heart strain as measured by hemodynamic
data may be estimated using the Septal angle. However, further
observation is required due to the relatively small number in this
group. Fijalkowska et al(6)
demonstrated that a NT-proBNP cut-off point at ≥1,400 pg/ml was
useful in identifying PH patients with poor long-term prognosis.
Notably, using ROC analysis, we observed a serum NT-proBNP level of
≥1,433 pg/ml has a similar value to a Septal angle of ≥67.5° for
predicting the PVR ≥1,000 (dynes.sec.mm−5) in PH and
CTEPH patients, suggesting the predictive value of a Septal angle
≥67.5° may also be used to evaluate the long-term prognosis in PH
patients.
Although the present observations indicate that the
CTPA-derived Septal angle is a useful parameter for evaluating the
severity and RV dysfunction in PH, there are possible limitations.
First, the retrospective design of our study allows for less
generalization from our results, so although the Septal angle
correlated with PVR and NT-proBNP, the correlations require further
verification by prospective observations. Secondly, although the
Septal angle appears to be useful in evaluating hemodynamics in
patients with CTEPH, it is unclear whether operability and surgical
success, defined as mortality and/or improvement of PVR, may be
predicted with sufficient accuracy since only 14 patients underwent
PEA. Our observations require verification from future studies of
larger numbers of patients to determine the usefulness of
preoperative CTPA in identifying RV dysfunction in high-risk CTEPH
patients and the association with postoperative hemodynamic
outcome, RV failure and mortality. Thirdly, the Septal angle
appears to be useful in evaluating the severity of PH and a Septal
angle ≥67.5° also may be used to evaluate the long-term prognosis.
However, the lack of prognostic data in a follow-up period may be
considered a major limitation of this study. The cut-off value of
septal angle is able to indicate the right ventricular function and
may assess the prognosis of patients with pulmonary hypertension.
However, this would require a larger and homogeneous cohort,
considering the numerous therapeutic options available at
present.
In summary, our results suggest that the measurement
of the Septal angle by CPTA is simple, noninvasive and may be a
useful parameter to assess the severity and RV dysfunction in PH
and its two subgroups.
Acknowledgements
The authors thank Dr Renyou Zhai for helpful
comments regarding hemodynamics and Dr Xiaoxia Peng for advice on
data management and for valuable suggestions about statistical
analysis. This study is supported by the Beijing Clinical Medical
Research Foundation (Z121107001012126).
References
|
1
|
McLaughlin VV, Archer SL, Badesch DB, et
al: ACCF/AHA 2009 expert consensus document on pulmonary
hypertension: a report of the American College of Cardiology
Foundation Task Force on Expert Consensus Documents and the
American Heart Association developed in collaboration with the
American College of Chest Physicians; American Thoracic Society,
Inc.; and the Pulmonary Hypertension Association. J Am Coll
Cardiol. 53:1573–1619. 2009.
|
|
2
|
Galiè N, Hoeper MM, Humbert M, et al; ESC
Committee for Practice Guidelines (CPG). Guidelines for the
diagnosis and treatment of pulmonary hypertension: the Task Force
for the Diagnosis and Treatment of Pulmonary Hypertension of the
European Society of Cardiology (ESC) and the European Respiratory
Society (ERS), endorsed by the International Society of Heart and
Lung Transplantation (ISHLT). Eur Heart J. 30:2493–2537. 2009.
|
|
3
|
Guillinta P, Peterson KL and Ben-Yehuda O:
Cardiac catheterization techniques in pulmonary hypertension.
Cardiol Clin. 22:401–415. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Seino Y, Ogawa A, Yamashita T, et al:
Application of NT-proBNP and BNP measurements in cardiac care: a
more discerning marker for the detection and evaluation of heart
failure. Eur J Heart Fail. 6:295–300. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pruszczyk P: N-terminal pro-brain
natriuretic peptide as an indicator of right ventricular
dysfunction. J Card Fail. 11(5 Suppl): S65–S69. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fijalkowska A, Kurzyna M, Torbicki A, et
al: Serum N-terminal brain natriuretic peptide as a prognostic
parameter in patients with pulmonary hypertension. Chest.
129:1313–1321. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Casserly B and Klinger JR: Brain
natriuretic peptide in pulmonary arterial hypertension: biomarker
and potential therapeutic agent. Drug Des Devel Ther. 29:269–287.
2009.PubMed/NCBI
|
|
8
|
Hoey ET, Gopalan D, Agrawal SK and
Screaton NJ: Cardiac causes of pulmonary arterial hypertension:
assessment with multidetector CT. Eur Radiol. 19:2557–2568. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ghuysen A, Ghaye B, Willems V, et al:
Computed tomographic pulmonary angiography and prognostic
significance in patients with acute pulmonary embolism. Thorax.
60:956–961. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Collomb D, Paramelle PJ, Calaque O, et al:
Severity assessment of acute pulmonary embolism: evaluation using
helical CT. Eur Radiol. 13:1508–1514. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nural MS, Elmali M, Findik S, et al:
Computed tomographic pulmonary angiography in the assessment of
severity of acute pulmonary embolism and right ventricular
dysfunction. Acta Radiol. 50:629–637. 2009. View Article : Google Scholar
|
|
12
|
Johnson TR, Nikolaou K, Becker A, Leber
AW, Rist C, Wintersperger BJ, Reiser MF and Becker CR: Dual-source
CT for chest pain assessment. Eur Radiol. 18:773–780.
2008.PubMed/NCBI
|
|
13
|
Revel MP, Faivre JB, Remy-Jardin M,
Delannoy-Deken V, Duhamel A and Remy J: Pulmonary hypertension:
ECG-gated 64-section CT angiographic evaluation of new functional
parameters as diagnostic criteria. Radiology. 250:558–566. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Doğan H, Kroft LJ, Huisman MV, van der
Geest RJ and de Roos A: Right ventricular function in patients with
acute pulmonary embolism: analysis with
electrocardiography-synchronized multi-detector row CT. Radiology.
242:78–84. 2007.PubMed/NCBI
|
|
15
|
Liu M, Ma Z, Guo X, Zhang H, Yang Y and
Wang C: Computed tomographic pulmonary angiography in the
assessment of severity of chronic thromboembolic pulmonary
hypertension and right ventricular dysfunction. Eur J Radiol.
80:e462–e469. 2011. View Article : Google Scholar
|
|
16
|
Liu M, Ma ZH, Guo XJ, et al: A septal
angle measured on computed tomographic pulmonary angiography can
noninvasively estimate pulmonary vascular resistance in patients
with chronic thromboembolic pulmonary hypertension. J Thorac
Imaging. 27:325–330. 2012. View Article : Google Scholar
|
|
17
|
Liu M, Ma Z, Guo X, Chen X, Yang Y and
Wang C: Cardiovascular parameters of computed tomographic pulmonary
angiography to assess pulmonary vascular resistance in patients
with chronic thromboembolic pulmonary hypertension. Int J Cardiol.
164:295–300. 2013. View Article : Google Scholar
|
|
18
|
Tramarin R, Torbicki A, Marchandise B,
Laaban JP and Morpurgo M; Working Group on Noninvasive Evaluation
of Pulmonary Artery Pressure. European Office of the World Health
Organization, Copenhagen. Doppler echocardiographic evaluation of
pulmonary artery pressure in chronic obstructive pulmonary disease.
A European multicentre study. Eur Heart J. 12:103–111. 1991.
|
|
19
|
Ganz W, Donoso R, Marcus HS, Forrester JS
and Swan HJ: A new technique for measurement of cardiac output by
thermodilution in man. Am J Cardiol. 27:392–396. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McMichael J and Sharpey-Schafer EP:
Cardiac output in man by a direct fick method: Effects of posture,
venous pressure change, atropine, and adrenaline. Br Heart J.
6:33–40. 1944. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bogaard HJ, Abe K, Vonk Noordegraaf A and
Voelkel NF: The right ventricle under pressure: cellular and
molecular mechanisms of right-heart failure in pulmonary
hypertension. Chest. 135:794–804. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Delcroix M, Vonk Noordegraaf A, Fadel E,
Lang I, Simonneau G and Naeije R: Vascular and right ventricular
remodelling in chronic thromboembolic pulmonary hypertension. Eur
Respir J. 41:224–232. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sitbon O, Humbert M, Nunes H, et al:
Long-term intravenous epoprostenol infusion in primary pulmonary
hypertension: prognostic factors and survival. J Am Coll Cardiol.
40:780–788. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Raymond RJ, Hinderliter AL, Willis PW,
Ralph D, et al: Echocardiographic predictors of adverse outcomes in
primary pulmonary hypertension. J Am Coll Cardiol. 39:1214–1219.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McGoon M, Gutterman D, Steen V, Barst R,
et al: Screening, early detection, and diagnosis of pulmonary
arterial hypertension: ACCP evidence based clinical practice
guidelines. Chest. 126(1 Suppl): 14S–34S. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Andreassen AK, Wergeland R, Simonsen S,
Geiran O, Guevara C and Ueland T: N-terminal pro-B-type natriuretic
peptide as an indicator of disease severity in a heterogeneous
group of patients with chronic precapillary pulmonary hypertension.
Am J Cardiol. 98:525–529. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nagaya N, Nishikimi T, Okano Y, et al:
Plasma brain natriuretic peptide levels increase in proportion to
the extent of right ventricular dysfunction in pulmonary
hypertension. J Am Coll Cardiol. 31:202–208. 1998. View Article : Google Scholar
|
|
28
|
McDonagh TA, Holmer S, Raymond I, Luchner
A, Hildebrant P and Dargie HJ: NT-proBNP and the diagnosis of heart
failure: a pooled analysis of three European epidemiological
studies. Eur J Heart Fail. 6:269–273. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yap LB, Ashrafian H, Mukerjee D, Coghlan
JG and Timms PM: The natriuretic peptides and their role in
disorders of right heart dysfunction and pulmonary hypertension.
Clin Biochem. 37:847–856. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
D'Armini AM, Cattadori B, Monterosso C, et
al: Pulmonary thromboendarterectomy in patients with chronic
thromboembolic pulmonary hypertension: hemodynamic characteristics
and changes. Eur J Cardiothorac Surg. 18:696–702. 2000. View Article : Google Scholar
|
|
31
|
Reesink HJ, Tulevski II, Marcus JT,
Boomsma F, et al: Brain natriuretic peptide as noninvasive marker
of the severity of right ventricular dysfunction in chronic
thromboembolic pulmonary hypertension. Ann Thorac Surg. 84:537–543.
2007. View Article : Google Scholar
|
|
32
|
Jamieson SW, Kapelanski DP, Sakakibara N,
et al: Pulmonary endarterectomy: experience and lessons learned in
1,500 cases. Ann Thorac Surg. 76:1457–1464. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Badesch DB, Champion HC, Sanchez MA, et
al: Diagnosis and assessment of pulmonary arterial hypertension. J
Am Coll Cardiol. 54(1 Suppl): S55–S66. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mukerjee D, Yap LB, Holmes AM, Nair D, et
al: Significance of plasma N-terminal pro-brain natriuretic peptide
in patients with systemic sclerosis-related pulmonary arterial
hypertension. Respir Med. 97:1230–1236. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Souza R, Jardim C, Julio Cesar Fernandes
C, Silveira Lapa M, et al: NT-proBNP as a tool to stratify disease
severity in pulmonary arterial hypertension. Respir Med. 101:69–75.
2007. View Article : Google Scholar : PubMed/NCBI
|