Introduction
It is well known that severe acute pancreatitis
(SAP) is associated with high mortality rates. To date, no specific
therapies have been developed. The annual global incidence of SAP
is 20–100 per 100,000, resulting in mortality rates of 20–30%. The
majority of the morbidity and mortality associated with SAP is the
result of complications associated primarily with bacterial
infections (1). The first peak in
mortality occurs within the first week subsequent to the onset of
symptoms and is characterized by systemic inflammatory response
syndrome (SIRS) associated with multiple organ damage (2). The second peak is often observed 3–4
weeks following admission. The main cause associated with this late
deterioration and systemic organ failure is bacterial
superinfection of the pancreas resulting in sepsis (3–6).
Bacterial translocation is considered the main cause of
superinfection resulting in pancreatic necrosis that peaks during
the first four days following the onset of symptoms (7). Subsequently, changes to intestinal
motility and flora, mucosal barrier function and immune responses
may lead to bacterial translocation, resulting in subsequent
superinfection and pancreatic necrosis (8,9). The
pathological mechanism and the route of bacterial translocation
associated with severe acute necrotizing pancreatitis are not
completely understood. Hematogenous or lymphatic dissemination of
bacteria (10,11) have been suggested, and it is
generally accepted that bacterial translocation from the gut is the
primary cause of secondary pancreatic infections.
Diamine oxidase (DAO) is an effective biomarker that
reflects the integrity and mucosal function of the small intestine.
As a measure of small intestine barrier function, changes in the
concentrations of DAO in the serum and the mucosa of the small
intestine may be determined.
Melatonin is secreted by the pineal gland, although
its main source has been observed to be the gastrointestinal tract
(12). Melatonin is known to be
important in the seasonal reproduction of certain species and in
the regulation of circadian rhythms. The level of melatonin in the
gastrointestinal tract is ~400-fold higher than the level of
melatonin in the pineal gland, and the concentrations of melatonin
in the gastrointestinal tract are 10–100-fold higher than in
plasma. Melatonin also has anti-inflammatory and antioxidant
properties that reduce ischemia/reperfusion injury and aid in
immune defense. Previous studies have demonstrated that melatonin
significantly decreased all studied acute pancreatitis
(AP)-associated inflammatory parameters, in addition to reducing
apoptosis and necrosis associated with pancreatic injury (13–17).
Col et al(13) observed
that intraperitoneal melatonin injections reduced the quantity of
malonyldialdehyde (MDA), and increased the levels of superoxide
dismutase (SOD) and glutathione (GSH), which are associated with
oxidative stress in pancreatic tissue. Additional studies (14–17)
have observed that melatonin reduced the occurrence and development
of AP, suggesting that melatonin may ameliorate AP severity through
its influence on cytokines, such as tumor necrosis factor
(TNF)-α.
Materials and methods
Animals
Twenty-seven clean-grade, male Sprague Dawley (SD)
rats weighing 200–250 g were purchased from the Experimental Animal
Center of Wenzhou Medical College (Wenzhou, China). The animals
were maintained under standard conditions of 12-h light/dark cycles
in a temperature-controlled room with free access to standard rat
pellets and water. All animals were maintained in the laboratory
for one week and were deprived of food for 12 h prior to
experimentation (rats had free access to water throughout the
experimental period).
Ethics statement
This experiment was approved by and performed in
accordance with the guidelines for animal use of the Experimental
Animal Center of Wenzhou Medical College.
Animal groups and procedures
SD rats were randomly divided into the sham
operation group (SO group, n=8), the SAP group (n=18) or the
melatonin treatment group (MT group, n=14). SAP was induced through
retrograde infusion of 4% taurocholate (1 ml/kg body weight;
Sigma-Aldrich, St. Louis, MO, USA) into the biliopancreatic duct,
following the clamping of the hepatic duct. In the SO group, the
procedure was terminated subsequent to cannulating the
biliopancreatic duct by penetrating the duodenum with a 24-gauge
catheter. In the MT group, melatonin (50 mg/kg body weight;
Sigma-Aldrich) was administered 30 min prior to the injection of
taurocholate. Twenty-four hours subsequent to SAP induction, rats
from each group were anesthetized with 10% chloral hydrate (30
mg/kg body weight), the abdomen was opened and the ileum tissues
adjacent to the cecum were rapidly collected and divided in two.
The first tissue sample from each rat was cut into several small
pieces (1×1 mm), immediately fixed in 2.5% glutaraldehyde in
phosphate-buffered saline (PBS, pH 7.2) and embedded in epoxy
resin, prior to ultra-thin sections being prepared using
conventional procedures. These sections were later examined with a
JEM-1230 transmission electron microscope (JEOL Ltd., Tokyo,
Japan). The second tissue sample was weighed and stored at −80°C in
PBS for later anaylsis of DAO levels using enzyme-linked
immunosorbent assay (ELISA). Blood samples (2 ml) from each rat in
each group were collected via a postcava puncture and split in two.
Half of the blood sample was allowed to clot for 20 min at room
temperature and centrifuged at 3,000 × g for 20 min, prior to the
serum being collected for the measurement of DAO levels using
ELISA. The second portion was collected in germ-free microtubes
with anticoagulants and stored at −20°C for the later
quantification of Escherichia coli (E. coli) O157
using real-time-fluorescence quantitative polymerase chain reaction
(RT-FQ-PCR). The rats were sacrificed by exsanguination at the end
of the experiment.
Transmission electron microscopy
The ileum tissues were cut into small pieces (1×1
mm) and immediately fixed in 2.5% glutaraldehyde in PBS and stored
at 4°C overnight. The ileum segments were then washed in 0.1 M PBS
three times (15 min each) and fixed in 1% osmium tetroxide for 1 h.
One hour later, the segments were washed in 0.1 M PBS a further
three times for 15 min each. The samples were then successively
dehydrated in 50, 70, 80 and 90% acetone, respectively, for 15 min
at each concentration, prior to being placed in 100% acetone twice
for 10 min each. Subsequently, the segments were embedded in
acetone and Epon resin (v/v=1:1), stored at 37°C for 2 h, embedded
in acetone and Epon resin (v/v=1:4) and stored at 37°C overnight.
The following day, the segments were embedded in Epon resin and
stored at 45°C for 2 h. The segments were then cut longitudinally
through the intestinal villi into 70–90 μm ultra-thin sections
using a Reichert ultra-thin microtome (Reichert Inc., Buffalo, NY,
USA). Subsequently, the ultra-thin sections were stained with
uranyl acetate and lead citrate, and examined using a JEM-1230
transmission electron microscope.
RT-FQ-PCR analysis
The postcava blood was removed from storage at −20°C
and placed into liquid nitrogen. E. coli O157 DNA in the
postcava blood was extracted using extraction solution (Da An Gene,
Zhongshan, China) in accordance with the manufacturer’s
instructions, and extraction was confirmed using agarose gel
electrophoresis. E. coli O157 DNA was amplified using Taq
DNA polymerase (Da An Gene). PCR was carried out using the
following primers: Forward primer 5′-CAGATCCGGCAAGGTATTGT-3′ and
reverse primer 5′-TGAGCGTTAAGCAGGTGATG-3′. The reporter dye
6-carboxyfluorescein (FAM) and the quencher dye Texas Red
(Sulforhodamine 101) were conjugated at the 5′ and 3′ ends of the
fluorescent probe, respectively. The reaction mixture (25 μl)
consisted of 3 μl Taq DNA polymerase, 1 μl 2.5 mmol/l
deoxyribonucleoside triphosphates (dNTPs), 5 μl DNA template, 1 μl
each primer, 1 μl Taqman probe and 13 μl MgCl2, and the
volume was adjusted with double-distilled water (ddH2O).
Genomic DNA purified from E. coli was used as a positive
control and ddH2O was used as a negative control. The
following PCR cycling conditions were used: 50°C for 2 min, 95°C
for 15 min, and 40 cycles at 94°C for 15 sec and 60°C for 45 sec.
The RT-PCR results were recorded using a 7500 Sequence Detection
system (Applied Biosystems Inc., Carlsbad, CA, USA).
Serum and ileum mucosal DAO level
measurements
The serum and ileum tissue samples were removed from
storage at −80°C and placed into liquid nitrogen. When thawed, the
samples were maintained at 2–8°C. The ileum tissues were
homogenized by hand or using a tissue grinder, centrifuged at 3,000
rpm for 20 min and the supernatants were then collected to measure
the DAO levels. Serum and ileum mucosal DAO assays were performed
using a rat DAO ELISA kit (Daweike Biotechnology, Shanghai, China)
according to the manufacturer’s instructions. Duplicate assays were
performed on all DAO specimens. The DAO assay range was 700–800
U/l.
Statistical analysis
Block randomization was used to assign animals into
the three groups. Data are expressed as the arithmetic mean ±
standard deviation (SD). Fisher probabilities in 2×2 tables were
used to analyze the early mortality rates of the SD rats and the
positive E. coli rates in the postcava blood. One-way
analysis of variance (ANOVA) was used to investigate the
differences among the three experimental groups, and comparisons
were performed between samples from the same experiment and
time-points to check for statistical significance. P≤0.05 was
considered to indicate a statistically significant difference.
Statistical analyses were performed using the SPSS software
(version 17.0; SPSS Inc., Chicago, IL, USA).
Results
Mortality rates
Ten rats in the SAP group died 8–12 h subsequent to
SAP induction (mortality rate of 55.56%). Three rats in the MT
group died 12–24 h subsequent to SAP induction, resulting in a
21.43% mortality rate. The mortality rate in the MT group was
significantly lower than that in the SAP group (P<0.05). No rats
in the SO group died.
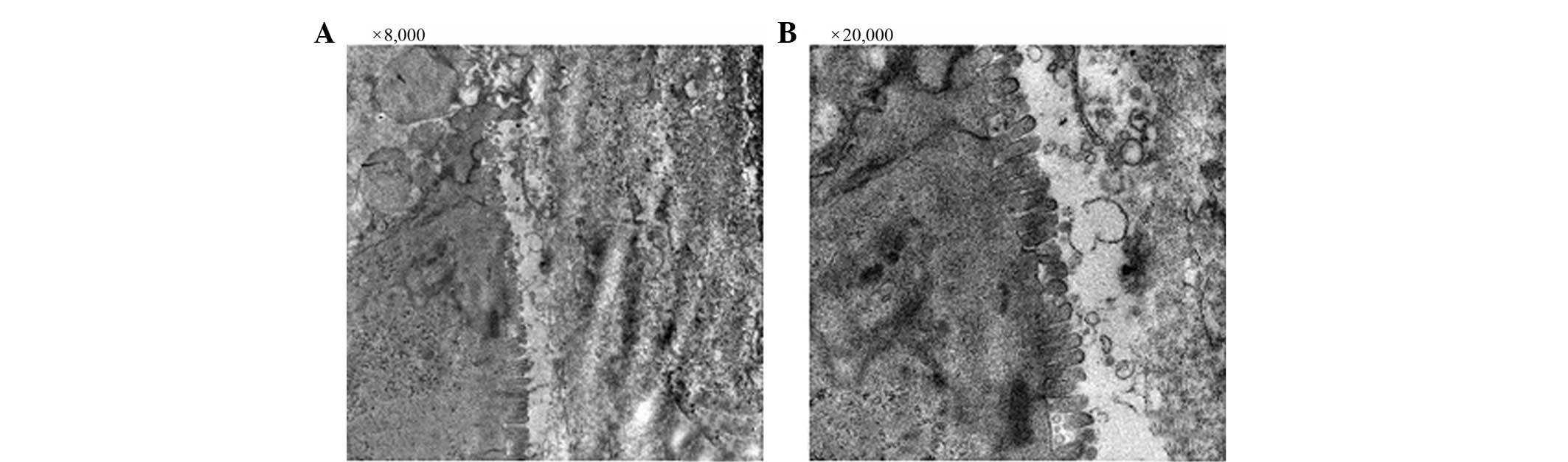
Transmission electron microscopy
The integrity of the intestinal villi and goblet
epithelial cells of the rats in the SO group was maintained
throughout the course of the experiment (Fig. 1A and B). The mitochondria,
endoplasmic reticulum, ribosomes and other cellular organelles were
normal following examination at a magnification of ×20,000
(Fig. 1B). The intestinal villi
and goblet epithelial cells of the SAP group presented with a loss
of integrity, and the intestinal villi were absent throughout the
sample examined (Fig. 2A and B).
The mitochondrial membrane and cristae were absent and cristae had
changed into vacuoles. The amount of rough endoplasmic reticulum
was increased and enlarged, and the ribosomes were distinctly
increased in size at a magnification of ×20,000 (Fig. 2B). The integrity of the intestinal
villi and goblet epithelial cells of rats in the MT group was
maintained (Fig. 3A and B). The
mitochondria, endoplasmic reticulum, ribosomes and other organelles
were unchanged (Fig. 3C).
Measurements of intestinal integrity
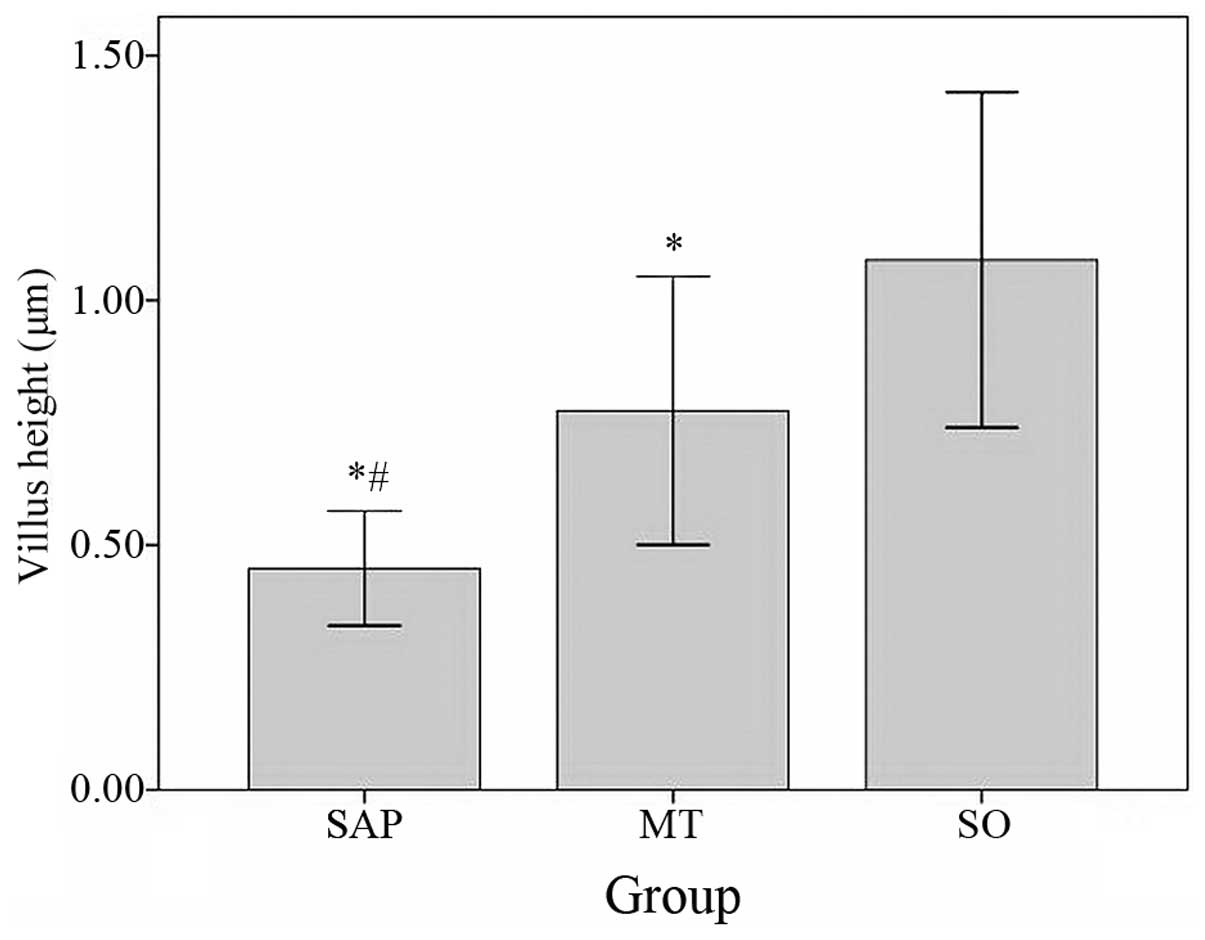
Compared with the villus height in the SO group
(1.08250±0.171193 μm), the villus height in the SAP group
(0.45250±0.058493 μm, P<0.05) and the MT group (0.77455±0.137067
μm, P<0.05) were significantly lower. The villus height in the
MT group was significantly higher than in the SAP group (P<0.05;
Fig. 4).
The crypt depth in the SO group (0.78500±0.171548
μm) was significantly increased compared with that in the SAP group
(0.16500±0.057570 μm, P<0.05) and the MT group (0.47182±0.133178
μm, P<0.05). The crypt depth of the MT group was significantly
deeper than in the SAP group (Fig.
5).
E. coli O157 quantification in postcava
blood
The concentration of E. coli DNA (Ct value)
in the postcava blood in the MT group was significantly lower than
the levels in the rats in the SAP group. No E. coli DNA was
detected in the animals in the SO group (Table I; Fig.
6). E. coli DNA in postcava blood was significantly
lower in the MT group compared to the SAP group (Table I).
 | Table IE. coli DNA in postcava
blood. |
Table I
E. coli DNA in postcava
blood.
| Groups | N | E. coli DNA
(n) | Positive rate
(%) | Ct-value |
|---|
| SAP | 8 | 0 | 0.00 | 0 |
| MT | 11 | 1 | 9.09 | 35.1081±3.2873 |
| SO | 8 | 5 | 62.50a |
29.4466±4.74451b |
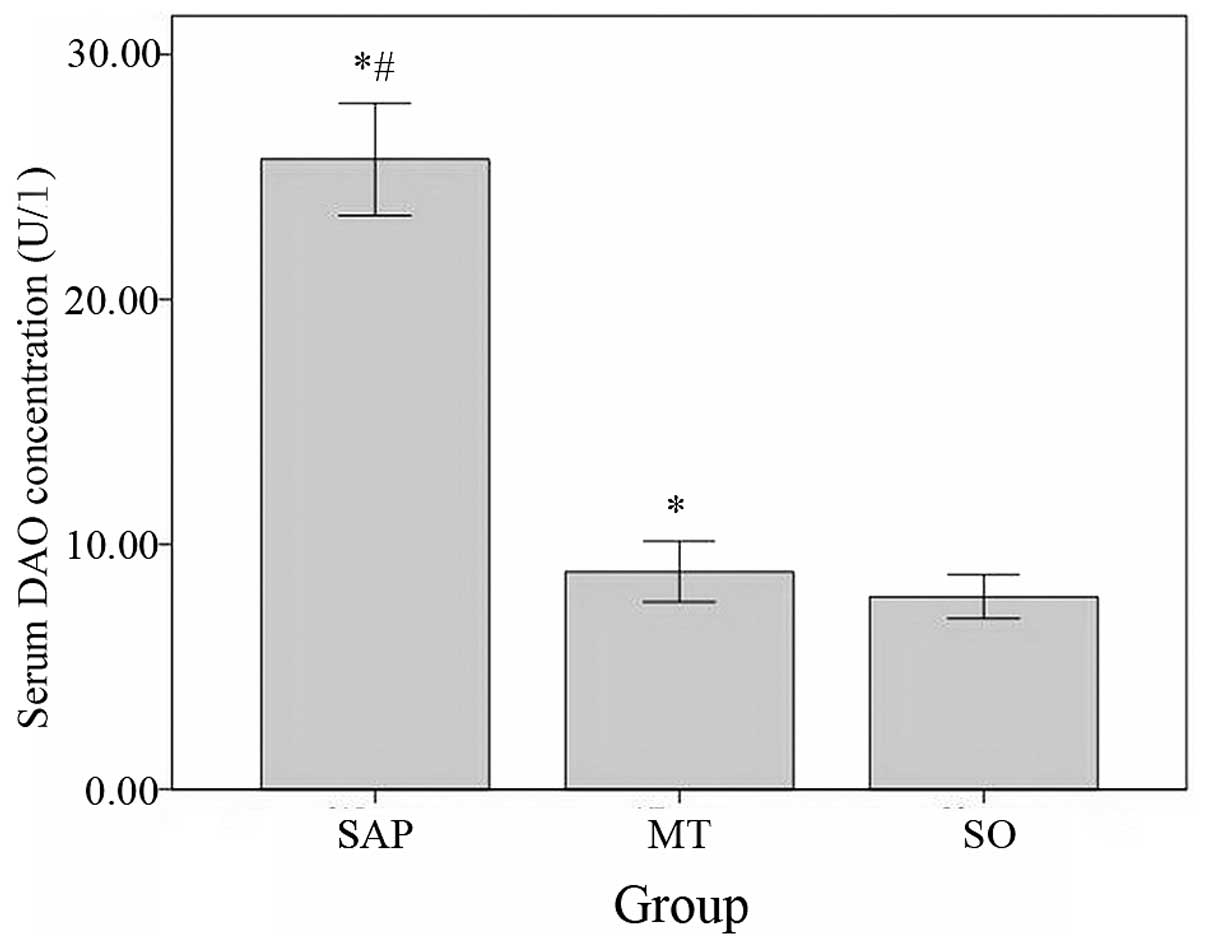
Serum and ileum mucosal DAO level
The levels of DAO in the serum were significantly
higher in the SAP group compared with the levels observed in the MT
group and significantly higher in the MT group than in the control
group (Fig. 7).
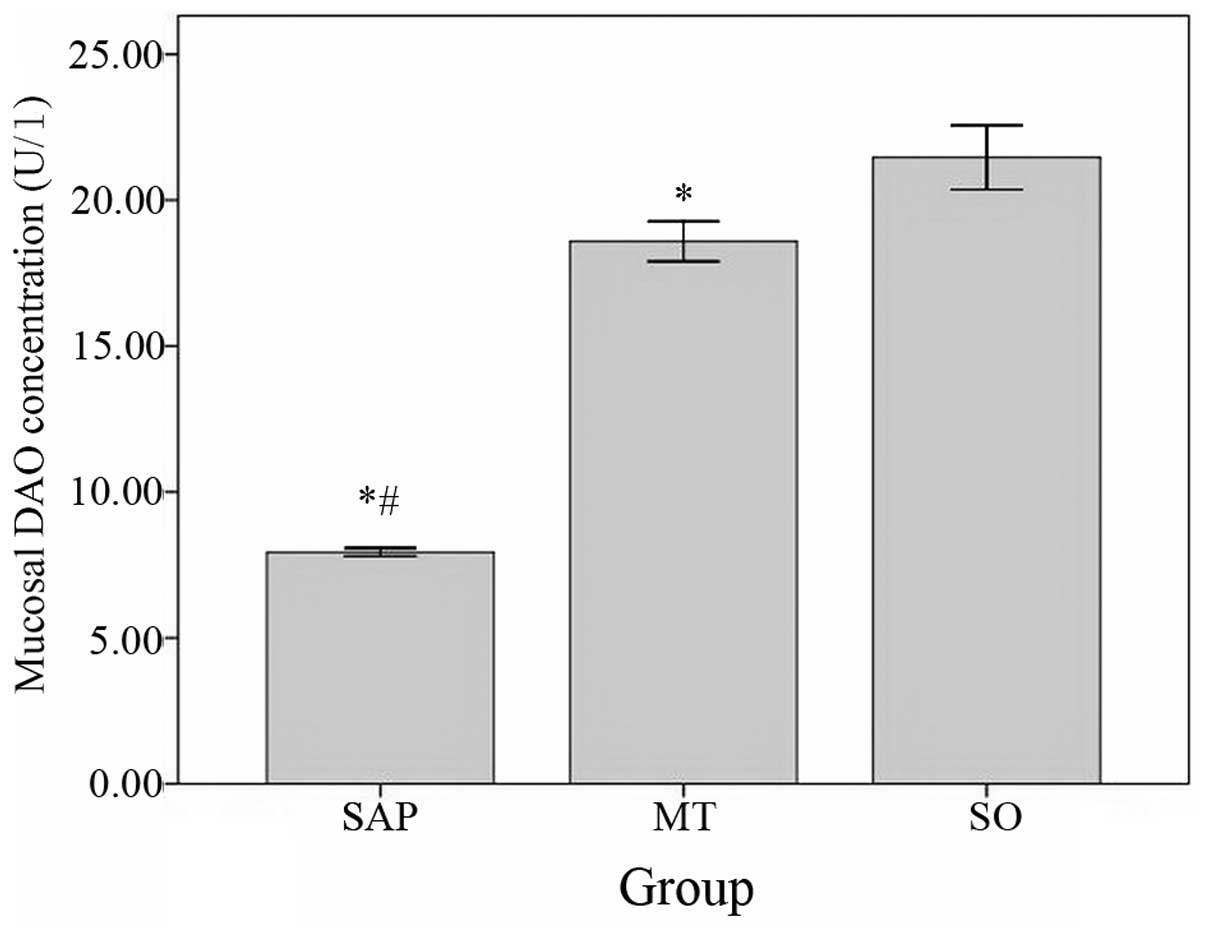
The levels of DAO in the ileum mucosa were
significantly lower in the SAP group than in the MT group and
significantly lower in the MT group than in the control group
(Fig. 8).
Discussion
Lichtman (18)
suggested that the clinical outcome of patients presenting with SAP
was significantly associated with bacteria crossing the intestinal
barrier and then invading organ systems, resulting in
superinfections associated with pancreatic necrosis. At present,
bacterial translocation is considered to be the main cause of
pancreatic superinfection and fatal sepsis (8,19).
Ammori et al(3) reported
that gut barrier function was disordered and that endotoxemia was
associated with SAP. Furthermore, Cicalese et al(9) observed that fluorescent microspheres
administered orally to rats prior to the induction of AP were able
to be detected later in different organ systems, including the
pancreas, liver or spleen. These experiments, as wells as other
studies (20,21), demonstrated that the gut was the
main source of bacterial translocation in AP. The aim of the
present study was to evaluate whether melatonin was able to reduce
bacterial translocation and ameliorate gut barrier dysfunction,
resulting in an improved clinical course associated with reduced
early mortality rates, in rats with SAP. It was therefore necessary
to detect systemic bacterial dissemination early, prior to the
development of systemic infections. Early dissemination was
monitored by testing blood samples that were frequently positive in
rats with pancreatitis, indicative of hematogenous dissemination.
Several hypotheses regarding the spread of intestinal bacteria have
been proposed. Although certain authors have suggested a lymphatic
spread of enteric bacteria, others have suggested that bacteria
cross the intestinal barrier and invade blood vessels (hematogenous
dissemination). Furthermore, transductal infections via the biliary
tract (either ascending or descending) and transperitoneal pathways
have been proposed (10,21). In the present study, it was
observed that melatonin treatment significantly reduced infection
of postcava blood, prevented gut barrier dysfunction and
subsequently reduced pancreatic superinfections. It was concluded
that bacterial translocation occurred via mesenteric lymph nodes
and subsequent hematogenous dissemination. This was in accordance
with previous studies, which showed that bacterial translocation
does not occur via transperitoneal pathways, but most likely via
lymphatic spread (22,23) followed by hematogenous
dissemination. Lichtman (18)
suggested that bacterial cell wall components (such as
lipopolysaccharide and peptidoglycan polysaccharide) allowed
enteric bacteria to cross the intestinal barrier into the
mesenteric lymph nodes, resulting in the subsequent spread of the
bacteria throughout the body causing sepsis and multiple organ
failure (MOF).
It is known that the small bowel has an important
pathophysiological role in the infection process associated with
pancreatic necrosis. Samel et al(20) observed that fluorescent bacteria
translocated from the small bowel lumen into the pancreas. Fritz
et al(24) suggested that
bacterial translocation occurred through the small bowel rather
than through the colon. A subsequent increase in intestinal
permeability facilitated bacterial translocation (25), resulting in apoptosis of intestinal
epithelial cells and/or alterations to tight junction integrity
(26). Although the pathogenesis
of intestinal bacterial translocation associated with SAP has yet
to be elucidated, several mechanisms have been proposed, including:
i) Altered permeability of the intestinal mucosa, ii) a disruption
of the indigenous gut flora, and iii) decreased host defenses. SAP
may be closely associated with these as well as other factors that
may promote bacterial translocation. Widdison et a1(27) described a reduced clearance of
E. coli from the circulation during SAP associated with
impaired phagocytic and reticuloendothelial function. Evidence of
bacterial translocation associated with enterogenic infections
resulting in AP and multiple organ dysfunction syndrome (MODS) has
led to a shift in focus onto the area of intestinal mucosal barrier
integrity as a key player in preventing SAP.
The intestinal mucosal barrier is able to prevent
the transport of harmful substances, including dangerous bacteria
and/or toxins, from penetrating the intestinal wall, and maintains
the stability of the internal environment (28,29).
SAP, as well as surgery, trauma, chemotherapy, radiotherapy or
severe infection, may damage the integrity and function of the
intestinal mucosa. In the present study, transmission electron
microscopy demonstrated that microvilli of the intestinal mucosa
had reduced widths and heights, tight junctions were damaged and
DAO levels were increased in SAP rats. Each of these parameters are
capable of leading to increases in intestinal permeability
(30–33), causing activation of endothelial
cells, translocation of enteric bacteria and endotoxins, and the
release of cytokines and inflammatory mediators that may result in
the onset of SIRS and MODS.
DAO is a high-activity intracellular enzyme that
metabolizes and catalyzes histamine, cadaverine and putrescine, and
is predominantly present within the intestinal mucosa, placenta and
kidney. However, it is also present in low levels in the plasma
(34). DAO oxidizes putrescine
into amino butyraldehyde and cyclized into pyrrole. The activity of
DAO is closely correlated with intestinal villi height and protein
synthesis. When the intestinal barrier is injured, intestinal
mucosal cells exfoliate into the gut lumen, decreasing the activity
of mucosal DAO. When DAO enters the lymphatic vessels and the blood
stream, the plasma DAO levels are increased. Therefore, high plasma
and low mucosal concentrations of DAO reflect impairment of the
intestinal tract function. It has been suggested that the combined
characterization of the ratio of urine lactulose to excretion and
plasma DAO levels may be used as measures of intestinal mucosal
function and integrity. The plasma DAO concentrations reflect the
intestinal permeability more effectively and rapidly (35).
In the present study, alterations to ileal mucosal
and serum DAO levels were characterized in order to evaluate the
function of the small intestinal barrier and the permeability
function in rats with SAP. The level of ileum mucosal DAO was
decreased and the level of serum DAO was increased in the SAP
group. These results indicated that damage to the intestinal
barrier resulted in increased intestinal permeability that occurred
during the early stages of SAP. These observations were confirmed
by the level of E. coli O157 (Ct value) detected in postcava
blood by RT-FQ-PCR in the SAP group.
In 1991, Lanas et al first proposed that
melatonin was an antioxidant, and it was subsequently tested in a
number of toxicity studies (36–38).
Melatonin readily protected the gastric and enteric mucosa from
damage caused by various factors, including ischemia/reperfusion
(39), stress (40) and ethanol (41). The present results suggested that
melatonin prevented (or reduced) the severity of experimental AP by
increasing antioxidant enzyme activity. To date, few studies have
examined the effects of melatonin on gut barrier dysfunction and
intestinal bacterial translocation, which is an important trigger
that drives the development of SIRS and MODS. The main observations
of this study were that melatonin reduced intestinal bacterial
translocation and reduced pancreatic infection and early mortality
rates by protecting the function and structure of the intestinal
mucosa.
The results of the present study indicated that
melatonin protected the small intestinal villi from damage caused
by taurocholate-induced SAP, subsequently preventing intestinal
barrier dysfunction and significantly reducing intestinal
permeability, which, in turn, prevented intestinal bacterial
translocation. The villus height in the MT group was significantly
higher than in the SAP group and the crypt depth of the MT group
was significantly deeper than in the SAP group. The level of ileum
mucosal DAO was decreased and the level of serum DAO was increased
in rats not treated with melatonin (SAP group). However,
alterations in the ileum mucosal and serum DAO levels were not
different in the MT group compared with rats in the SO group. The
level of E. coli O157 also differed between groups. This
study demonstrated that melatonin treatment of rats presenting with
SAP protected intestinal mucosal cells against mechanical and
chemical damage, attenuated damage to the small intestines, reduced
intestinal bacterial translocation and reduced early mortality
rates. Thus, melatonin may reduce intestinal bacterial
translocation by alleviating intestinal injury.
In conclusion, the results of the study demonstrated
that intestinal bacterial translocation may be associated with
damage to the intestinal mucosal barrier. Melatonin is potentially
capable of reducing intestinal bacterial translocation by
preventing damage to the intestinal mucosa.
Acknowledgements
This study was supported by the Zhejiang Provincial
Natural Science Foundation (grant no. Q12H030005).
References
|
1
|
Petrov MS, Shanbhag S, Chakraborty M,
Phillips AR and Windsor JA: Organ failure and infection of
pancreatic necrosis as determinants of mortality in patients with
acute pancreatitis. Gastroenterology. 139:813–820. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bettinger JR and Grendell JH:
Intracellular events in the pathogenesis of acute pancreatitis.
Pancreas. 6(Suppl 1): S2–S6. 1991. View Article : Google Scholar
|
|
3
|
Ammori BJ, Fitzgerald P, Hawkey P and
McMahon MJ: The early increase in intestinal permeability and
systemic endotoxin exposure in patients with severe acute
pancreatitis is not associated with systemic bacterial
translocation: molecular investigation of microbial DNA in the
blood. Pancreas. 26:18–22. 2003. View Article : Google Scholar
|
|
4
|
Gregoric P, Sijacki A, Stankovic S, et al:
SIRS score on admission and initial concentration of IL-6 as severe
acute pancreatitis outcome predictors. Hepatogastroenterology.
57:349–353. 2010.PubMed/NCBI
|
|
5
|
Slavin J and Neoptolemos JP: Antibiotic
prophylaxis in severe acute pancreatitis - what are the facts?
Langenbecks Arch Surg. 386:155–159. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gloor B, Müller CA, Worni M, Martignoni
ME, Uhl W and Büchler MW: Late mortality in patients with severe
acute pancreatitis. Br J Surg. 88:975–979. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tarpila E, Nyström PO, Franzén L and Ihse
I: Bacterial translocation during acute pancreatitis in rats. Eur J
Surg. 159:109–113. 1993.PubMed/NCBI
|
|
8
|
van Minnen LP, Blom M, Timmerman HM,
Visser MR, Gooszen HG and Akkermans LM: The use of animal models to
study bacterial translocation during acute pancreatitis. J
Gastrointest Surg. 11:682–689. 2007.PubMed/NCBI
|
|
9
|
Cicalese L, Sahai A, Sileri P, et al:
Acute pancreatitis and bacterial translocation. Dig Dis Sci.
46:1127–1132. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Runkel NS, Rodriguez LF and Moody FG:
Mechanisms of sepsis in acute pancreatitis in opossums. Am J Surg.
169:227–232. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
de las Heras G, Forcelledo JL, Gutiérrez
JM, et al: Selective intestinal bacterial decontamination in
experimental acute pancreatitis. Gastroenterol Hepatol. 23:461–465.
2000.(In Spanish).
|
|
12
|
Bubenik GA, Hacker RR, Brown GM and Bartos
L: Melatonin concentrations in the luminal fluid, mucosa, and
muscularis of the bovine and porcine gastrointestinal tract. J
Pineal Res. 26:56–63. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Col C, Dinler K, Hasdemir O, Buyukasik O
and Bugdayci G: Oxidative stress and lipid peroxidation products:
effect of pinealectomy or exogenous melatonin injections on
biomarkers of tissue damage during acute pancreatitis.
Hepatobiliary Pancreat Dis Int. 9:78–82. 2010.
|
|
14
|
Chen HM, Hsu JT, Chen JC, Ng CJ, Chiu DF
and Chen MF: Delayed neutrophil apoptosis attenuated by melatonin
in human acute pancreatitis. Pancreas. 31:360–364. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jaworek J, Bonio J, Leja-Szpa A, et al:
Sensory nerves in central and peripheral control of pancreatic
integrity by leptin and melatonin. J Physiol Pharmacol. 53:51–74.
2002.PubMed/NCBI
|
|
16
|
Jaworek J, Leja-Szpak A, Bonior J, et al:
Protective effect of melatonin and its precursor L-tryptophan on
acute pancreatitis induced by caerulein overstimulation or
ischemia/reperfusion. J Pineal Res. 34:40–52. 2003. View Article : Google Scholar
|
|
17
|
Gülben K, Ozdemir H, Berberoğlu U, et al:
Melatonin modulates the severity of taurocholate-induced acute
pancreatitis in the rat. Dig Dis Sci. 55:941–946. 2010.PubMed/NCBI
|
|
18
|
Lichtman SM: Bacterial [correction of
baterial] translocation in humans. J Pediatr Gastroenterol Nutr.
33:1–10. 2001.
|
|
19
|
Arendt T, Wendt M, Olszewski M,
Falkenhagen U, Stoffregen C and Fölsch UR: Cerulein-induced acute
pancreatitis in rats - does bacterial translocation occur via a
transperitoneal pathway? Pancreas. 15:291–296. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Samel S, Lanig S, Lux A, et al: The gut
origin of bacterial pancreatic infection during acute experimental
pancreatitis in rats. Pancreatology. 2:449–455. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yasuda T, Takeyama Y, Ueda T, et al:
Breakdown of intestinal mucosa via accelerated apoptosis increases
intestinal permeability in experimental severe acute pancreatitis.
J Surg Res. 135:18–26. 2006. View Article : Google Scholar
|
|
22
|
Wazna E and Górski A: Bacterial
translocation and its clinical significance. Postepy Hig Med Dosw
(Online). 59:267–275. 2005.(In Polish).
|
|
23
|
Marotta F, Geng TC, Wu CC and Barbi G:
Bacterial translocation in the course of acute pancreatitis:
beneficial role of nonabsorbable antibiotics and lactitol enemas.
Digestion. 57:446–452. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fritz S, Hackert T, Hartwig W, et al:
Bacterial translocation and infected pancreatic necrosis in acute
necrotizing pancreatitis derives from small bowel rather than from
colon. Am J Surg. 200:111–117. 2010. View Article : Google Scholar
|
|
25
|
Wang X, Andersson R, Soltesz V, Leveau P
and Ihse I: Gut origin sepsis, macrophage function, and oxygen
extraction associated with acute pancreatitis in the rat. World J
Surg. 20:299–307. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hać S, Dobosz M, Kaczor JJ, et al:
Neutrophil engagement and septic challenge in acute experimental
pancreatitis in rats. World J Gastroenterol. 11:6459–6465.
2005.PubMed/NCBI
|
|
27
|
Widdison AL, Karanjia ND and Reber HA:
Routes of spread of pathogens into the pancreas in a feline model
of acute pancreatitis. Gut. 35:1306–1310. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Garside P, Millington O and Smith KM: The
anatomy of mucosal immune responses. Ann N Y Acad Sci. 1029:9–15.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kiyono H, Kweon MN, Hiroi T and Takahashi
I: The mucosal immune system: from specialized immune defense to
inflammation and allergy. Acta Odontol Scand. 59:145–153. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Meriläinen S, Mäkelä J, Koivukangas V, et
al: Intestinal bacterial translocation and tight junction structure
in acute porcine pancreatitis. Hepatogastroenterology. 59:599–606.
2012.PubMed/NCBI
|
|
31
|
Besselink MG, van Santvoort HC, Renooij W,
et al: Intestinal barrier dysfunction in a randomized trial of a
specific probiotic composition in acute pancreatitis. Ann Surg.
250:712–719. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Takahashi Y, Fukushima J, Fukusato T, et
al: Prevalence of ischemic enterocolitis in patients with acute
pancreatitis. J Gastroenterol. 40:827–832. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Penalva JC, Martínez J, Laveda R, et al: A
study of intestinal permeability in relation to the inflammatory
response and plasma endocab IgM levels in patients with acute
pancreatitis. J Clin Gastroenterol. 38:512–517. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Takagi K, Nakao M, Ogura Y, Nabeshima T
and Kunii A: Sensitive colorimetric assay of serum diamine oxidase.
Clin Chim Acta. 226:67–75. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Luan ZG, Zhang H, Ma XC, Zhang C and Guo
RX: Role of high-mobility group box 1 protein in the pathogenesis
of intestinal barrier injury in rats with severe acute
pancreatitis. Pancreas. 39:216–223. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Galano A, Tan DX and Reiter RJ: Melatonin
as a natural ally against oxidative stress: a physicochemical
examination. J Pineal Res. 51:1–16. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hardeland R, Tan DX and Reiter RJ:
Kynuramines, metabolites of melatonin and other indoles: the
resurrection of an almost forgotten class of biogenic amines. J
Pineal Res. 47:109–126. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Peyrot F and Ducrocq C: Potential role of
tryptophan derivatives in stress responses characterized by the
generation of reactive oxygen and nitrogen species. J Pineal Res.
45:235–246. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cuzzocrea S, Costantino G, Mazzon E,
Micali A, De Sarro A and Caputi AP: Beneficial effects of melatonin
in a rat model of splanchnic artery occlusion and reperfusion. J
Pineal Res. 28:52–63. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kato K, Murai I, Asai S, et al: Central
effect of melatonin against stress-induced gastric ulcers in rats.
Neuroreport. 8:2305–2309. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Melchiorri D, Sewerynek E, Reiter RJ,
Ortiz GG, Poeggeler B and Nisticò G: Suppressive effect of
melatonin administration on ethanol-induced gastroduodenal injury
in rats in vivo. Br J Pharmacol. 121:264–270. 1997. View Article : Google Scholar : PubMed/NCBI
|