Introduction
Colorectal cancer (CRC) is a cancer that develops
from uncontrolled cell growth in the colon or rectum (parts of the
large intestine), or in the appendix (1). Genetic analysis has shown that colon
and rectal tumors are essentially the same type of cancer (2). The symptoms of CRC typically include
rectal bleeding and anemia, which may occur with weight loss and
changes in bowel habits (3). The
majority of the cases of CRC occur due to lifestyle and increasing
age; only a minority of cases are associated with underlying
genetic disorders (4). The disease
typically starts in the lining of the bowel and, if left untreated,
may grow into the muscle layers underneath and then through the
bowel wall (5). Cases of CRC that
are confined within the wall of the colon are often curable with
surgery, while cancer that has spread widely around the body is
usually incurable. In such instances, disease management focuses on
extending the life of the patient using chemotherapy and improving
the patient's quality of life (6).
CRC is the third most frequently diagnosed type of cancer in males
and the second most frequently diagnosed type of cancer in females,
and was estimated to account for >1.2 million new cancer cases
and 608,700 mortalities in 2008 (7). At present, there is a focus on
chemotherapy for tumors (8).
Despite the fact that considerable progress has been made in recent
years, the pathogenesis and treatment of CRC remain unclear.
S100A3 is a matricellular protein, which is
expressed in numerous tissues and cell types (9). The S100A3 protein is a protein that
in humans is encoded by the S100A3 gene. The protein encoded by the
S100A3 gene is a member of the S100 family of proteins containing
two EF-hand calcium-binding motifs (10–13).
Over the last decade it has become increasingly apparent that
S100A3 is an important mediator, although it is unclear whether
S100A3 is important in CRC and whether it is possible to inhibit
S100A3 with drug treatment. At present, fluorouracil is one of the
standard chemotherapeutic drugs used in the treatment of CRC
(14). In the past decade, the
treatment options for CRC have expanded and include additional
chemotherapeutic agents and targeted therapies (cetuximab,
panitumumab and bevacizumab) (15). The proper use of these therapies
has had a major impact on the prognoses of patients (16).
In recent years, data concerning the treatment of
cancer with traditional Chinese medicine have had a considerable
influence with regard to the identification of specific molecular
markers and pathway aberrations that may guide treatment decisions
(17). However, it has not yet
been elucidated whether traditional Chinese medicine is able to
inhibit the expression of S100A3 and prevent the symptoms of CRC.
Cantharidin (also its acid form cantharidinate) has been used in
traditional Chinese medicine (18,19).
Cantharidinate induces cell cycle arrest and triggers apoptosis in
various types of tumor cells, including hepatoma, myeloma, oral
buccal carcinoma, leukemia, gastric cancer, TSGH-8301 human bladder
carcinoma, Colo205 CRC, A549 human lung cancer and intestinal
epithelial cells (20–23).
In the present study, we investigated whether S100A3
is important in CRC and whether cantharidinate may be used to
inhibit the expression of S100A3.
Materials and methods
Patients and tissue specimens
Twenty patients, comprising 12 males and 8 females,
with an average age of 68.25 years (range, 21–87 years) were
included in this study. Human CRC tissue specimens were obtained by
surgical resection from May 2011 to June 2012 in the Jilin
University Second Hospital (Changchun, China). The study was
approved by the Ethics Committee of Jilin University Second
Hospital (no. 2012-43) and patient consent was obtained. Tissue
microarrays (TMAs) were constructed. The histological grade of the
tumor and its site (colon or sigmoid colon) were recorded.
Histopathological examination
The specimens and cells were examined under a light
microscope (Eclipse TE-2000-U, equipped with an attached digital
camera SXM1200F, Nikon, Tokyo, Japan) following hematoxylin and
eosin (H&E) staining.
Immunohistochemical staining
Paraffin-embedded slices, measuring 4 μm in
thickness, were probed with anti-human S100A3 monoclonal antibody
(Sigma, St. Louis, MO, USA; 1:300 diluted for use) at 4°C
overnight. The sections were then immersed in 0.3%
H2O2 in absolute methanol for 15 min to block
endogenous peroxidase. The color was developed using the chromagen
3,3′-diaminobenzidine (DAB) with ABC immunohistochemistry kits from
Beijing Biosynthesis Biotechnology Co., Ltd. (Beijing, China). The
slices were subsequently counterstained with hematoxylin, mounted
on glass coverslips and sealed with neutral resin.
UCT-116 cell culture and treatment
UCT-116 human CRC cell lines were donated by the
Jilin University Institute of Regenerative Medicine. UCT-116 cells
were routinely cultured in Dulbecco's modified Eagle medium (DMEM;
Gibco-BRL, Invitrogen Life Technologies, Gaithersburg, MD, USA)
supplemented with 20% fetal bovine serum (FBS) and 50 U/ml
antibiotics under the conditions of 5% CO2 at 37°C.
Following trypsinization to passage the cells, the cells were
incubated in DMEM with 0.5% FBS for 24 h and then treated with 2.5
μmol/l cantharidinate (verified by Professor B. Liu, Jilin
University Second Hospital) or 2.5 μmol/l fluorouracil in DMEM with
0.5% FBS, respectively, or were left untreated as control cells for
48 h.
Quantitative polymerase chain reaction
(qPCR) analysis
Total RNA (mRNA) was extracted from colorectal
cancer and adjacent non-tumorous tissues using TRIzol®
reagent (Invitrogen Life Technologies, Carlsbad, CA, USA), in
accordance with the manufacturer's instructions. Total RNA (1 μg)
was reverse transcribed to complementary DNA (cDNA) with oligo (dT)
primers. The sense and antisense primers for S100A3 were designed
according to the mRNA sequence (GenBank accession no. NM-002960.1)
and synthesized by Shanghai Sangon Biological Engineering Co. Ltd.
(Shanghai, China). Amplified PCR fragments spanning different exons
were used to prevent the amplification of contaminated genomic DNA.
The primer sequences of S100A3 were as follows: sense,
5′-GACCATCTGGTTCAGGTTCC-3′ and antisense,
5′-ACATTCCCGAAACTCAGTCG-3′. The PCR products were 200 bp in length.
The housekeeping gene reduced glyceraldehyde-phosphate
dehydrogenase (GAPDH) was used as an internal control, with the
primer sequences as follows: sense, 5′-CCAGGTGGTCTCCTCTGACTT-3′ and
antisense, 5′-GTTGCTGTAGCCAAATTCGTTGT-3′.
Statistical analysis
The statistical analysis of the data was performed
using SPSS statistical software version 11 for Windows (SPSS, Inc.,
Chicago, IL, USA). All data are presented as the mean ± standard
error of the mean. Statistical comparisons were conducted using the
Student's t-test; P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinicopathological features and patient
outcome
The present study was performed on a TMA constructed
from the surgical resection samples of patients with CRC with a
range of grades of differentiation. The samples were collected as
part of a trial comparing the expression of S100A3 in carcinoma and
control tissues. The demographics of the patients are shown in
Table I, along with the
clinicopathological features of the colon and sigmoid and the
grades of differentiation. There was an incidence of 8.3% for
grades IIB, IIC, IIIB and IIIA CRC, respectively, in males. In
females, the incidence of grades IIB and IIC CRC was zero, and the
incidence of grades IIIB and IIIA was 25.0 and 12.5%, respectively.
The incidence of cancer in the colon and sigmoid colon was 75 and
25% in males, and 50 and 50% in females, respectively.
 | Table IClinicopathological features of the
cohort of patients with colorectal cancer (n=20). |
Table I
Clinicopathological features of the
cohort of patients with colorectal cancer (n=20).
| Variable | Male (n=12) | Female (n=8) |
|---|
| Age (years) |
| Mean | 63.50 | 73.38 |
| Range | 21–80 | 63–87 |
| Minimum (%) | 21 (12.5) | 63 (25.0) |
| Maximum (%) | 80 (12.5) | 87 (37.5) |
| Grade of
differentiation (n) |
| IIB (%) | 1 (8.3) | 0 (0) |
| IIC (%) | 1 (8.3) | 0 (0) |
| IIIB (%) | 1 (8.3) | 2 (25.0) |
| IIIA (%) | 1 (8.3) | 1 (12.5) |
| Colon (%) | 9 (75.0) | 4 (50.0) |
| Sigmoid (%) | 3 (25.0) | 4 (50.0) |
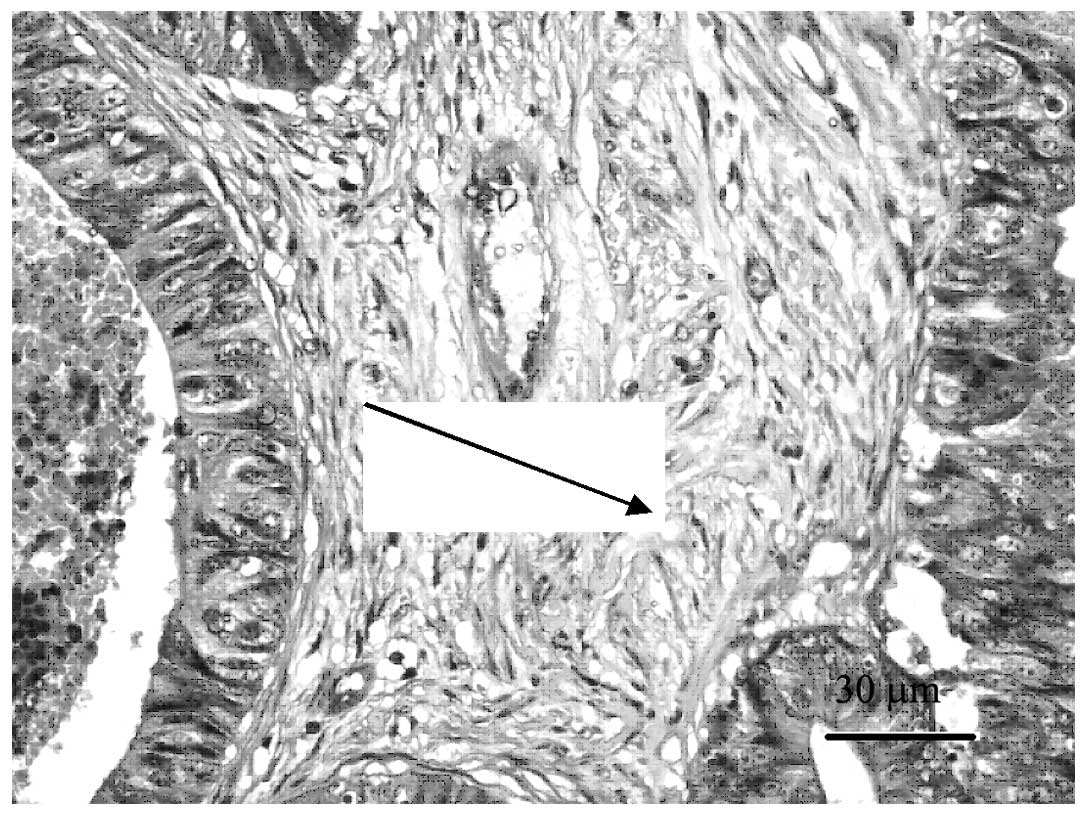
H&E of human CRC tissues
Following conventional H&E staining, the CRC
TMAs were observed to have representative histological structures
when viewed under a microscope (Fig.
1). The immunohistochemical staining of the microarray samples
was representative (Fig. 2).
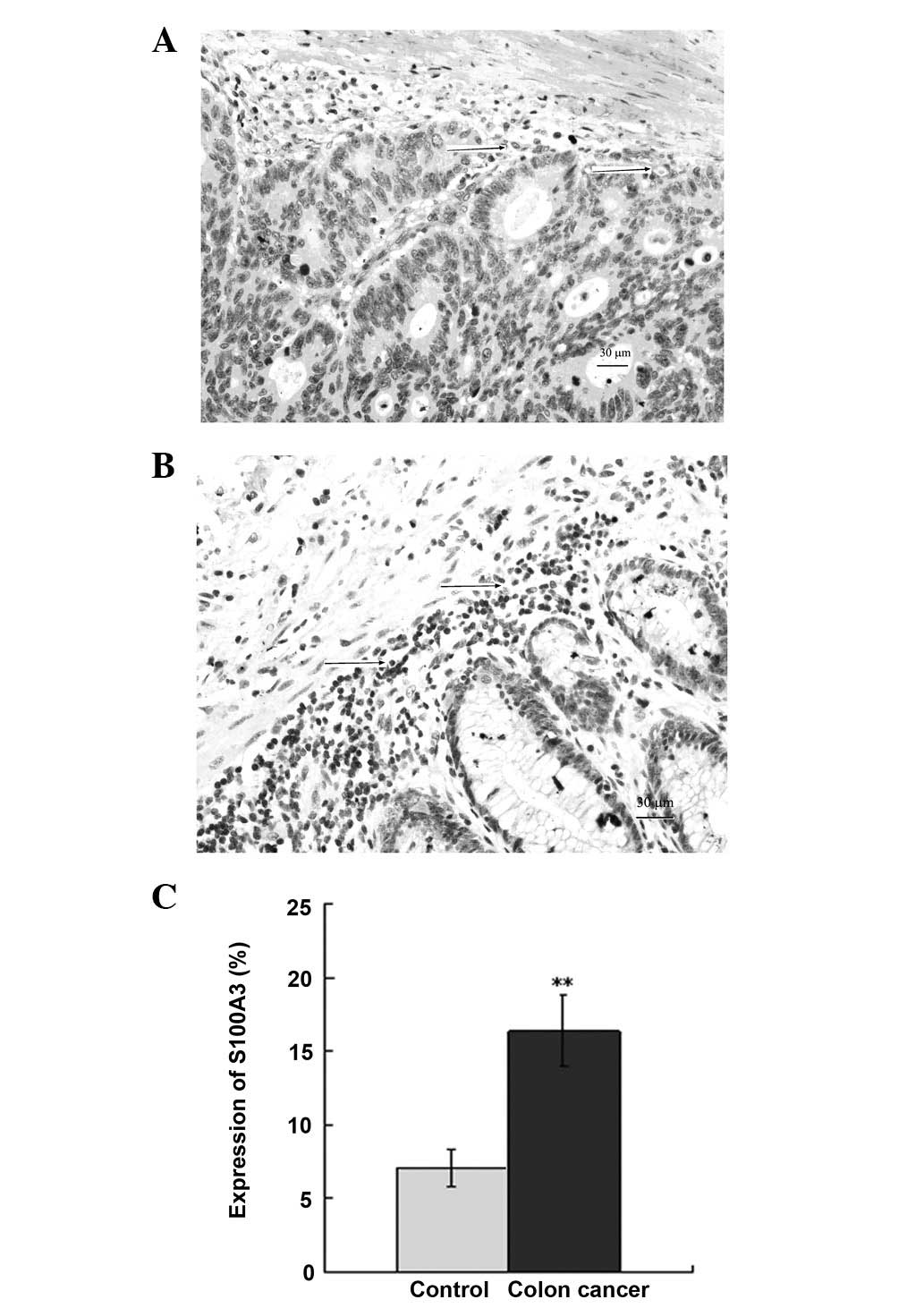
Immunohistochemical staining of S100A3 in
human CRC tissues
Immunohistochemical staining was used to assess the
protein expression of S100A3 in human CRC tissues. The results
showed that S100A3 was expressed in the membrane and cytoplasm in
the normal tissue of the patients with CRC (Fig. 2A). The expression of S100A3
increased notably in the CRC tissues (Fig. 2B), with the expression
predominantly in the tumor and tumor interstitial regions. There
was a significant difference between the expression of S100A3 in
the CRC and normal tissues (P<0.01; Fig. 2C).
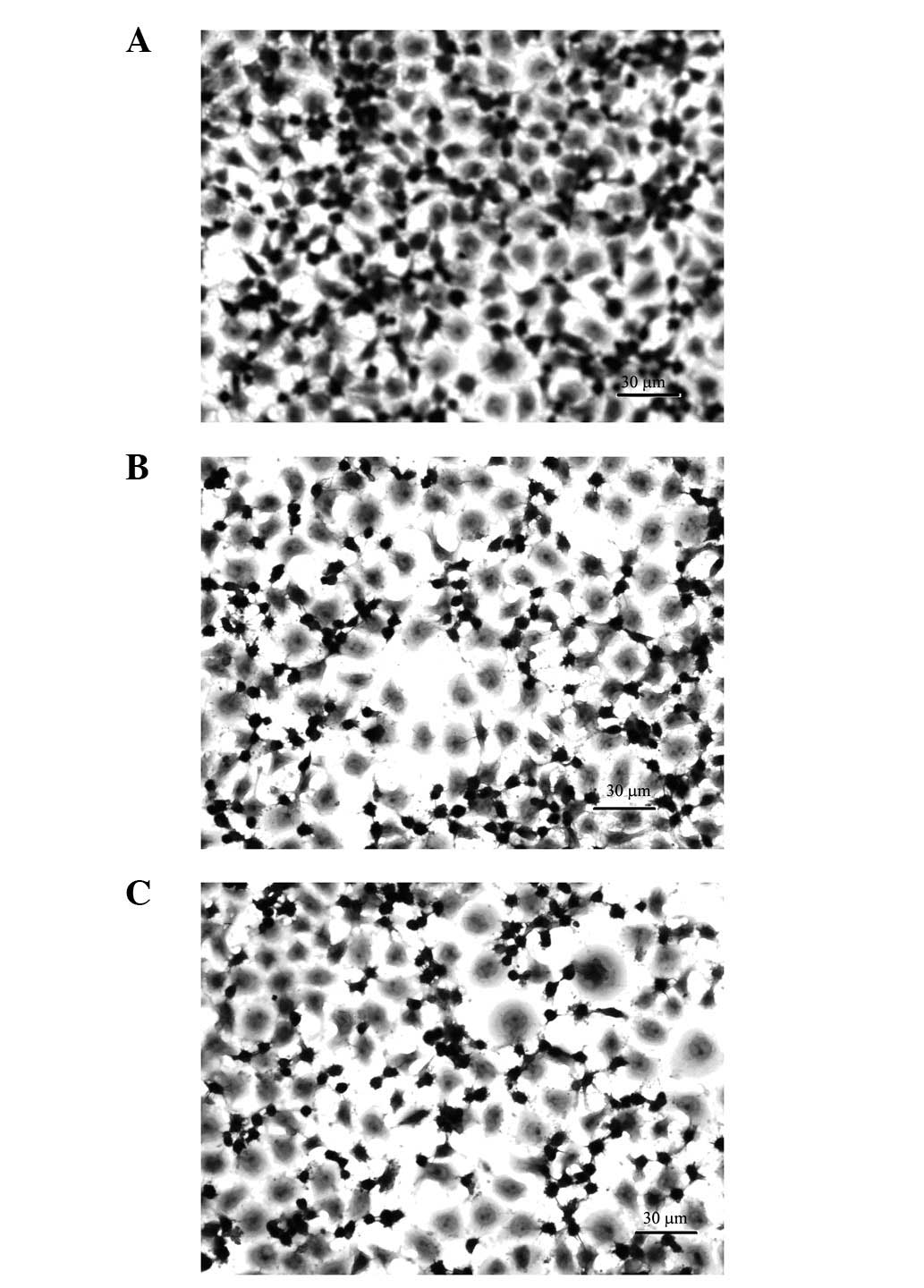
H&E staining of UCT-116 cells
The pathological changes in the different groups of
UCT-116 human CRC cells are shown in Fig. 3. The quantity of UCT-116 cells
decreased 48 h subsequent to the application of fluorouracil and
cantharidinate (Fig. 3B and C).
This result suggested that cantharidinate may reduce the time
required for chemotherapy and subsequently decrease the risk of the
treatment for human CRC.
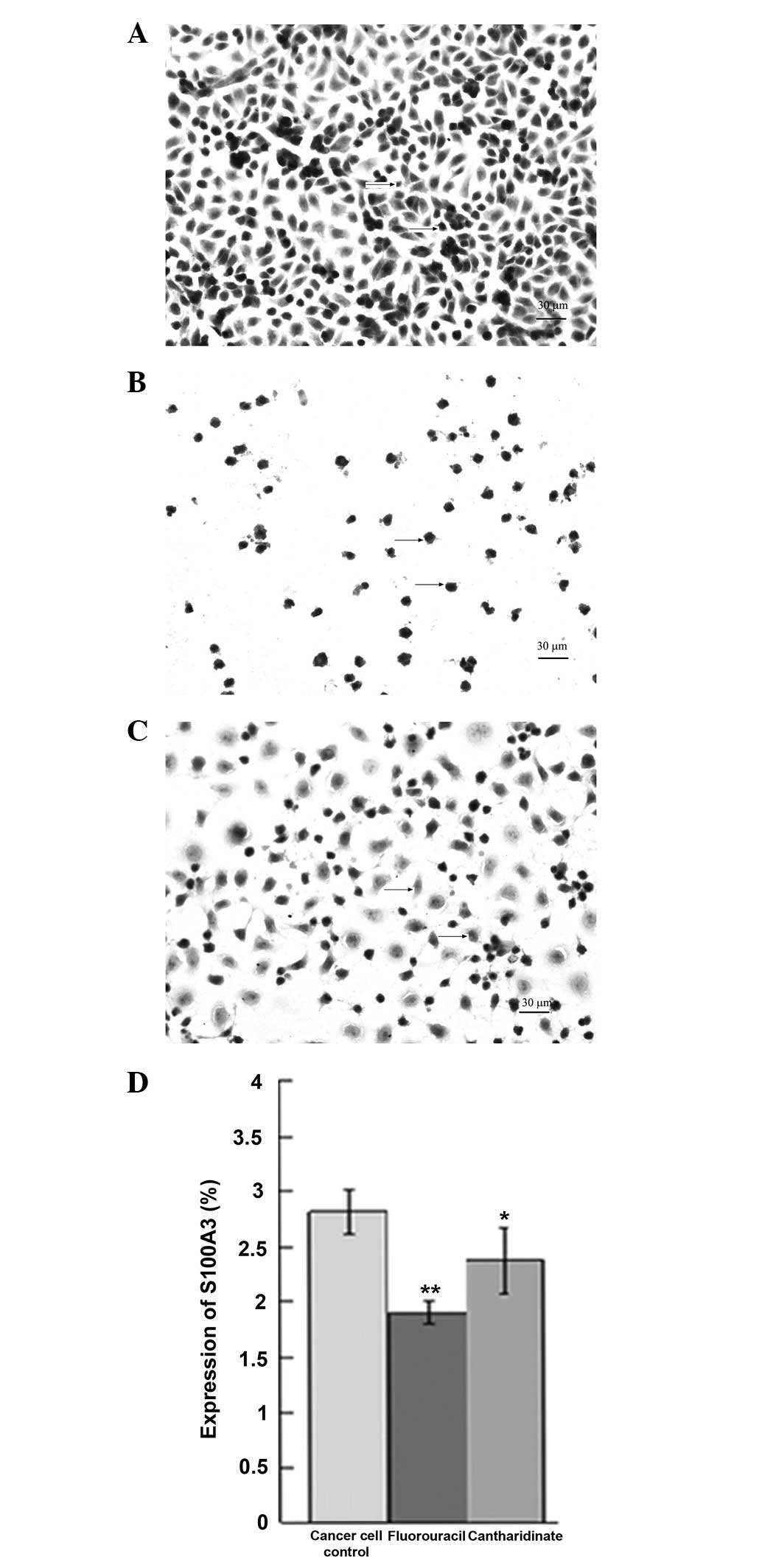
Immunohistochemical staining detects
S100A3 expression in UCT-116 cells
Fig. 4 shows the
S100A3 expression in UCT-116 cells, as observed using
immunohistochemical staining. A high level of S100A3 protein was
detected in the untreated UCT-116 cell controls (Fig. 4A and D). The cantharidinate and
fluorouracil treatments each reduced the level of S100A3 in the
UCT-116 cells significantly (P<0.05; Fig. 4B–D). The protein expression of
S100A3 increased by 2.4-fold in human CRC cells compared with the
expression level in normal control cells (P<0.01). These results
suggest that cantharidinate is able to inhibit the expression of
S100A3 and, therefore, may have the ability to block tumor
growth.
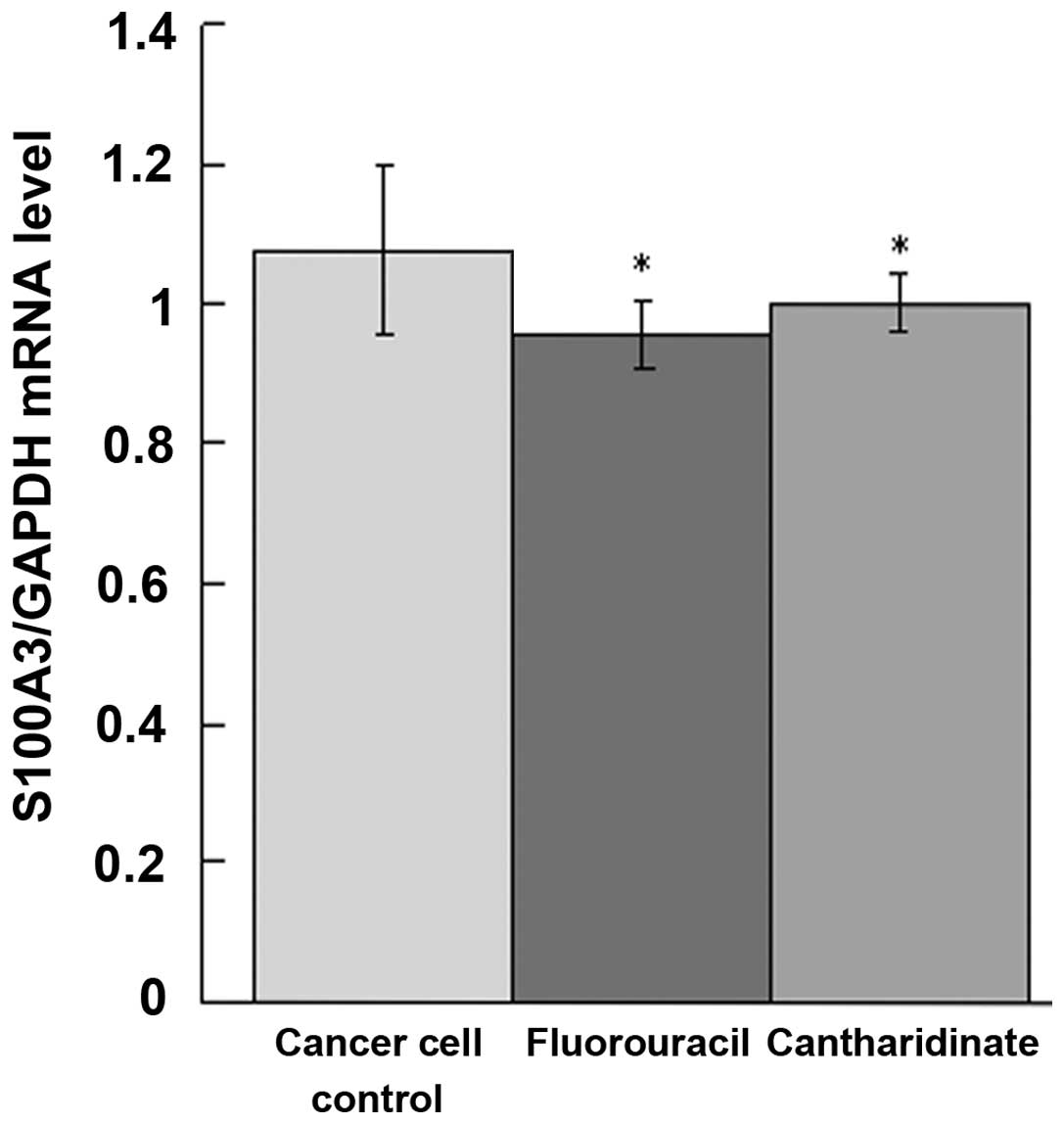
qPCR analysis of result
To determine whether the S100A3 mRNA level in the
UCT-116 cells changed following the application of cantharidinate,
qPCR analysis was performed. As shown in Fig. 5, the expression of S100A3 mRNA in
the cantharidinate group decreased to 0.88-fold that of the
untreated cancer cell controls (P<0.05). The level of S100A3 in
the cantharidinate group was similar to that in the fluorouracil
group. This result suggested that the ability of cantharidinate to
kill tumor cells may be due to its ability to inhibit S100A3 mRNA
expression (Fig. 5).
Discussion
CRC is the third most frequently diagnosed cancer in
the world. It is more common in developed countries (24), with ~60% of cases being diagnosed
in the developed world (25).
However, the pathogenesis on CRC remains unclear. The prevention of
genetic mutations and treatment of mutant genes is of critical
significance. Studies have demonstrated that S100A3 belongs to a
family of structurally and functionally associated proteins that
are widely distributed in tumors. There has been a surge in studies
that have produced results indicating that the dysregulated
expression and function of S100A3 contributes to pathological
conditions, such as cancer metastasis, celiac disease and diseases
associated with defective assembly (10–13).
It is unclear whether S100A3 is significant in CRC.
The present study demonstrated that the level of S100A3 was
increased in the process of tumor occurrence and progression, and
that S100A3 expression in human CRC was inhibited by
cantharidinate. A desirable property of an anticancer drug is the
ability to induce the death of tumor cells with few side-effects on
normal cells (16,26). The present study demonstrated that
cantharidinate has inhibitory activity against S100A3 in human CRC.
The effects of cantharidinate were similar to those of
fluorouracil. Cantharidinate was able to inhibit the proliferation
of human CRC cells with an IC50 value of 2.5 μM and
exhibited little cytotoxicity in normal cells (data not shown). The
present study demonstrated that cantharidinate reduced the mRNA and
protein expression of S100A3 in human CRC cells. To the best of our
knowledge, this is the first study that has shown that the mRNA and
protein expression levels of S100A3 are downregulated by
cantharidinate (18–23).
In conclusion, S100A3 is important in human CRC.
Cantharidinate is able to inhibit the expression of S100A3 and may
be considered as a novel additional drug that may be used to
control the expression of S100A3 in human CRC and the growth of
human CRC.
References
|
1
|
Deng X, Cao Y, Liu Y, Li F, et al:
Overexpression of Evi-1 oncoprotein represses TGF-β signaling in
colorectal cancer. Mol Carcinog. 52:255–264. 2013.PubMed/NCBI
|
|
2
|
Potter JD: Colorectal cancer: molecules
and populations. J Natl Cancer Inst. 91:916–932. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Domínguez-Ayala M, Díez-Vallejo J and
Comas-Fuentes A: Missed opportunities in early diagnosis of
symptomatic colorectal cancer. Rev Esp Enferm Dig. 104:343–349.
2012.PubMed/NCBI
|
|
4
|
Fearon ER: Molecular genetics of
colorectal cancer. Annu Rev Pathol. 6:479–507. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chan EC, Barry MJ, Vernon SW and Ahn C:
Brief report: physicians and their personal prostate
cancer-screening practices with prostate-specific antigen. A
national survey. J Gen Intern Med. 21:257–259. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Simoglou C, Gymnopoulou E, Simoglou L,
Gymnopoulou M, Nikolaou K and Gymnopoulos D: Surgery for colorectal
cancer in the small town of Komotini. J Multidiscip Healthc.
5:273–276. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kotzev I, Mirchev M, Manevska B, Ivanova I
and Kaneva M: Risk and protective factors for development of
colorectal polyps and cancer (Bulgarian experience).
Hepatogastroenterology. 55:381–387. 2008.PubMed/NCBI
|
|
8
|
Posner MR: Paradigm shift in the treatment
of head and neck cancer: the role of neoadjuvant chemotherapy.
Oncologist. 10(Suppl 3): S11–S19. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fritz G, Mittl PR, Vasak M, Grutter MG and
Heizmann CW: The crystal structure of metal-free human EF-hand
protein S100A3 at 1.7-A resolution. J Biol Chem. 277:33092–33098.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kizawa K, Takahara H, Unno M and Heizmann
CW: S100 and S100 fused-type protein families in epidermal
maturation with special focus on S100A3 in mammalian hair cuticles.
Biochimie. 93:2038–2047. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kizawa K, Inoue T, Yamaguchi M, Kleinert
P, Troxler H, Heizmann CW and Iwamoto Y: Dissimilar effect of
perming and bleaching treatments on cuticles: advanced hair damage
model based on elution and oxidation of S100A3 protein. J Cosmet
Sci. 56:219–226. 2005.PubMed/NCBI
|
|
12
|
Kizawa K, Unno M, Takahara H and Heizmann
CW: Purification and characterization of the human cysteine-rich
S100A3 protein and its pseudo citrullinated forms expressed in
insect cells. Methods Mol Biol. 963:73–86. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kizawa K, Jinbo Y, Inoue T, Takahara H,
Unno M, Heizmann CW and Izumi Y: Human S100A3 tetramerization
propagates Ca2+/Zn2+ binding states. Biochim
Biophys Acta. 1833:1712–1719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
van Hazel GA, Pavlakis N, Goldstein D,
Olver IN, Tapner MJ, Price D, Bower GD, Briggs GM, Rossleigh MA,
Taylor DJ and George J: Treatment of fluorouracil-refractory
patients with liver metastases from colorectal cancer by using
yttrium-90 resin microspheres plus concomitant systemic irinotecan
chemotherapy. J Clin Oncol. 27:4089–4095. 2009.
|
|
15
|
Gerber DE: Targeted therapies: a new
generation of cancer treatments. Am Fam Physician. 77:311–319.
2008.PubMed/NCBI
|
|
16
|
Dehmer GJ, Douglas JS Jr, Abizaid A, Berg
JW, Day J, Hall R, Leon MB, Hijazi ZM, Marchlinski F, Park SJ and
Popma JJ: SCAI/ACCF/HRS/ESC/SOLACI/APSIC statement on the use of
live case demonstrations at cardiology meetings: assessments of the
past and standards for the future. Heart Rhythm. 7:1522–1535. 2010.
View Article : Google Scholar
|
|
17
|
Liu HG and Huang HX: Overview
pharmacokinetic about traditional Chinese medicine in recent 10
years. Zhongguo Zhong Yao Za Zhi. 32:2346–2348. 2007.(In
Chinese).
|
|
18
|
Honkanen RE: Cantharidin, another natural
toxin that inhibits the activity of serine/threonine protein
phosphatases types 1 and 2A. FEBS Letters. 330:283–286. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Deng LP, Dong J, Cai H and Wang W:
Cantharidin as an antitumor agent: a retrospective review. Curr Med
Chem. 20:159–166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang YP, Ni CH, Lu CC, Chiang JH, Yang
JS, Ko YC, Lin JP, Kuo JH, Chang SJ and Chung JG: Suppressions of
migration and invasion by cantharidin in TSGH-8301 human bladder
carcinoma cells through the inhibitions of matrix
metalloproteinase-2/−9 signaling. Evid Based Complement Alternat
Med. 2013:1902812013.PubMed/NCBI
|
|
21
|
Zhan YP, Huang XE, Cao J, Lu YY, Wu XY,
Liu J, Xu X, Xu L, Xiang J and Ye LH: Clinical study on safety and
efficacy of Qinin® (cantharidin sodium) injection combined with
chemotherapy in treating patients with gastric cancer. Asian Pac J
Cancer Prev. 13:4773–4776. 2012.PubMed/NCBI
|
|
22
|
Kim YM, Ku MJ, Son YJ, Yun JM, Kim SH and
Lee SY: Anti-metastatic effect of cantharidin in A549 human lung
cancer cells. Arch Pharm Res. 36:479–484. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yeh CH, Yang YY, Huang YF, Chow KC and
Chen MF: Induction of apoptosis in human Hep3B hepatoma cells by
norcantharidin through a p53 independent pathway via TRAIL/DR5
signal transduction. Chin J Integr Med. 18:676–682. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Haggar FA and Boushey RP: Colorectal
cancer epidemiology: incidence, mortality, survival, and risk
factors. Clin Colon Rectal Surg. 22:191–197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
BenZion I and Helveston EM: Use of
telemedicine to assist ophthalmologists in developing countries for
the diagnosis and management of four categories of ophthalmic
pathology. Clin Ophthalmol. 1:489–495. 2007.PubMed/NCBI
|
|
26
|
Gerber DE: Targeted therapies: a new
generation of cancer treatments. Am Fam Physician. 77:311–319.
2008.PubMed/NCBI
|