Introduction
Heart failure (HF) is a modern epidemic and a
significant public health problem. Patients with HF are frequently
hospitalized and have a high mortality rate. Regardless of the
remarkable advances in diagnosis and therapy over the past decade,
the prognosis of patients with HF remains poor, with mortality
rates approaching 20% per year (1). With changing epidemiological and
socioeconomical developments, the epidemiology characteristics of
HF in developing and developed countries are becoming increasingly
similar, such that coronary heart disease as HF etiology is
increasingly prominent in China (2,3).
However, the overall profile and prognosis of patients with HF and
reduced ejection fraction (HFrEF) is very limited (4). Most Chinese cardiologists are
challenged with the high mortality rate of patients with HFrEF. The
present study retrospectively analyzed a cohort of 685 Chinese
patients to clarify the overall profile and prognosis of HFrEF. A
left ventricular ejection fraction (LVEF) of ≤45% is defined as a
significantly reduced LVEF (4).
Subjects and methods
Study groups
A total of 748 patients were admitted to the
Department of Cardiology, Henan Provincial People’s Hospital
(Zhengzhou, China) from June 14, 2007 to January 27, 2012. Patients
were diagnosed with HF according to the modified Framingham
criteria for HF (5) and an LVEF of
≤45% was determined by echocardiography during hospitalization.
Patients were excluded from this study if they had recent acute
coronary syndrome, acute viral myocarditis, congenital heart
disease or severe heart valve disease. In addition, patients that
had other concomitant diseases that are associated with a reduced
life expectancy, including malignant tumors, severe hematological
system disorders, chronic respiratory failure and end-stage
cirrhosis, were excluded. Sixty-three patients (9%) who had
incomplete clinical data or were lost during the follow-up period,
were also excluded from this study. The study population consisted
of 685 patients with HF, divided into two groups: patients with an
LVEF of ≤35% (n=371) or an LVEF of 36–45% (n=314). Moreover, if the
patient was hospitalized more than once due to HF, only the data
from the first hospitalization was analyzed. This study was
conducted in accordance with the Declaration of Helsinki and with
approval from the ethics committee and the Institutional Review
Board of Henan Provincial People’s Hospital (Zhengzhou, China).
Written informed consent was obtained from all participants.
Data extraction
The data relating to the demographic status of
patients, including age, gender, body weight, height, place of
residence, admission date, cause of admission, background
(concurrent) diseases, drug use during hospitalization and drug
prescription on discharge, were recorded systematically from the
medical records during hospitalization. Body mass index (BMI) was
calculated using the following equation: BMI = body weight
(kg)/[height (m)]2. Moreover, the estimated glomerular
filtration rate (eGFR) was calculated as described in a previous
study (6) using the following
equation: eGFR = 186 × SCr−1.154 × age−0.203 (x 0.742 if female)
ml/min/1.73 m2.
Study endpoints
The study endpoints included registration for total
mortality and sudden or pump failure death. Mortality was defined
as sudden if it occurred within 24 h in the absence of pre-existing
progressive circulatory failure or other causes of mortality, as
well as if a witnessed death occurred within 60 min of the
emergence of new symptoms. Pump failure death was defined as those
occurring due to refractory progressive end-stage HF.
Follow-up
Information regarding the clinical outcome was
collected from the patients, dependents of patients or referring
physician via telephone interviews, letters or clinical visits.
Statistical analysis
Statistical analyses were performed using SPSS
software, version 13.0 (SPSS, Inc., Chicago, IL, USA). Data are
presented as the mean ± standard deviation or median for continuous
data and the significance was analyzed using a two-sampled t-test.
Categorical variables were described in terms of frequencies and
percentages and tested using a Chi-square or a Fisher’s exact test
when the theoretical frequency was ≥1 or <5. Cox proportional
hazards regression analysis for time of death was used to identify
the factors associated with the increased risk of mortality. A
forward step method was used to define the final model and the
independent predictors of mortality. Results are presented as
hazard ratios (HR) and 95% confidence intervals (CI) for each
covariate in the model. The Kaplan-Meier survival curves were
plotted and the groups were compared using the log-rank test. All
P-values were calculated from a 2-tailed test and P<0.05 was
considered to indicate a statistically significant difference.
Results
Clinical parameters
The baseline characteristics of the 685 patients in
this study are shown in Table I.
Patients with an LVEF of ≤35% had a significantly higher heart rate
(87±19 versus 80±15 bpm, P=0.000), increased incidence of
ventricular tachycardia (18 versus 12%, P=0.021), New York Heart
Association (NYHA) classes III (41 versus 32%, P=0.017) and IV (30
versus 17%, P=0.000) upon admission, than the patients with LVEFs
of 36–45%. Compared to ejection fraction of 36% −45% of the
patients, the ejection fraction ≤35% of patients with low serum
sodium (140±4 versus 141±5 mmol/l, P=0.049). Furthermore, patients
with an LVEF of ≤35% were less likely to be receiving aspirin (62
versus 73%, P=0.003), nitrates (45 versus 54%, P=0.018), statins
(32 versus 49%, P=0.000) and clopidogrel (10 versus 23%, P=0.000),
but more likely to be taking diuretics (93 versus 85%, P=0.001),
digoxin (89 versus 70%, P=0.000), spironolactone (86 versus 78%,
P=0.009), coenzyme Q10 (33 versus 23%, P=0.004) and stem cells (13
versus 6%, P = 0.004). In addition, patients with an LVEF of ≤ 35%
had a higher incidence rate of left bundle branch block (9% vs. 5%,
P=0.041), as well as a larger left ventricular end-diastolic
dimension (69±9 versus 64±8 mm, P=0.000).
 | Table IClinical characteristics of the study
patients. |
Table I
Clinical characteristics of the study
patients.
| Parameter | All patients
(n=685) | LVEF ≤35%
(n=371) | LVEF 36–45%
(n=314) | P-value |
|---|
| Male | 462 (67) | 255 (69) | 207 (66) | 0.434 |
| Age (year) | 57±16 | 56±16 | 59±15 | 0.032 |
| BMI
(kg/m2) | 24±4 | 23±4 | 24±4 | 0.004 |
| SBP (mmHg) | 124±21 | 122±21 | 128±21 | 0.000 |
| DBP (mmHg) | 80±13 | 79±14 | 81±13 | 0.104 |
| HR (beats per
min) | 84±18 | 87±19 | 80±15 | 0.000 |
| Hemoglobin (g/l) | 128±19 | 129±19 | 126±19 | 0.012 |
| ALT (U/l) | 66±272 | 83±350 | 46±127 | 0.066 |
| AST (U/l) | 60±379 | 78±506 | 38±107 | 0.136 |
| Uric acid
(μmol/l) | 416±134 | 422±137 | 409±129 | 0.206 |
| Serum sodium
(mmol/l) | 140±5 | 140±4 | 141±5 | 0.049 |
| Ischemic
cardiomyopathy | 287 (42) | 123 (33) | 164 (52) | 0.000 |
| Atrial
fibrillation | 121 (18) | 63 (17) | 58 (19) | 0.610 |
| Hypertension | 223 (33) | 95 (26) | 128 (41) | 0.000 |
| Diabetes
mellitus | 118 (17) | 60 (16) | 58 (19) | 0.427 |
| Smoking | 190 (28) | 100 (27) | 90 (29) | 0.619 |
| Ventricular
tachycardiaa | 102 (15) | 66 (18) | 36 (12) | 0.021 |
| ICD implant | 15 (2) | 11 (3) | 4 (1) | 0.132 |
| CRT implant | 23 (3) | 14 (4) | 9 (3) | 0.511 |
| Stem cell | 68 (10) | 48 (13) | 20 (6) | 0.004 |
| KD stage |
| 1 (≥90) | 256 (37) | 140 (38) | 116 (37) | 0.831 |
| 2 (60–89) | 293 (43) | 163 (44) | 130 (41) | 0.504 |
| 3 (30–59) | 124 (18) | 62 (17) | 62 (20) | 0.304 |
| 4 (15–29) | 6 (1) | 2 (1) | 4 (1) | 0.421 |
| 5 (<15) | 6 (1) | 4 (1) | 2 (1) | 0.693 |
| NYHA class |
| II | 267 (39) | 107 (29) | 160 (51) | 0.000 |
| III | 253 (37) | 152 (41) | 101 (32) | 0.017 |
| IV | 165 (24) | 112 (30) | 53 (17) | 0.000 |
| Medications at
discharge |
| ACE
inhibitor/ARB | 569 (83) | 309 (83) | 260 (83) | 0.866 |
| β-blockers | 555 (81) | 302 (81) | 253 (81) | 0.783 |
| Digoxin | 550 (80) | 331 (89) | 219 (70) | 0.000 |
| Diuretics | 609 (89) | 343 (93) | 266 (85) | 0.001 |
| Nitrates | 333 (49) | 165 (45) | 168 (54) | 0.018 |
| Spirolactone | 563 (82) | 318 (86) | 245 (78) | 0.009 |
| Aspirin | 458 (67) | 230 (62) | 228 (73) | 0.003 |
| Statins | 272 (40) | 118 (32) | 154 (49) | 0.000 |
| Clopidogrel | 110 (16) | 37 (10) | 73 (23) | 0.000 |
| Coenzyme Q10 | 196 (29) | 123 (33) | 73 (23) | 0.004 |
| Sinus rhythm | 544 (79) | 296 (80) | 248 (79) | 0.796 |
| Conduction
block |
| LBBB | 50 (7) | 34 (9) | 16 (5) | 0.041 |
| RBBB | 31 (5) | 16 (4) | 15 (5) | 0.771 |
| LVEF (%) | 34±7 | 28±6 | 40±2 | 0.000 |
| LVEDD (mm) | 68±9 | 69±9 | 64±8 | 0.000 |
Survival analysis
The patients were followed up for a median of 31
months (range, 8–61 months). A total of 191 mortalities (28%) were
recorded, 127 of which were due to pump failure (19%) and 42 were
sudden deaths (6%). The all-cause mortality rate was 37% (n=137)
among patients with an LVEF of ≤35%, which was significantly higher
compared with 17% (n=54) in patients with LVEFs of 36–45%
(P=0.000). Pump failure death occurred in 25 and 12% of patients
with LVEFs of ≤35 and 36–45%, respectively (P=0.000). Moreover,
sudden death occurred in 8 and 4% of patients with LVEFs of ≤35 and
36–45%, respectively (P=0.046). The unadjusted mortality rates from
all causes and the Kaplan-Meier estimated survival for the two
groups are summarized in Table
II. The Kaplan-Meier estimated survival curve for all patients
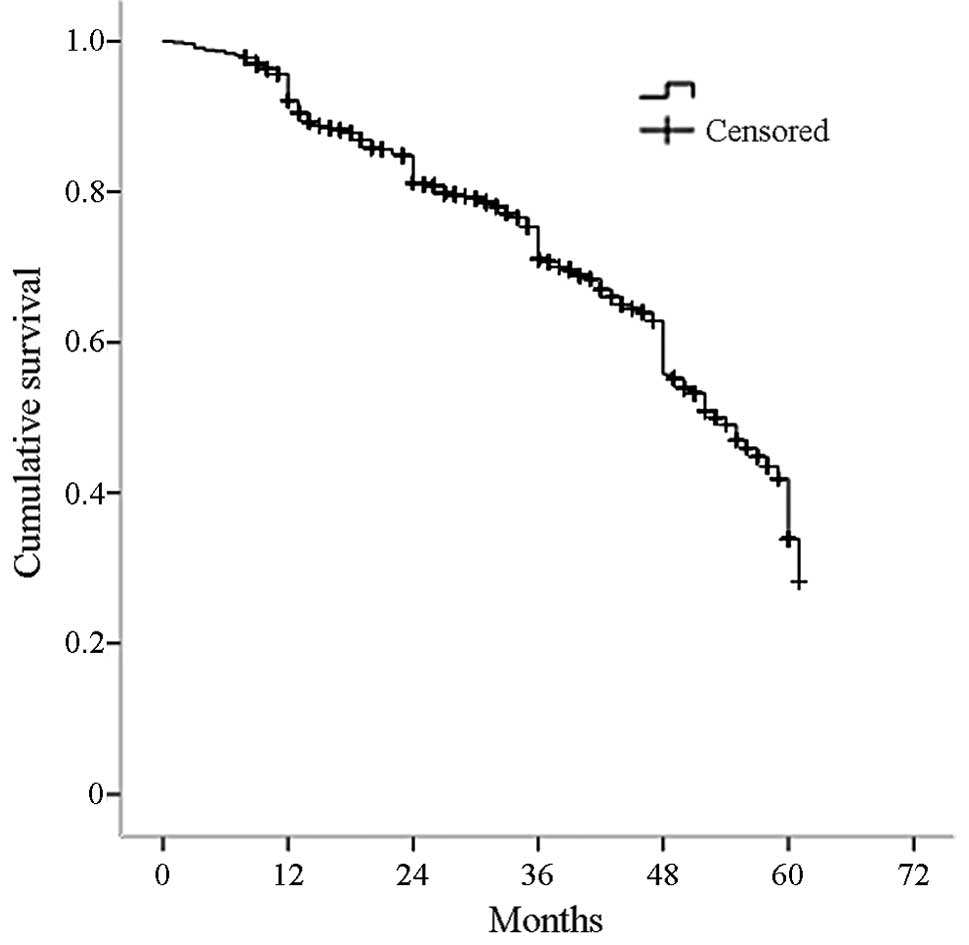
is shown in Fig. 1, with 3, 4 and
5-year survival rates of 71, 56 and 34%, respectively. The
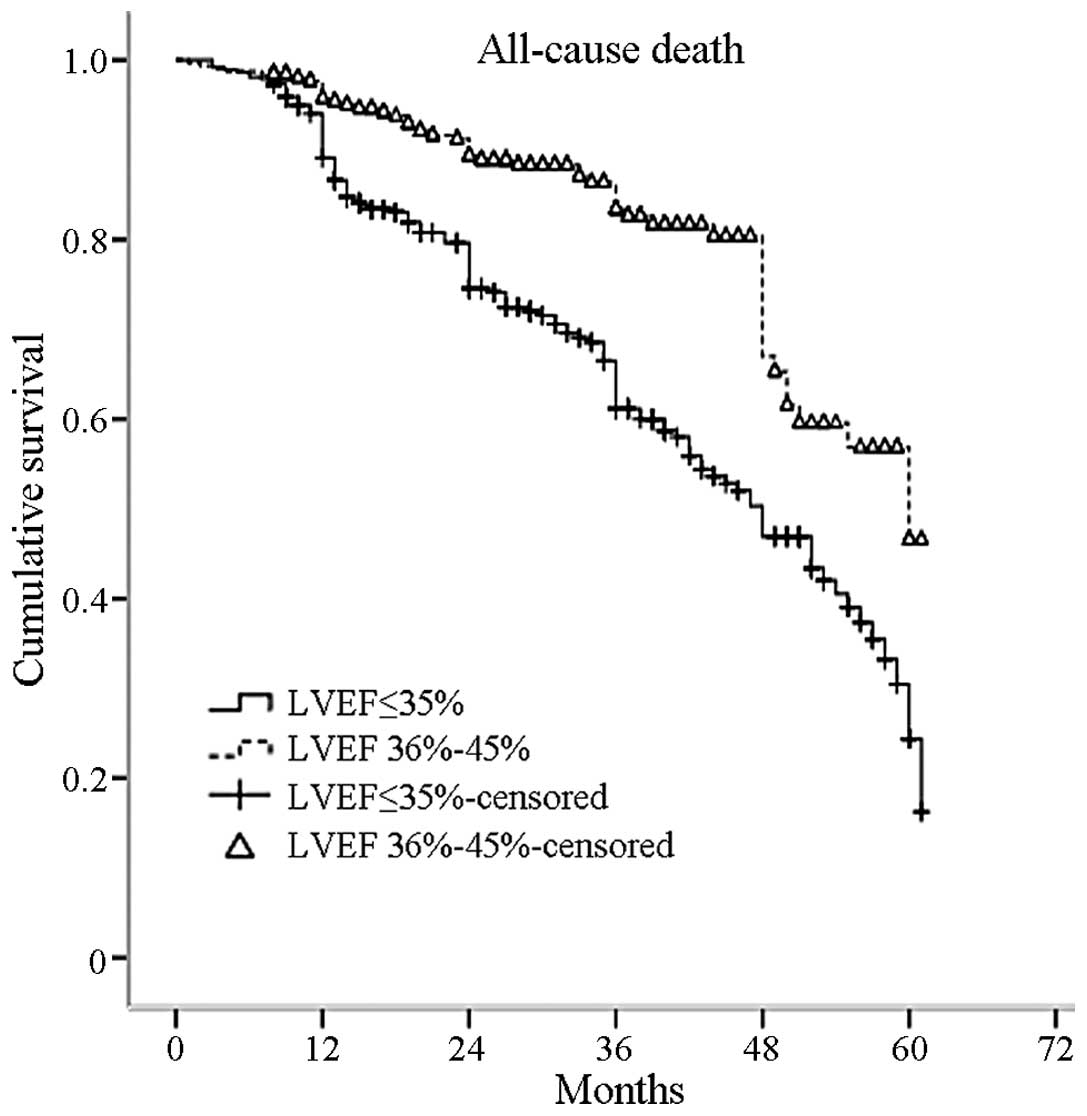
Kaplan-Meier estimated survival curves for the two groups are shown
in Fig. 2, with 3, 4 and 5-year
survival rates of 61, 47 and 25%, respectively, in patients with an
LVEF of ≤35% and 83, 66 and 46%, respectively, in patients with an
LVEF of 36–45% (P=0.000 log-rank test).
 | Table IIUnadjusted all-cause mortality and
Kaplan-Meier estimated survival rates. |
Table II
Unadjusted all-cause mortality and
Kaplan-Meier estimated survival rates.
| Survival rates
(%) | All patients
(n=685) (%) | LVEF ≤35% (n=371)
(%) | LVEF 36–45% (n=314)
(%) | P-value |
|---|
| All-cause
mortality | 191 (28) | 137 (37) | 54 (17) | 0.000 |
| 3-year
survival | 71 | 61 | 83 | 0.000a |
| 4-year
survival | 56 | 47 | 66 | - |
| 5-year
survival | 34 | 25 | 46 | - |
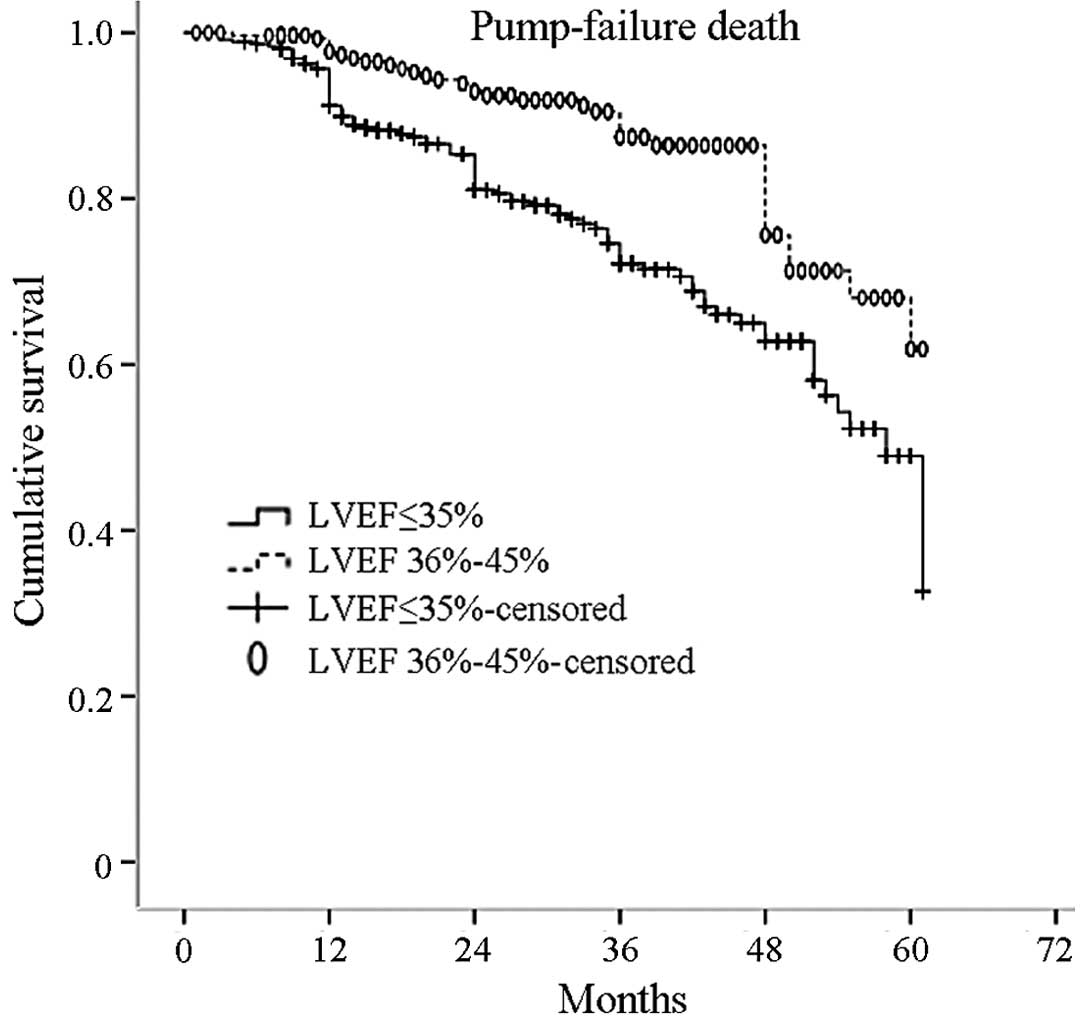
Kaplan-Meier curves describing the cumulative
survival probability of time to occurrence of pump failure death
for the two groups, is represented in Fig. 3 (P=0.000 log-rank test).
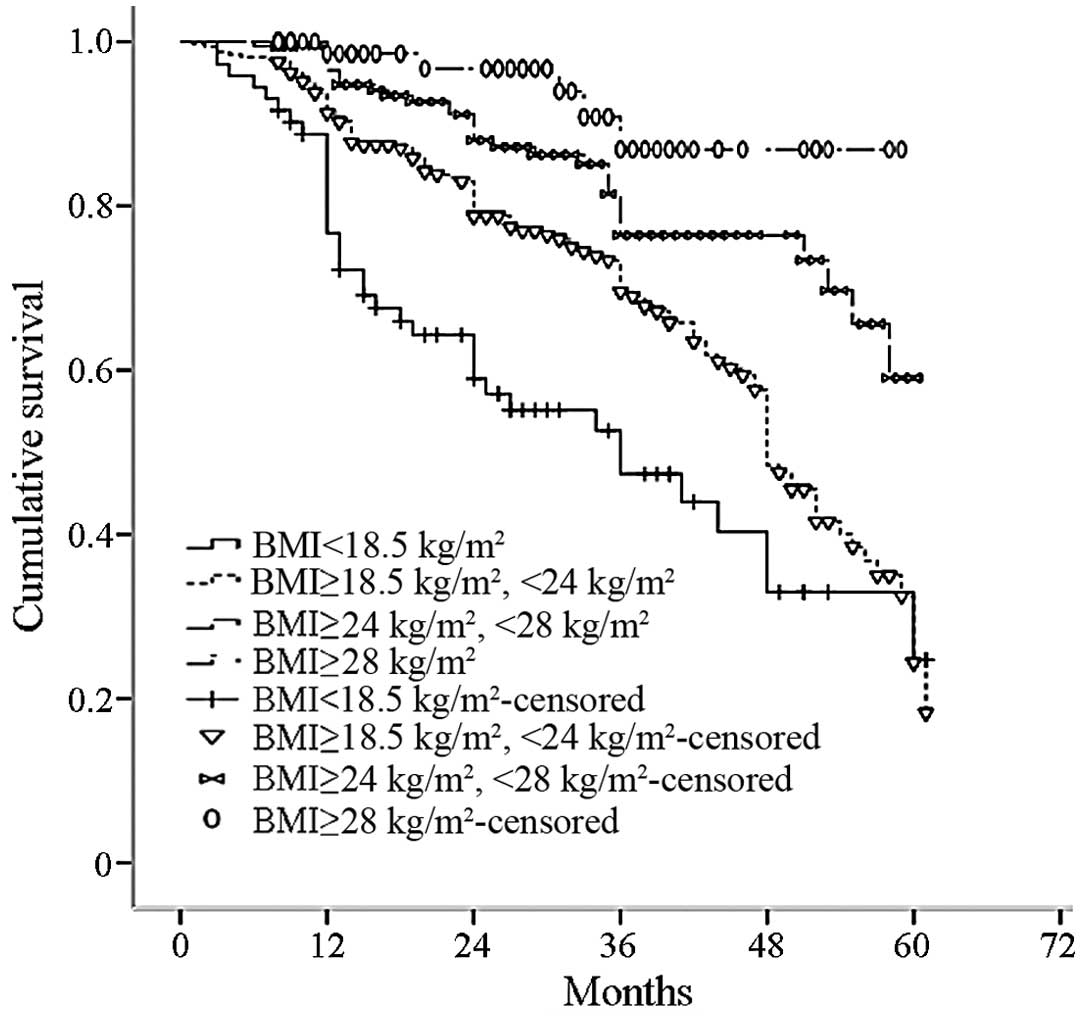
Using the recommended BMI classified according to
the Working Group of China Obesity (7), the patients were divided into four
groups: Low weight (BMI <18.5 kg/m2), normal weight
(18.5≤BMI<24.0 kg/m2), overweight (24.0≤BMI<28.0
kg/m2) and obese (BMI ≥28.0 kg/m2). The
all-cause mortality rate significantly increased with a reduction
in BMI (P=0.000; Fig. 4).
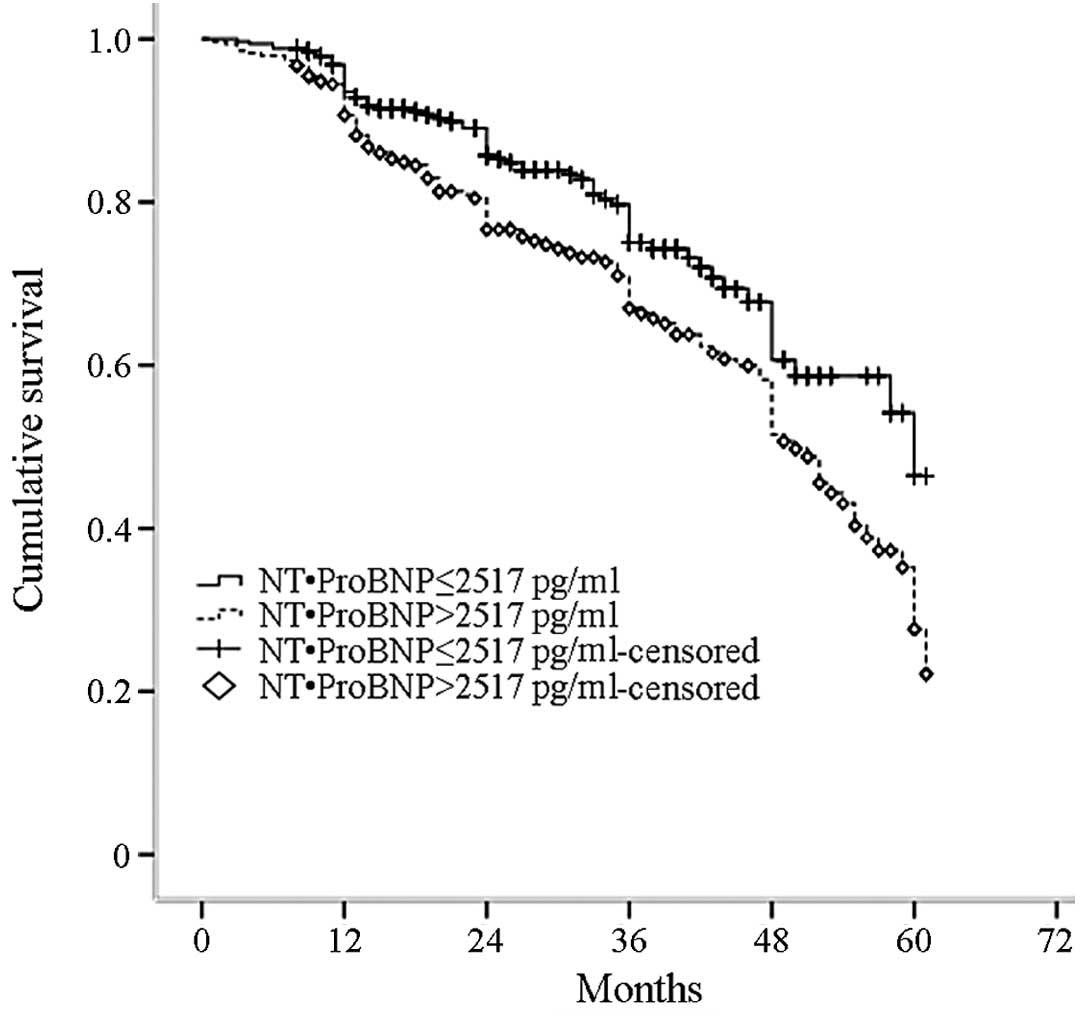
The N-terminal pro-brain natriuretic peptide
(NT-proBNP) median level was 2,517 pg/ml. The study population was
divided into two groups: NT-ProBNP ≤2,517 and >2,517 pg/ml. The
all-cause mortality rate significantly increased with an increasing
NT-ProBNP median level (P=0.003; Fig.
5).
The predictors of the all-cause mortality rate among
the study patients were advanced age and BMI, as well as the lack
of oral β-blockers at discharge and NYHA functional class. The
results of these multivariate analyses are reported in Table III.
 | Table IIIPredictors of all-cause mortality
after Cox analyses. |
Table III
Predictors of all-cause mortality
after Cox analyses.
| Parameter | Hazard Ratio (95%
CI) | P-value |
|---|
| Age (per increased
1 year) | 1.03
(1.02–1.04) | 0.000 |
| NYHA (per increased
1 class) | 1.59
(1.32–1.92) | 0.000 |
| β-blockers at
discharge | 0.69
(0.50–0.95) | 0.021 |
| BMI | 0.58
(0.48–0.72) | 0.000 |
| LVEF 36–45% | 0.52
(0.38–0.71) | 0.000 |
Medication status quo
During the follow-up period, 24% of the patients who
continued to receive angiotension-converting enzyme
inhibitor/angiotensin receptor blocker (ACEI/ARB) treatment and 23%
of those on β-blockers were taking the maximum tolerated dose. In
addition, 17 and 9% were taking the recommended target doses, 47
and 20%, were taking ≥50 to <100% of the target dose, 30 and 36%
were receiving ≥25 to <50% of the target dose and 7 and 35% were
taking <25% of the target dose.
Discussion
The present study examined the clinical
characteristics and outcomes of Chinese patients with HF and an
LVEF of ≤45%. The main findings were as follows: The predictors for
all-cause mortality were advanced age, NYHA class, BMI and lack of
β-blockers at discharge; the all-cause mortality for the entire
study population was 28% during the median follow-up period of 31
months and the 5-year survival rate of patients with HF and LVEFs
of ≤35 and 36–45%, were 25 and 46%, respectively; and up to 42% of
the patients had ischemic cardiomyopathy and >80% of patients
were taking β-blockers, ACEI/ARB, aspirin and diuretics at
discharge.
The European Society of Cardiology (ESC) integrated
data from 51 countries and demonstrated there are ≥15 million cases
of HF among 1 billion patients, as well as a considerable number of
patients with asymptomatic heart dysfunction (8). The American Heart Association (AHA)
identified that >5 million patients have HF in the United
States, a number that continues to increase by 550,000 patients per
year1. Furthermore, in Japan, the 3-year mortality of
patients with HF was reported in 2008 to be 29.2% (9). The situation in Europe is not
optimistic, with a 4-year survival rate of only 50% and 40% of the
patients admitted to hospitals due to HF are readmitted or die
within 1 year of treatment (10,11).
In the Framingham Heart study, 75% of men and 62% of women
succumbed during the 5-year follow-up time (12). In the current study, the 5-year
survival rate of patients was 34%, similar to the findings by
Rochester and colleagues in 1991 (13), who noted a 5-year survival rate of
33% among patients with HF during the 10-year follow-up period. The
predictors of mortality demonstrated in the current study are
consistent with previous reports (9,14).
McDonagh et al(15)
confirmed that, even for asymptomatic patients, the BNP mass
concentration was slightly elevated (≥17.9 ng/l), the risk of
mortality increased by two-fold (hazard ratio of 2.2) and that LVEF
is an important predictor of mortality. The COPERNICUS (16) NT-pro BNP substudy indicated that
NT-pro BNP levels above the median (>1,767 pg/ml) on admission
were independently associated with an increased risk of all-cause
mortality during follow-up. Simultaneously, the meta-analysis
conducted by Doust et al(17) showed that the BNP mass
concentration is closely related to the prognosis of patients with
HF. For each additional 100 ng/l increase in BNP mass
concentration, a 35% increase in the relative risk of mortality was
observed. Additionally, Zamora et al(18) assessed the relationship between BMI
and survival over a long-term follow-up period of ischemic and
non-ischemic HF and concluded that the obesity paradox was only
observed in patients with non-ischemic HF. In the MERIT-HF
(19) and CIBIS-II (20) trials, β-blockers reduced mortality
and sudden cardiac death (SCD), and metoprolol and bisoprolol
reduced the all-cause mortality by 34%. A further study (21) demonstrated that carvedilol
significantly reduced mortality by 35% (P=0.0014). The results of
the three large-scale clinical trials indicate that β-blockers
significantly improved the prognosis, showing a significant
reduction in total mortality (34–35%) and sudden death (41–45%). It
may be suggested that β-blockers are indispensible in the treatment
of HF; thus, patients should be prescribed the recommended dose to
suppress excessive activation of the sympathetic nervous system,
improve cardiac remodeling and reduce sudden death. However, in
clinical practice, the application of ACEI/ARB, β-blockers and
heart failure treatment guidelines still have a diverse gap;
therefore, these therapies should be used widely and consistently.
The Chinese Medical Society of Cardiology retrospectively analyzed
and compared results of 42 hospitals in 1980, 1990 and 2000 heart
failure hospitalization records, and observed that in 2000 the ACEI
use ratio was 40.4% in China. The use of β-blockers was <20%,
far less than in the United States and Europe in the 1990s (from 60
to 90%). In addition, ARB use was only 4.5% (22). Cao et al(23) examined 17 regions in China and
demonstrated that 10% of patients received high-dose digoxin
(≥0.125 mg/day), 90% of symptomatic patients with chronic HF were
prescribed diuretics and 80% of patients received ACEI. The
recommended dose application rate was only 2%, the use of
β-blockers was 40% and the rate of application of the recommended
dose was only 1%. The present study had a small sample size and was
a single-center study; therefore, the results may not be applicable
to other regions of China.
In China, patients with HFrEF have a poor prognosis,
particularly those with an LVEF of ≤35%. Cardiologists should aim
to improve the prognosis of HF among Chinese patients and focus on
the importance of diagnosis and treatment. Guidelines for the
practical application and recommended use of treatment agents
should be provided and efforts made to reach the recommended target
dose or the maximum tolerated dose in patients.
References
|
1
|
Rosamond W, Flegal K, Friday G, et al;
American Heart Association Statistics Committee and Stroke
Statistics Subcommittee. Heart disease and stroke statistics - 2007
update: a report from the American Heart Association Statistics
Committee and Stroke Statistics Subcommittee. Circulation.
115:e69–e171. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mendez GF and Cowie MR: The
epidemiological features of heart failure in developing countries:
a review of the literature. Int J Cardiol. 80:213–219. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
World Health Organization. Cardiovascular
disease: prevention and control (EB/OL). http://www.who.int/dietphysicalactivity/publications/facts/cvd/en/uri.
Accessed March 07, 2010
|
|
4
|
Cheng Z, Zhu K, Chen T, et al: Poor
prognosis in chronic heart failure patients with reduced ejection
fraction in China. Congest Heart Fail. 18:165–172. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McKee PA, Castelli WP, McNamara PM and
Kannel WB: The natural history of congestive heart failure: the
Framingham study. N Engl J Med. 285:1441–1446. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
National Kidney Foundation. K/DOQI
clinical practice guidelines for chronic kidney disease:
evaluation, classification, and stratification. Am J Kidney Dis.
39(2 Suppl 1): S1–S266. 2002.PubMed/NCBI
|
|
7
|
Zhou B; Cooperative Meta-Analysis Group of
China Obesity Task Force. Predictive values of body mass index and
waist circumference to risk factors of related diseases in Chinese
adult population. Chinese Journal of Epidemiology. 23:5–10.
2002.(In Chinese).
|
|
8
|
Dickstein K, Cohen-Solal A, Filippatos G,
et al: ESC guidelines for the diagnosis and treatment of acute and
chronic heart failure 2008: the Task Force for the diagnosis and
treatment of acute and chronic heart failure 2008 of the European
Society of Cardiology. Developed in collaboration with the Heart
Failure Association of the ESC (HFA) and endorsed by the European
Society of Intensive Care Medicine (ESICM). Eur J Heart Fail.
10:933–989. 2008.
|
|
9
|
Kawashiro N, Kasanuki H, Ogawa H, Matsuda
N and Hagiwara N; Heart Institute of Japan - Department of
Cardiology (HIJC) Investigators. Clinical characteristics and
outcome of hospitalized patients with congestive heart failure:
results of the HIJC-HF registry. Circ J. 72:2015–2020. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cowie MR, Wood DA, Coats AJ, et al:
Survival of patients with a new diagnosis of heart failure: a
population based study. Heart. 83:505–510. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stewart S, MacIntyre K, Hole DJ, Capewell
S and McMurray JJ: More ‘malignant’ than cancer? Five-year survival
following a first admission for heart failure. Eur J Heart Fail.
3:315–322. 2001.
|
|
12
|
Ho KK, Anderson KM, Kannel WB, Grossman W
and Levy D: Survival after the onset of congestive heart failure in
Framingham Heart Study subjects. Circulation. 88:107–115. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Senni M, Tribouilloy CM, Rodeheffer RJ, et
al: Congestive heart failure in the community: trends in incidence
and survival in a 10-year period. Arch Intern Med. 159:29–34.
1999.PubMed/NCBI
|
|
14
|
Levy WC, Mozaffarian D, Linker DT, et al:
The Seattle Heart Failure Model: prediction of survival in heart
failure. Circulation. 113:1424–1433. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McDonagh TA, Cunninghanl AD, Morrison CE,
et al: Left ventricular dysfunction, natriuretic peptides, and
mortality in an urban population. Heart. 86:21–26. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hartmann F, Packer M, Coats AJ, et al:
Prognostic impact of plasma N-terminal pro-brain natriuretic
peptide in severe chronic congestive heart failure: a substudy of
the Carvedilol Prospective Randomized Cumulative Survival
(COPERNICUS) trial. Circulation. 110:1780–1786. 2004. View Article : Google Scholar
|
|
17
|
Doust JA, Pietrzak E, Dobson A and
Glasziou P: How well does B-type natriuretic peptide predict death
and cardiac events in patients with heart failure: systematic
review. BMJ. 330:6252005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zamora E, Lupón J, de Antonio M, et al:
The obesity paradox in heart failure: Is etiology a key factor? Int
J Cardiol. 166:601–605. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
No authors listed. Effect of metoprolol
CR/XL in chronic heart failure: Metoprolol CR/XL Randomised
Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet.
353:2001–2007. 1999. View Article : Google Scholar
|
|
20
|
No authors listed. The Cardiac
Insufficiency Bisoprolol Study II (CIBIS-II): a randomized trial.
Lancet. 353:9–13. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Packer M, Fowler M, Rouleau J, et al:
COPERNICUS (carvedilol prospective randomized cumulative survival
trial): A multicenter randomized double-blind placebo-controlled
study to determine the effect of carvedilol on mortality in severe
congestive heart failure. Cardiovasc Drugs Ther. 13:241999.
|
|
22
|
Society of Cardiology, Chinese Medical
Association. Retrospective investigation of hospitalized patients
with heart failure in some parts of China in 1980, 1990 and 2000.
Chinese Journal of Cardiology. 30:450–454. 2002.(In Chinese).
|
|
23
|
Cao YM, Hu DY, Wang HY and Wu Y: A survey
of medical therapies for chronic heart failure in primary hospitals
in China. Chinese Journal of Internal Medicine. 45:907–909.
2006.(In Chinese).
|