Introduction
Diabetes is a serious chronic disease threatening
human health. The incidence of type II diabetes is increasing
rapidly on a global scale, and the population with the disease is
expected to rise from 190 million in 2000 to 360 million in 2030
(1). Of the 285 million patients
with diabetes worldwide (2), the
prevalence rate of adult type II diabetes is 9.7%, including a
20.4% prevalence rate in individuals >60 years of age (3). Type II diabetes is closely associated
with dietary factors and cohort studies have observed that the
intake of dairy products reduces the risk of type II diabetes
occurring in healthy individuals (4,5). The
related meta-analysis further indicated that an intake of dairy
products is able to reduce the risk of the prevalence of type II
diabetes, and it has been suggested that the intake of whey protein
may increase the sensitivity to insulin in normal mice and improve
lipid metabolism (6). In addition
to impacting the metabolism of glucose and lipids, diabetes also
affects the metabolism of protein and amino acids. The uptake of
glucose and the insulin activity of skeletal muscles may be
increased when the body’s amino acid concentration is sufficient,
further promoting the synthetic metabolism of proteins (7). As such, the intake of whey protein
increases the synthesis of muscle proteins (8). Amino acids are crucial materials in
the activities of the body. As signaling molecules, amino acids
have been shown to be important in the signal transduction of
insulin secretion and glucose metabolism (9). In the present study, the amino acid
levels in the whey proteins and the plasma of diabetic mice were
analyzed to further investigate the mechanism of action of whey
proteins on the prevention and treatment of type II diabetes.
The common methods used to detect amino acids are
high-pressure liquid chromatography (HPLC), amino acid analyzers
and multiple technologies involving liquid chromatography
mass-spectrometry and mass spectrometry. Amino acid analyzers are
simple to operate, do not require pre-column derivatization and may
be used for batch testing; however, the cost is high, the detection
time is relatively long and the resolution is low. The multiple
technologies involving liquid chromatography-mass spectrometry and
mass spectrometry are convenient and accurate; however, the cost is
high. The HPLC method has numerous advantages, including a low
cost, good reproducibility and high sensitivity, and, therefore,
HPLC is often used as a general method for the detection of amino
acids (10–12). In the present study, HPLC was used
to detect the composition and content of the amino acids in whey
protein and the plasma amino acid levels of mice were analyzed
following gavage with whey protein. Furthermore, the mechanism of
action of whey protein in the prevention of diabetes was explored.
Different concentrations of whey protein were administered to ICR
mouse models of type I and II diabetes. The composition and content
of amino acids in whey protein were detected by HPLC, and the
effect of the whey protein on plasma amino acid levels in the
diabetic mice was subsequently analyzed.
Materials and methods
Experimental animals
Eight-week-old male ICR mice of specific pathogen
free (SPF) class were purchased from the Shanghai Laboratory Animal
Center, Chinese Academy of Sciences (Shanghai, China). The mice
were raised in a 12-h light/dark cycle in standardized animal rooms
with 40–70% relative humidity and a continuous room temperature of
18–22°C. The animal use protocol was reviewed and approved by the
Institutional Animal Care and Use Committee (IACUC) of Shanghai
Jiao Tong University School of Medicine (Shanghai, China).
Model preparation
Solutions of streptozotocin (STZ; Sigma, St. Louis,
MO, USA) were prepared using 0.1 mol/l citrate buffer (pH 4.2).
Sodium citrate (2.1 g) was dissolved in 100 ml double distilled
water to prepare solution A and 2.94 g trisodium citrate was
dissolved in 100 ml double distilled water to prepare solution B.
Solutions A and B were subsequently mixed with a volume ratio of
1:1.321 and the pH value was adjusted to 4.2 using 10% aqueous
sodium bicarbonate, in order to prepare the desired citrate buffer.
STZ (100 mg) was subsequently dissolved in 10 ml citrate buffer and
filter-sterilized, prior to use. The entire preparation process was
performed in an ice box.
Animals were acclimated for one week prior to the
experiment. A single intraperitoneal injection of STZ (100 mg STZ
solution per 1 kg body weight) was administered to the ICR mice, in
order to prepare the type II diabetes model. The dose was 160 mg/kg
for the preparation of the type I diabetes model (7,8).
When the fasting glucose concentration was detected to be >5
mmol/l, three days later, the model was considered to be
successfully prepared to meet the requirements of the
experiment.
Animal grouping and gavage
Three groups of mice were used in the experiment:
Normal, a type I diabetes model and a type II diabetes model. The
32 mice in each group were then divided into four subgroups (n=8).
Solutions of whey protein (0.5 ml; Australian concentrated whey
protein powder with 80% purity; Davisco Foods International, Inc.,
Eden Prairie, MN, USA) with concentrations of 0, 10, 20 and 40%
were subsequently administered to the mice in each subgroup,
respectively. The aqueous solutions of whey proteins were prepared
and administered at ~4:00 pm every day for four weeks; the
experiment had a duration of four weeks. The eyeballs were then
removed following the fasting of the mice and the blood plasma was
cryopreserved.
HPLC analysis
Solution preparation
Triethylamine solution was prepared by mixing 1.4 ml
triethylamine with 8.6 ml acetonitrile and phenethyl isothiocyanate
(PITC) acetonitrile solution was prepared by mixing one bottle (25
μl) of phenethyl isothiocyanate fat with 2 ml acetonitrile. For the
mobile phase A, 15.2 g sodium acetate was dissolved in 1,850 ml
water and the pH value was adjusted to 6.5 using glacial acetic
acid. Following this, 140 ml acetonitrile was added and the
resulting composition was mixed and filtered using a 0.45-μm
membrane. For mobile phase B, 80% acetonitrile was used. In order
to prepare the norleucine internal standard solution, 10 mg
norleucine was dissolved and mixed in 10 ml 0.1 mol/l hydrochloric
acid solution. The pure acetonitrile used in the HPLC was obtained
from Concord Technology Co., Ltd. (Tianjin, China).
Sample treatments
Whey protein powder (2 mg) was dissolved in 1 ml
Tris-HCI (pH 8.0), in order to prepare 2 mg/ml whey protein
solution. To hydrolyze the whey proteins, 3.5 μl trypsin, 10 μl
peptidase and 100 μl α-chymotrypsin were added to the prepared
solution. The solution was mixed and warmed at 37°C in a water bath
for 24 h and then stored at 4°C prior to use. To obtain a
precipitate of the plasma proteins, 80 μl plasma samples were added
to 100 μl perchloric acid with a concentration of 12 mol/l and
centrifuged at 1,000 × g for 10 min, prior to the supernatant being
transferred to a new centrifuge tube and stored at 4°C for later
use.
Derivatization of the standard and
sample solutions
Aliquots (200 μl) of the amino acid standard
solutions and sample solutions were accurately measured and added
to 1.5 ml Eppendorf tubes. An internal standard solution of
norleucine (20 μl), 100 μl triethylamine acetonitrile and 100 μl
PITC fat acetonitrile were added. After mixing and reacting at room
temperature for 1 h, 400 μl n-hexane was added. The resulting
mixture was then incubated with shaking for 10 min, prior to the
derivative products (phenylthiocarbamoyl-amino acid solution,
PTC-AA) being collected from the underlayer, filtered with a
0.45-μm needle filter and stored at 4°C for use. The amino acid
analysis package was from Tianjin Bonna-Agela Technologies Co.,
Ltd. (Tianjin, China).
Chromatography conditions and the
separation and determination of amino acids
The column used was a Venusil-AA amino acid analysis
column measuring 4.6 mm × 250 mm × 5 μm and was obtained from
Tianjin Bonna-Agela Technologies Co., Ltd. Wavelength detection was
performed at 254 nm and the column temperature was 40°C. The mobile
phase gradient is shown in Table
I. The separation and determination of the amino acids was
performed by injecting 2 μl filtrate into the HPLC column, and
monitoring the chromatograms.
 | Table IGradient of the mobile phase. |
Table I
Gradient of the mobile phase.
| Time (min) | Mobile phase A
(%) | Mobile phase B
(%) |
|---|
| 0.0 | 100 | 0 |
| 2.0 | 100 | 0 |
| 15.0 | 90 | 10 |
| 25.0 | 70 | 30 |
| 33.0 | 55 | 45 |
| 33.1 | 0 | 100 |
| 38.0 | 0 | 100 |
| 38.1 | 100 | 0 |
| 45.0 | 100 | 0 |
Determination of recovery rates
Three mixed branched-chain amino standards of valine
(Val), leucine (Leu) and isoleucine (Ile) were accurately prepared
and diluted using 0.1% hydrochloric acid solution, in order to
prepare standard solutions with concentrations of 0, 50, 100, 200,
400 and 1,000 μmol/l. Val, Leu and Ile mixed standard solution (50,
200 and 1,000 μmol/l) was added to the same sample with a known
concentration to determine the recovery rates.
The amino acid compositions and contents in the whey
proteins and the plasma were determined by HPLC, and the amino acid
levels were analyzed. In this study, norleucine was used as an
internal standard to calculate the content of the other amino
acids. The correction factor (f) was calculated based on the
obtained peak response values of the control solution in the
control substances and the internal standard substances, according
to the following formula: f=(As/ms)/(Ar/mr), where As and Ar
represent the peak area or peak height of the internal standard and
the control, respectively, and ms and mr indicate the amount of the
internal standard and the control, respectively. Following this,
the samples that contained the internal standard were injected into
the HPLC column and the chromatograms were recorded. According to
the peak response value of the component contained in the internal
standard solution being tested, the content (MI) was calculated by
the flowing formula: MI=f × Ai/(As/ms), where Ai and As represent
the peak area or peak height of the to-be-tested substances and the
internal standard substance, respectively, and ms indicates the
amount of the added internal standard. When required, the content
was converted to percentage content of the labeled amount according
to the dilution multiple, sample volume and labeled amount or
converted into the percentage content according to the dilution
multiple and the sample volume.
Statistical analysis
All data are presented as the mean ± standard
deviation. The statistical analysis was performed using SPSS 13.0
statistical software (SPSS, Inc., Chicago, IL, USA) and significant
differences were analyzed using one way analysis of variance
(ANOVA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Amino acid analysis of the standard
solution
According to the protocol stated in Materials and
methods, the amino acid standards were analyzed using the
appropriate column for HPLC. Standard amino acid analysis
chromatogram shown in Fig. 1A, the
whey protein is rich in branched chain amino acids. Analysis of the
branched-chain amino acids in the present study showed that Val
peaked at 23.58 min and that the peak times for Ile and Leu were
similar, at 26.52 and 26.83 min, respectively. This method
effectively separated each amino acid in the standard mixture. The
peak for each amino acid was approximately symmetrical and the
distribution curve was normal.
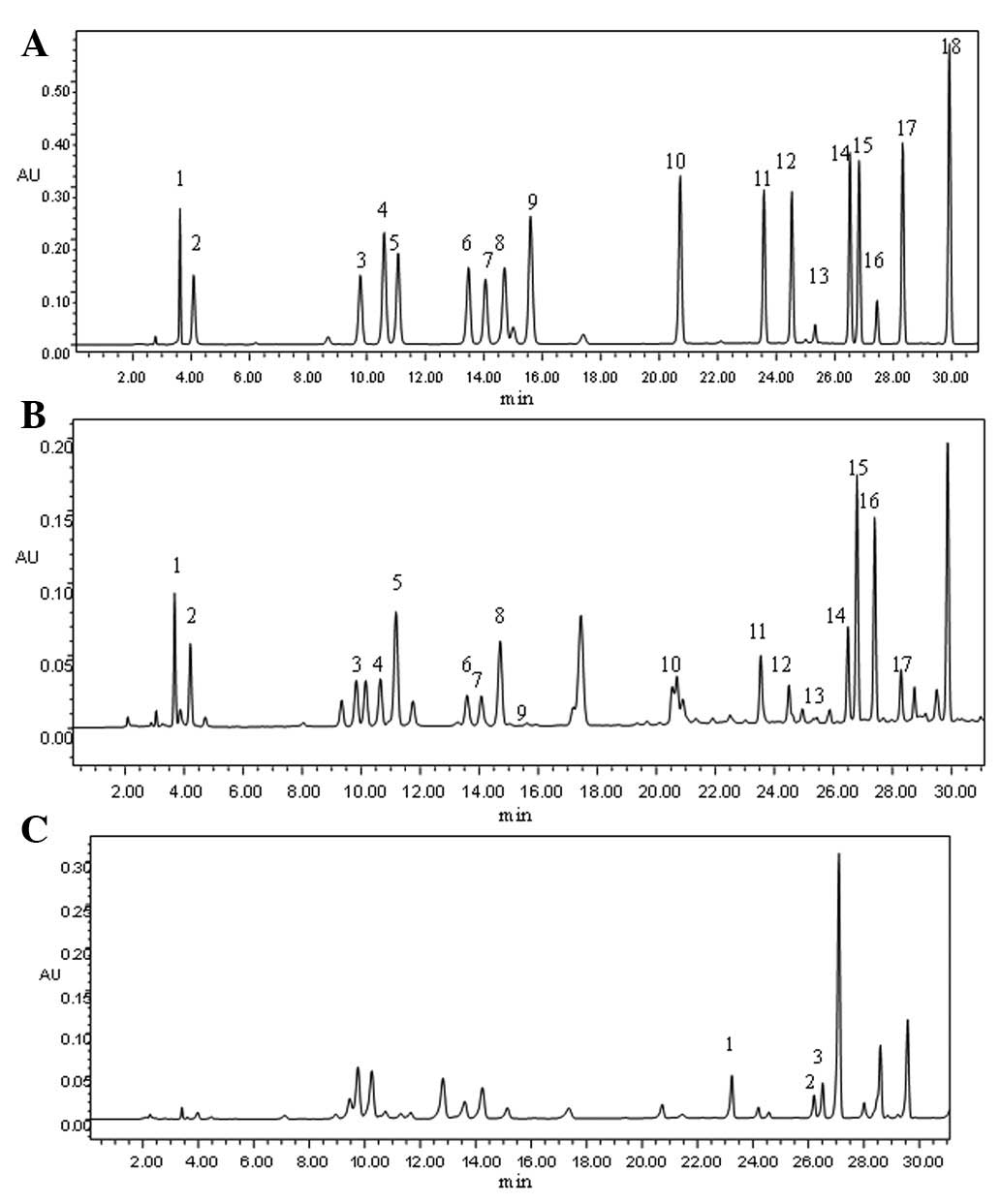 | Figure 1Amino acid chromatography in various
samples. (A) Chromatography of the 18 amino acid standards: 1,
aspartic acid (Asp); 2, glutamic acid (Glu); 3, serine (Ser); 4,
glycine (Gly); 5, histidine (His); 6, arginine (Arg); 7, threonine
(Thr); 8, alanine (Ala); 9, proline (Pro); 10, tyrosine (Tyr); 11,
valine (Val); 12, methionine (Met); 13, cystine (Cys); 14,
isoleucine (Ile); 15, leucine (Leu); 16, norleucine (Nle); 17,
phenylalanine (Phe) and 18, lysine (Lys). (B) Amino acid
chromatography of a 2 mg/ml solution of whey protein: 1, Asp; 2,
Glu; 3, Ser; 4, Gly; 5, His; 6, Arg; 7, Thr; 8, Ala; 9, Pro; 10,
Tyr; 11, Val; 12, Met; 13, Cys; 14, Ile; 15, Leu; 16, Nle; 17, Phe
and 18, Lys. (C) Chromatography of plasma amino acids in the normal
mouse group: 1, Val; 2, Ile; 3, Leu and 4, Internal standard
(Nle). |
Analysis of recovery rate
The recovery rates of the branched-chain amino acids
were determined. Three concentrations (50, 200 and 1,000 μmol/l)
were selected at which to measure the recovery rates of Val, Ile
and Leu. As shown in Table II,
the recovery rates of three branched-chain amino acids were all
high, and the majority were >90%. The highest recovery rates
were obtained when the concentration was 1,000 μmol/l.
 | Table IIRecovery rate from solutions
containing different concentrations of Val, Leu and Ile. |
Table II
Recovery rate from solutions
containing different concentrations of Val, Leu and Ile.
| Recovery rate
(%) |
|---|
|
|
|---|
| Concentration
(μmol/l) | Valine | Isoleucine | Leucine |
|---|
| 50 | 92.96 | 84.61 | 94.08 |
| 200 | 107.56 | 92.77 | 91.98 |
| 1000 | 103.54 | 100.08 | 103.40 |
Analysis of whey protein
The amino acid components of the whey proteins were
measured following processing with trypsin, peptidase and
α-chymotrypsin. The component chromatogram and the amino acid
content in the typical whey protein solution are shown in Fig. 1B. The correction factor of the
internal standards in the present study was 0.0859, and the content
of the amino acids was calculated using the formula shown in
Materials and methods. The whey protein was rich in numerous types
of amino acids, with the lysine content being measured as the
highest (17.16%). The three branched-chain amino acids (Val, Ile
and Leu) accounted for 25.65% of the total amino acid content
(~14.40% Leu, 5.93% Ile and 5.32% Val).
Analysis of plasma samples
The plasma samples were treated using the methods
stated previously. The three branched-chain amino acids in the
plasma samples were observed to be well separated following the
amino acid chromatography (Fig.
1C). As shown in Fig. 2, the
levels of branched-chain amino acids in the plasma increased in the
normal and model mice. Furthermore, the increase in the levels of
the branched amino acids was correlated with the concentration of
the whey protein used for the gavage. In addition, the levels of
branched-chain amino acids in the blood of the model mice were
higher than those in the normal mice, although the differences were
not observed to be significant (Table III).
 | Table IIIPlasma levels of branched-chain amino
acids in the normal and model groups following whey protein
gavage. |
Table III
Plasma levels of branched-chain amino
acids in the normal and model groups following whey protein
gavage.
| A, Normal group |
|---|
|
|---|
| Branched-chain amino
acids (mmol/l) |
|---|
|
|
|---|
| Whey protein (%) | Ile | Leu | Val | BCAA |
|---|
| 0 | 50.01±22.31 | 53.27±18.84 | 78.99±33.64 | 182.26±43.78 |
| 10 | 51.44±25.10 | 58.82±17.07 | 87.12±36.87 | 197.39±59.15 |
| 20 | 55.19±27.40 | 71.19±19.32 | 100.26±37.21 | 226.65±55.27 |
| 40 | 68.12±29.35 | 74.18±24.23 | 108.58±40.57 | 250.88±64.57 |
|
| B, 100 mg/kg STZ
model group |
|
| Branched-chain amino
acids (mmol/l) |
|
|
| Whey protein (%) | Ile | Leu | Val | BCAA |
|
| 0 | 56.03±19.00 | 64.19±16.14 | 89.13±31.54 | 209.35±58.74 |
| 10 | 60.45±24.70 | 77.25±27.85 | 107.72±33.08 | 245.42±67.14 |
| 20 | 65.12±28.00 | 88.61±24.94 | 109.51±42.75 | 263.23±71.07 |
| 40 | 78.13±34.20 | 90.19±28.96 | 119.27±52.36 | 287.59±106.72 |
|
| C, 160 mg/kg STZ
model group |
|
| Branched-chain amino
acids (mmol/l) |
|
|
| Whey protein (%) | Ile | Leu | Val | BCAA |
|
| 0 | 52.10±17.05 | 60.86±24.51 | 108.03±41.25 | 220.98±67.04 |
| 10 | 77.33±25.36 | 83.26±30.07 | 120.00±43.09 | 280.59±76.15 |
| 20 | 70.15±16.34 | 79.05±32.64 | 139.89±50.07 | 289.09±82.47 |
| 40 | 77.50±28.76 | 91.10±36.47 | 166.73±60.41 | 335.33±92.36 |
Discussion
Streptozotocin (STZ) is a reagent that is commonly
used globally in the preparation of diabetic models. The
intraperitoneal injection of STZ into ICR mice selectively destroys
the pancreatic β cells of the mice. This method of model
preparation has the advantages of low toxicity to body tissues, a
high experimental animal survival rate and being simple, convenient
and easy to perform. The prepared model exhibits symptoms such as
high blood sugar levels, low weight, increased appetite, polydipsia
and polyuria. The type II diabetic model may be prepared by the
administration of STZ to 8-week-old ICR mice by intraperitoneal
injection at a dose of 100 mg/kg body weight (13). In the study by Giarratana et
al(14), it was proposed that
a high-dose injection of STZ (200 mg per 1 kg body weight) was
capable of inducing a type I diabetic model (14). In the present study, 100 mg STZ per
1 kg body weight was intraperitoneally injected to prepare the type
II diabetic model, while 160 mg/kg was used to prepare the type I
diabetic model.
A previous study has demonstrated that glucose and
lipid metabolism in the blood is improved in normal mice following
whey protein administration (15).
As important substances for improving the protein synthesis of
skeletal muscle, amino acids are likely to be widely involved in
the metabolic changes (16). In
order to further investigate the mechanism of action of whey
protein, the composition and content of amino acids in whey protein
was determined in the present study. A number of methods are
commonly used to detect amino acid derivatives, including the
o-phthalaldehyde (OPA) method, the PITC fat method and the
2,4-dinitrofluorobenzene method. The OPA method has been shown to
elicit a fast response; however, it has been reported that the
fluorescence of lysine and cystine derivatives is weak, the
sensitivity is low, the glycine and lysine derivatives are unstable
and the salt in the samples affects the effect of the derivatives.
Furthermore, residual reagents have been shown to exert a marked
impact on the column in the 2,4-dinitrofluorobenzene method, which
further affects the test results. The PITC method has demonstrated
the advantages of rapid analysis, high sensitivity and good
repeatability (8). In the current
study, in order to investigate the role of the amino acids,
particularly branched-chain amino acids, present in whey protein,
HPLC was used to determine the amino acid composition of the whey
proteins and the amino acid levels in mice plasma. With the
characteristics of rapid and highly accurate analysis, high
separation efficiency, good detection performance and diverse
applications, HPLC has been widely used in various fields from its
inception in the 1960s and is, at present, an indispensable means
of separation. In the present study, the amino acid composition and
content were analyzed using HPLC. The 18 amino acid standards were
well separated, and the peak of each amino acid was approximately
symmetrical, with a normal distribution curve, indicating that HPLC
effectively separated the amino acids.
With the characteristics of a full range of amino
acids, an amino acid composition similar to the composition of
amino acids essential to humans and an easy digestibility, whey
protein exhibits a high bioavailability. Compared with the levels
recommended by the Food and Agriculture Organization (FAO)/World
Health Organization (WHO) for the amino acid content of three main
proteins (whey and soy protein and casein) (17), whey protein has been shown to be
rich in amino acids, particularly branched-chain amino acids, with
the levels Val, Leu and Ile all being higher than those recommended
by FAO/WHO. The levels of Leu and Ile in whey proteins are higher
than in casein and soy proteins (18,19).
In addition, the levels of histidine, threonine, methionine,
cystine, phenylalanine and lysine were all higher than the
recommended amounts.
In the present study, 18 amino acids were well
separated from the whey protein samples, demonstrating that whey
protein contains the full range of amino acids. The high levels of
lysine and arginine, in particular, may stimulate the secretion of
anabolic hormones to promote the growth of muscles. As a good
source of sulfur-containing amino acids, such as cysteine and
methionine, lysine and arginine may maintain the level of
glutathione (GSH), which acts as an antioxidant. As whey protein is
rich in branched-chain amino acids, the levels of branched-chain
amino acids in the mouse plasma were analyzed following gavage with
whey proteins, in order to further explore the role of the
branched-chain amino acids. The levels of Leu, Ile and Val in the
whey protein used in this study were 14.40, 5.93 and 5.32% of the
total amino acid content, respectively, i.e., the branched-chain
amino acid content was 25.65%. This was consistent with the results
from a previous study (20).
The irreversible absorption of biological
macromolecules from the plasma on the reversed phase column may
lead to an increase in column pressure and reductions in column
efficiency and the usage-life of the column. Therefore, it is
necessary for macromolecules in the plasma to be removed prior to
analysis. The method commonly used to remove proteins includes the
addition on perchloric acid and centrifugation. According to
preliminary experiments, perchloric acid was most efficacious at
removing proteins under the conditions of the present study
(21).
The levels of branched-chain amino acids in the
plasma were increased following gavage with whey protein, with a
higher concentration of whey protein leading to higher levels of
branched-chain amino acids. In addition, the levels of
branched-chain amino acids in the blood of the model mice were
higher than those in the normal mice. This may have been associated
with the previously reported effect of branched-chain amino acids
in the improvement of insulin resistance (22).
In the present study, HPLC was demonstrated to
successfully separate the amino acids in the whey proteins. The
intake of whey protein increased the levels of amino acids,
particularly branched-chain amino acids, in the mouse plasma. This
study provided a foundation for future studies in which the
mechanism of action of whey protein in the prevention of diabetes
is investigated. Further studies are required to investigate the
mechanism of action of the branched-chain amino acids that are
abundant in the whey proteins.
References
|
1
|
Wild S, Roglic G, Green A, Sicree R and
King H: Global prevalence of diabetes: estimates for the year 2000
and projections for 2030. Diabetes Care. 27:1047–1053.
2004.PubMed/NCBI
|
|
2
|
International Diabetes Federation. Type 2
diabetes epidemic: a global education. Lancet. 374:16542009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang W, Lu J, Weng J, et al: Prevalence of
diabetes among men and women in China. N Engl J Med. 362:1090–1101.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Choi HK, Willett WC, Stampfer MJ, Rimm E
and Hu FB: Dairy consumption and risk of type 2 diabetes mellitus
in men: a prospective study. Arch Intern Med. 165:997–1003. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu S, Choi HK, Ford E, et al: A
prospective study of dairy intake and the risk of type 2 diabetes
in women. Diabetes Care. 29:1579–1584. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tong X, Dong JY, Wu ZW, Li W and Qin LQ:
Dairy consumption and risk of type 2 diabetes mellitus: a
meta-analysis of cohort studies. Eur J Clin Nutr. 65:1027–1031.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Solerte SB, Fioravanti M, Locatelli E, et
al: Improvement of blood glucose control and insulin sensitivity
during a long-term (60 weeks) randomized study with amino acid
dietary supplements in elderly subjects with type 2 diabetes
mellitus. Am J Cardiol. 101:82E–88E. 2008. View Article : Google Scholar
|
|
8
|
Coker RH, Miller S, Schutzler S, Deutz N
and Wolfe RR: Whey protein and essential amino acids promote the
reduction of adipose tissue and increased muscle protein synthesis
during caloric restriction-induced weight loss in elderly, obese
individuals. Nutr J. 11:1052012. View Article : Google Scholar
|
|
9
|
Xia T, Cheng Y, Zhang Q, et al: S6K1 in
the central nervous system regulates energy expenditure via
MC4R/CRH pathways in response to deprivation of an essential amino
acid. Diabetes. 61:2461–2471. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Woolfitt AR, Solano MI, Williams TL,
Pirkle JL and Barr JR: Amino acid analysis of peptides using
isobaric-tagged isotope dilution LC-MS/MS. Anal Chem. 81:3979–3985.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Smith JC and Figeys D: Recent developments
in mass spectrometry-based quantitative phosphoproteomics. Biochem
Cell Biol. 86:137–148. 2008.PubMed/NCBI
|
|
12
|
Langrock T, Czihal P and Hoffmannn R:
Amino acid analysis by hydrophilic interaction chromatography
coupled on-line to electrospray ionization mass spectrometry. Amino
Acids. 30:291–297. 2006. View Article : Google Scholar
|
|
13
|
Ito M, Kondo Y, Nakatani A, Hayashi K and
Naruse A: Characterization of low dose streptozotocin-induced
progressive diabetes in mice. Environ Toxicol Pharmacol. 9:71–78.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Giarratana N, Penna G and Adorini L:
Animal models of spontaneous autoimmune disease: type 1 diabetes in
the nonobese diabetic mouse. Methods Mol Biol. 380:285–311. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nilsson M, Stenberg M, Frid AH, et al:
Glycemia and insulinemia in healthy subjects after
lactose-equivalent meals of milk and other food proteins: the role
of plasma amino acids and incretins. Am J Clin Nutr. 80:1246–1253.
2004.PubMed/NCBI
|
|
16
|
Veldhorst MA, Nieuwenhuizen AG,
Hochstenbach-Waelen A, et al: Dose-dependent satiating effect of
whey relative to casein or soy. Physiol Behav. 96:675–682. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gilani GS, Xiao C and Lee N: Need for
accurate and standardized determination of amino acids and
bioactive peptides for evaluating protein quality and potential
health effects of foods and dietary supplements. J AOAC Int.
91:894–900. 2008.
|
|
18
|
Xi P, Jiang Z, Zheng C, Lin Y and Wu G:
Regulation of protein metabolism by glutamine: implications for
nutrition and health. Front Biosci. 16:578–597. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Katsanos CS, Chinkes DL, Paddon-Jones D,
Zhang XJ, Aarsland A and Wolfe RR: Whey protein ingestion in
elderly persons results in greater muscle protein accrual than
ingestion of its constituent essential amino acid content. Nutr
Res. 28:651–658. 2008. View Article : Google Scholar
|
|
20
|
Walzem RL, Dillard CJ and German JB: Whey
components: millennia of evolution create functionalities for
mammalian nutrition: what we know and what we may be overlooking.
Crit Rev Food Sci Nutr. 42:353–375. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pi LG, Tang AG, Mo XM, Luo XB and Ao X:
More rapid and sensitive method for simultaneous determination of
tryptophan and kynurenic acid by HPLC. Clin Biochem. 42:420–425.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takeshita Y, Takamura T, Kita Y, et al:
Beneficial effect of branched-chain amino acid supplementation on
glycemic control in chronic hepatitis C patients with insulin
resistance: implications for type 2 diabetes. Metabolism.
61:1388–1394. 2012. View Article : Google Scholar : PubMed/NCBI
|
















