Introduction
Mesenchymal stromal cells (MSCs) are originally
isolated from bone marrow (BM) (1). MSCs show proliferation without the
loss of undifferentiated phenotype and retain the ability to
differentiate into several mesenchymal lineages, such as bone,
cartilage, adipose and muscle tissues (2). In addition to BM, MSCs have also been
isolated from adipose tissue (3),
placenta (4), amniotic fluid
(5) and fetal tissues (6). The percentage of MSCs in BM is low
(0.001-0.01% of the mononuclear cell fraction). By contrast,
adipose tissue contains a ~500-fold percentage of MSCs than BM and
the process of tissue collection is simple (7).
An important characteristic of MSCs is their
immunomodulatory capacity. MSCs suppress the proliferation of T
cells upon allogeneic (8–19) or mitogenic stimulation (11), promote apoptosis of activated T
cells (12) and enhance the
generation of regulatory T cells (13). MSCs also inhibit the proliferation
of B cells and natural killer cells (14,15).
Several factors have been implicated in the immunomodulatory
effects of MSCs, including prostaglandin E2 (PGE2),
transforming growth factor-β1 (TGF-β1) and indoleamine
2,3-dioxygenase (IDO) (15,16).
In experimental models, administration of MSCs resulted in the
prevention of graft-versus-host disease (GvHD) (17) in prolonged skin graft survival
(18). The use of MSCs as a
cellular therapy has been examined in clinical trials to treat GvHD
(19) and Crohn’s disease
(20). MSCs express intermediate
levels of major histocompatibility complex (MHC) class I molecules
and very low levels of class II (21), which may be recognized by
alloreactive T cells. Notably, the immunomodulatory capacity of
adipose tissue-derived mesenchymal stromal cells (ASCs) is higher
than that of BM-derived MSCs (22).
In the present study, the direct and indirect
effects of ASCs on alloreactively stimulated mouse spleen cells
were observed. In addition, the interferon-γ (IFN-γ)-producing
capability of the spleen cells, the population of activated
CD69+ cells among CD45+ leukocytes and the
functions of MHC molecules on ASCs were investigated.
Materials and methods
Experimental animals
Thirty male BALB/c mice were purchased from Chubu
Kagaku Shizai Co., Ltd. (Nagoya, Japan) and had access to Oriental
MF solid chow (Oriental Yeast Co., Tokyo, Japan) and water ad
libitum. This study was approved by the Animal Ethics Committee
of Asahi University (Gifu, Japan; grant no. 07–016).
Harvest and primary culture of ASCs
Four-week-old male BALB/c mice (weight, 15–20g) were
sacrificed by cervical dislocation. The inguinal fat pads were
harvested and washed with phosphate-buffered saline (PBS). They
were excised, finely minced and then digested with 0.1% collagenase
(Wako Pure Chemical Industries, Ltd., Osaka, Japan) for 40 min at
37°C. After digestion, they were filtered through a cell strainer
(BD Biosciences, San Jose, CA, USA). An equal volume of starting
medium (FM-start™; Toyobo Co., Ltd., Osaka, Japan) was added to the
cell suspension, which was then centrifuged at 270 × g for 5 min.
Cells were resuspended with 10 ml starting medium, plated on 100-mm
tissue culture plates and then incubated at 37°C in 5%
CO2. The medium was replaced every 3 days and the
non-adherent cells were discarded. The cells were harvested at
80–90% confluence with 0.25% trypsin/0.1%
ethylenediaminetetraacetic acid (EDTA; Invitrogen Life
Technologies, Grand Island, NY, USA), collected by centrifugation
at 190 × g for 5 min at room temperature, then passaged at a ratio
of 1:3. The cells were cultured in FM-medium™ (Toyobo Co., Ltd.) at
37°C, 5% CO2. Of the cultured ASCs, passage 3 were used
in this study.
Phenotype and differentiation capacity of
ASCs
The capacity of ASCs to differentiate along
adipogenic and osteogenic lineages was assessed as previously
described (23). Briefly, for
adipogenic differentiation, cells were induced by adding
1-methyl-3-isobutylxanthine (0.5 mM), insulin (10 μM), indomethacin
(200 μM) and dexamethasone (1 μM) to Dulbecco’s modified Eagle’s
medium (DMEM; Wako Pure Chemical Industries, Ltd.), containing 10%
fetal bovine serum (FBS; Invitrogen) and 1% antibiotic antimycotic
solution (10,000 U/ml penicillin, 10,000 μg/ml streptomycin and 25
μg/ml amphotericin B; Gibco-BRL). This medium was replaced every
3–4 days for 2 weeks. Adipogenesis was measured by the accumulation
of neutral lipids in fat vacuoles, observed using Oil red O
staining.
For osteogenic differentiation, cells were grown in
minimum essential medium-α (MEM-α; Wako Pure Chemical Industries,
Ltd.,) supplemented with ascorbic acid (50 μg/ml) and
glycerophosphate (10 mM) containing 10% FBS (JRH Biosciences,
Lenexa, KS, USA) and 1% Pen Strep (penicillin, 10,000 U/ml and
streptomycin, 10,000 μg/ml; Gibco-BRL). This medium was replaced
every 3–4 days for 3 weeks. Differentiated cells were examined by
Alizarin red (Wako Pure Chemical Industries, Ltd.) staining.
Preparation of mouse spleen cells
Twenty-four-week-old male BALB/c mice were
sacrificed by cervical dislocation. The spleen was removed. Spleen
cells were isolated by smashing the tissue with stainless steel
mesh in RPMI-1640 medium (Sigma-Aldrich, St. Louis, MO, USA)
containing 10% FBS (Biowest SAS, Nuaillé, France), 50 μM
2-mercaptoethanol (Nacalai Tesque, Inc., Kyoto, Japan) and 1%
antibiotic antimycotic solution (Gibco-BRL). Cells were collected
by centrifugation at 430 × g for 5 min and then resuspended with 10
ml red blood cell lysis buffer [10 mM Tris-HCl (pH 7.3) containing
140 mM NH4Cl and 1 mM EDTA]. After incubation for 5 min
at room temperature, the cells were washed three times with
RPMI-1640 medium and centrifuged at 1,500 rpm for 5 min. The spleen
cells were re-suspended with RPMI-1640 medium and filtered using a
cell strainer (BD Biosciences) to remove the residue.
Analysis of cytokine production by spleen
cells
Spleen cells were suspended in RPMI-1640
supplemented with 10% FBS, 50 μM 2-mercaptoethanol and 1%
antibiotic antimycotic solution. Cell suspension
(4×106/ml) was added (0.1 ml/well, in triplicate) to a
96-well plate, to which 4×105 of anti-CD3 and anti-CD28
antibody-coated (anti-CD3/CD28) beads (Dynabeads® Mouse
T-Activator CD3/CD28; Invitrogen Life Technologies) were added.
Additionally, ASCs (0–16,000 cells/well) were added to the wells.
To examine the indirect effects of ASCs on the stimulated spleen
cells, Transwell chambers (pore size, 0.4 μm; Corning Inc.,
Corning, NY, USA) 24-well plates were used. The spleen cell
suspension together with anti-CD3/CD28 beads were transferred to
the upper chambers and ASCs were added to the bottom chambers of a
Transwell.
The cells were incubated for 48 h in 5%
CO2 at 37°C, and then the supernatant was harvested by
centrifugation at 1,710 × g for 5 min and stored at −80°C.
Production of IFN-γ in the supernatant of cell culture was assayed
by enzyme-linked immunosorbent assay using BD OptE1A Set Mouse
IFN-γ (BD Biosciences).
Flow cytometry
A four-colored panel was used to analyze the
stimulated spleen cells. The spleen cells were harvested from the
culture and transferred to a Falcon tube by thorough resuspension
with a pipette. The cells were washed twice with PBS and stained
with anti-mouse antibodies (mAbs), including phycoerythrin
(PE)-conjugated mAb specific for CD4 (clone GK1.5), peridinin
chlorophyll-a protein-cyanine 5.5 (PerCP-Cy™5.5)-conjugated mAb
specific for CD45 (clone 104) and allophycocyanin (APC)-conjugated
mAb specific for CD69 (clone H1.2F3; eBioscience, San Diego, CA,
USA). Fluorescein isothiocyanate (FITC)-conjugated mAb specific for
CD8 (KT15) was purchased from Immunotech (Marseille, France). Cells
were re-suspended in PBS containing 2% FBS, 1 mM EDTA and 1% sodium
azide, then analyzed by flow cytometry (FACSCalibur; BD Bioscience)
with Cell Quest software (BD Bioscience).
Knockdown of β2-microglobulin (β2M) in
ASCs
β2M siRNA duplex (Silencer® select
Pre-designed siRNA; Ambion, Invitrogen Life Technologies) was used
to knockdown the representative gene in ASCs. Sense and antisense
sequences of β2M siRNA duplex were: 5′-gcc uca cau uga aau cca
att-3′ and 5′-uug gau uuc aau gug agg cgg-3′. Briefly, ASCs
(1×104) were seeded in a 96-well plate with 0.1 ml
Opti-MEM (Invitrogen Life Technologies). Then, 6 pmol siRNA and 1
μl Lipofectamine (Invitrogen Life Technologies) were added. Cells
with reagents were incubated for 20 min in 5% CO2 at
37°C, then 10 μl FBS was added. The mixture was incubated for 4 h,
and then the medium was replaced with DMEM containing 10% FBS.
After 48 h, transfected ASCs were used for further experiments.
Quantitative polymerase chain reaction
(qPCR)
Knockdown of endogenous β2M in ASCs was confirmed by
semi-qPCR. The whole-cell RNA extraction and semi-qPCR technique
were conducted as previously described (24). Primer sequences were as follows:
β2M, forward 5′-gca ggc gta tgt atc agt ctc agt-3′ and reverse
5′-gag aat ggg aag ccg aac ata ct-3′; ribosomal protein S5 (RPS5),
forward 5′-aga aga ctc aac acg cat tgg gc-3′ and reverse 5′-gca ctc
agc gat ggt ctt gat gt-3′. The expression levels of β2M mRNA were
normalized as a ratio to that of RPS5-mRNA.
Statistics
Data are expressed as mean ± standard deviation.
Student’s t-test was applied to determine the significance of
differences between two groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
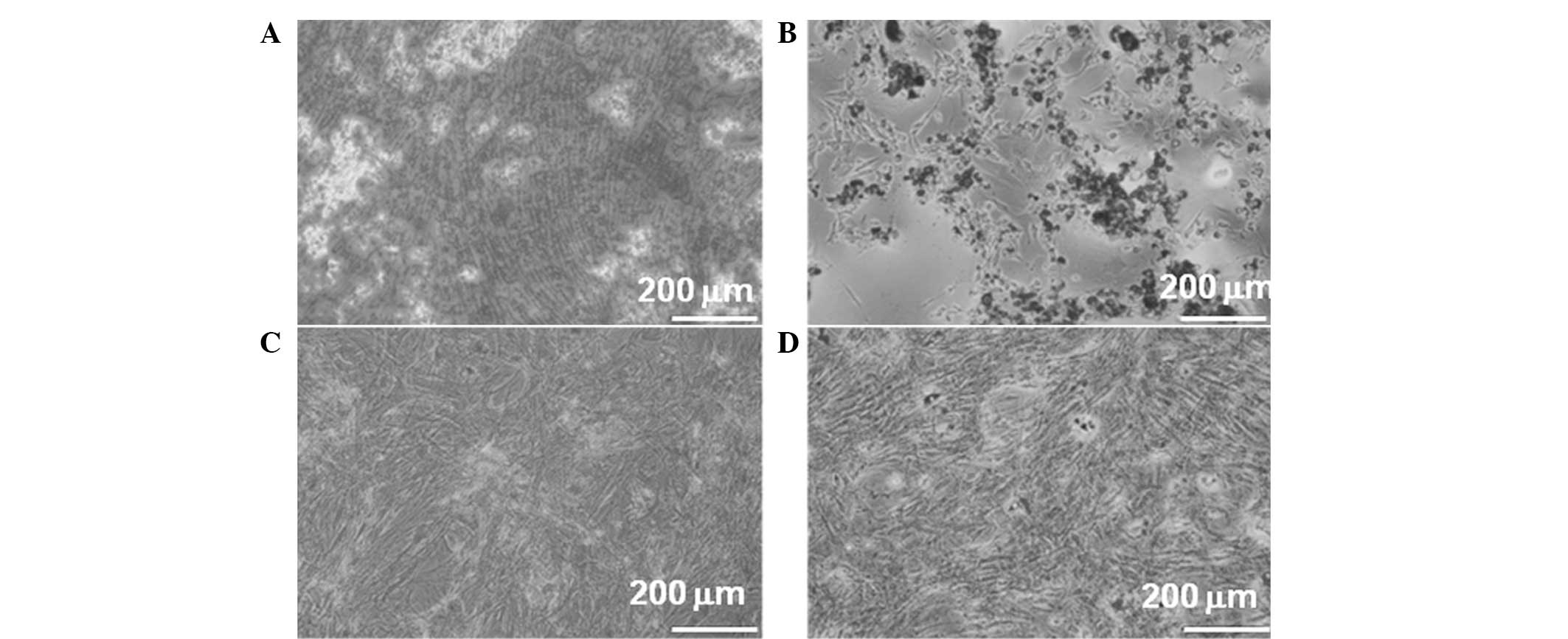
In vitro differentiation of ASCs
ASCs were tested for their capacity to differentiate
toward the osteogenic and adipogenic lineages. The cells treated
with osteogenic medium underwent a morphological change
demonstrating calcium deposition (Fig.
1A). In the adipogenic medium, the cells may have been induced
toward adipogenic differentiation as shown by the accumulation of
lipid vacuoles (Fig. 1B). However,
no apparent changes were observed in untreated ASCs (Fig. 1C and D).
These results demonstrated that most of the cells
harbor characteristic phenotypes of ASCs to differentiate along
adipogenic and osteogenic lineages.
Effects of ASCs on alloreactively
stimulated spleen cells
It has been identified that MSCs mainly control the
Th1 response (25). Among the
acute inflammatory molecules, the current study focused on IFN-γ,
as this cytokine is a major product of Th1 cells, which reduces the
Th2 phenotype and stimulates several key functions to activate
macrophages and anti-tumor reaction (26). Our preliminary experiments revealed
that the production of IFN-γ by the anti-CD3/CD28 bead-stimulated
spleen cells was significantly upregulated by 48 h and the elevated
levels continued until 96 h (data not shown). Therefore, spleen
cells (4×105) and anti-CD3/CD28 beads (Invitrogen Life
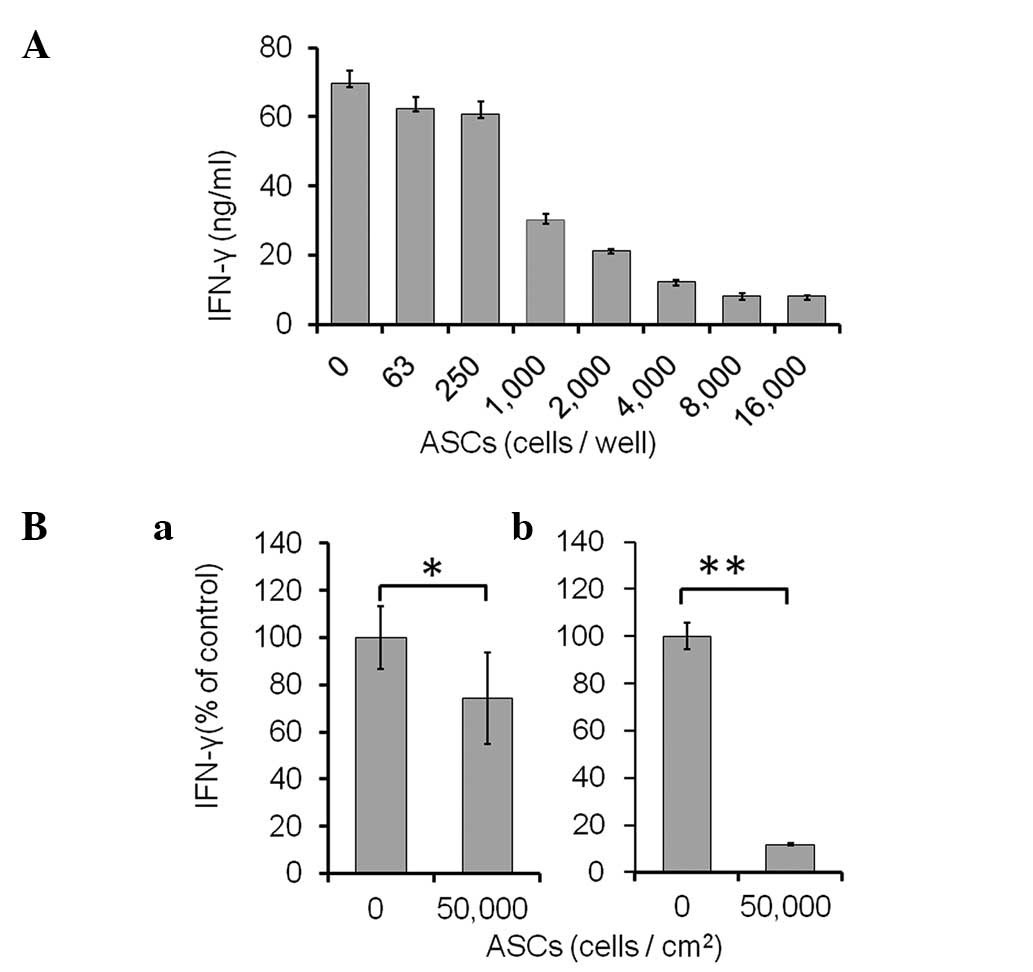
Technologies) were co-cultured with ASCs (0–16,000 cells/well) for
48 h (Fig. 2A). As shown in
Fig. 2A, the production of IFN-γ
was markedly suppressed by the ASCs in a dose-dependent manner.
ASCs (n=8,000) showed maximal suppression and the suppressive level
was unchanged up to 16,000 ASCs.
In addition to the direct co-culture assay,
Transwell assays were performed to determine whether cell-to-cell
contact was necessary for the suppression of activated spleen cells
by ASCs (Fig. 2B). ASCs were
plated in the lower wells; Transwell inserts containing the
anti-CD3/CD28 beads and spleen cells were placed over each well.
After 48 h, the volume of secreted IFN-γ was reduced to 74.4±19.4%
of control (without ASCs; P<0.05) in contactless Transwell
culture (Fig. 2Ba). However, the
concentration of secreted IFN-γ was markedly reduced to 11.8±0.3%
(P<0.01) in the direct co-culture (Fig. 2Bb).
The results revealed that ASCs require direct
cell-to-cell contact to maximally suppress IFN-γ production by
activated spleen cells. However, even in the absence of cell
contact, ASCs partially suppressed the IFN-γ production via one or
more secreted factors.
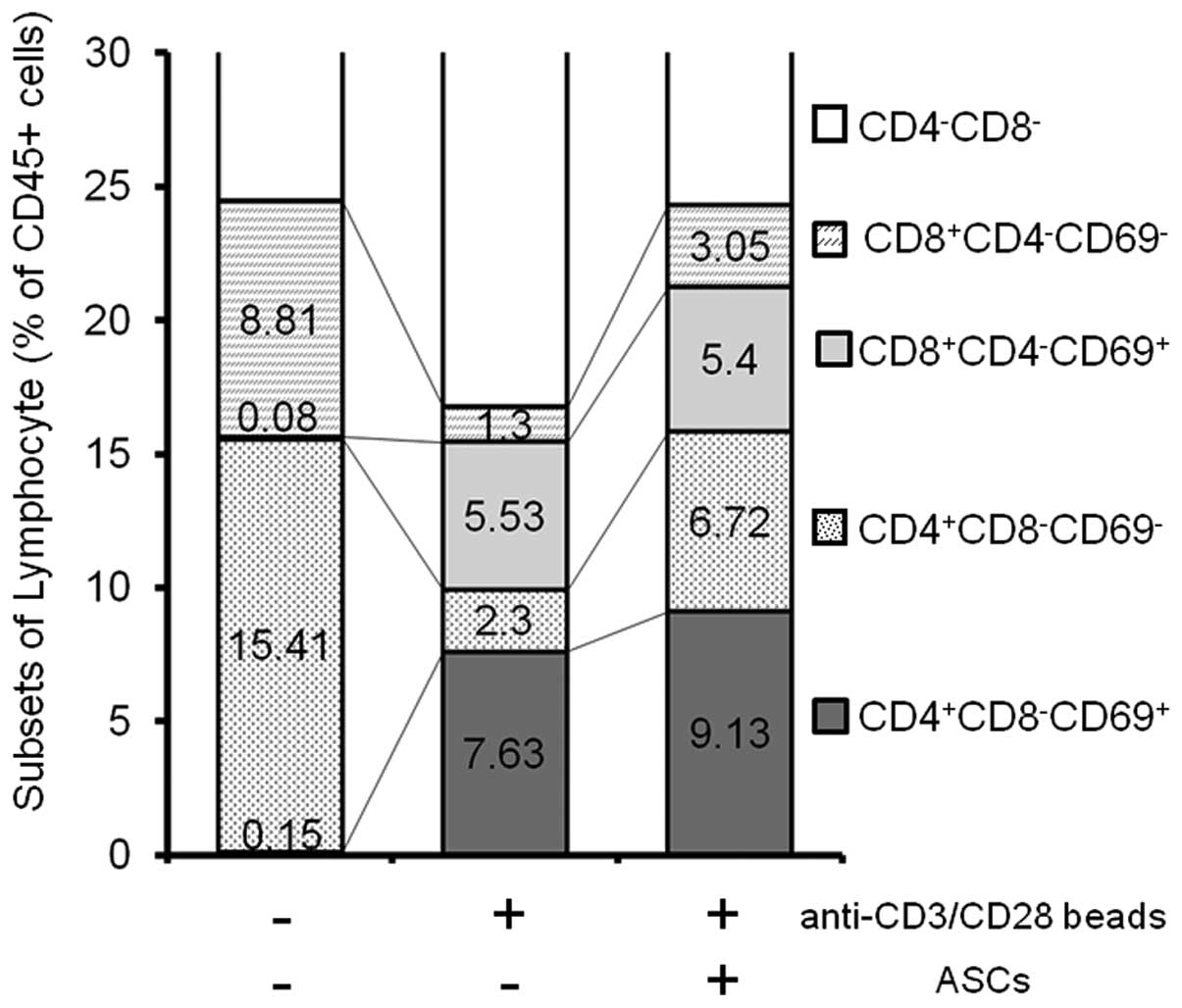
CD69+ activated spleen T cells
in the presence and absence of ASCs
To investigate whether the activation status of T
cells is changed by ASCs or not, the expression of CD69 on
stimulated spleen cells was analyzed by flow cytometry (Fig. 3). CD69 is the earliest inducible
cell surface glycoprotein acquired during lymphoid activation,
which is involved in lymphocyte proliferation and function
(27). In order to select all
leukocytes, CD45+ cells were first gated (100%). CD69
positive/negative fractions among CD4+/CD8−
and CD4−/CD8+ T subsets were compared. The
results are summarized in Fig. 3.
No CD69+ cells were detected in CD45+
un-stimulated spleen cells. Following stimulation with the
anti-CD3/CD28 beads, CD69+ populations emerged in the
CD4+/CD8− and CD4−/CD8+
T subsets, as 7.6% and 5.5%, respectively. In the presence of ASCs,
the production of IFN-γ was greatly suppressed; however, certain
populations of CD69+ continued to exist in the
CD4+/CD8− and CD4−/CD8+
subsets, as 9.1% and 5.4%, respectively.
These results suggested that the suppressive effects
of ASCs on the activated spleen cells did not interfere with the
signaling loop mediated by T cell receptors.
Role of MHC class I molecules on the
immunosuppressive function of ASCs
It has been identified that the immunosuppressive
effects of MSCs involve a nonclassical MHC, HLA-G (28,29).
HLA-G is expressed in membrane-bound and soluble isoforms (29). In order to examine the association
of MHC molecules and the suppressive effects of mouse ASCs,
knockdown of endogenous β2M in ASCs was performed, as the molecule
is a component of MHC class I molecules, and also necessary for
cell surface expression of MHC class I molecules and stability
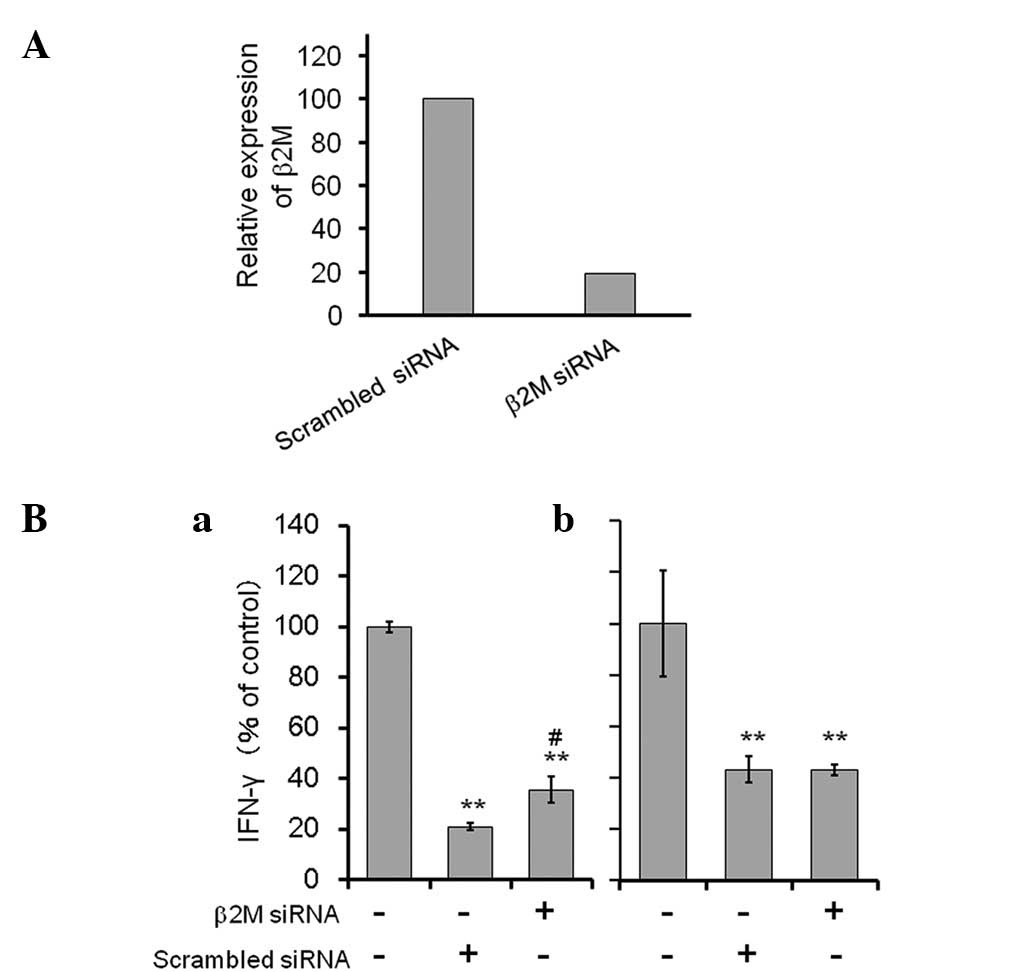
(30). As shown in Fig. 4A, β2M siRNA-transfected ASCs
expressed a lower level of endogenous β2M mRNA (<20%) compared
with that of the non-specific scrambled RNA-transfected cells.
In the direct co-culture of the
anti-CD3/CD28-stimulated spleen cells and non-specific scrambled
RNA-transfected ASCs, the level of IFN-γ production was
considerably reduced (to 21% of the control). By contrast, IFN-γ
production was significantly recovered (to 36% of the control) in
the co-culture using β2M siRNA transfectants. Furthermore, in the
contactless co-culture using a Transwell, the level of IFN-γ
produced by the spleen cells was unchanged between those treated
with β2M siRNA-transfected ACSs and those treated with control
siRNA transfected ASCs.
The results suggest that the suppressive function of
ASCs on spleen cells is directly mediated by an MHC class I
complex.
Discussion
In the present study, it was demonstrated that mouse
ASCs markedly suppressed IFN-γ production by anti-CD3/CD28
bead-stimulated spleen cells; however, certain populations of
CD69+ existed in CD4+/CD8− and
CD4−/CD8+ subsets, even in the presence of
ASCs. This observation is similar to that previously reported for a
co-culture with BM-derived MSCs, where proliferation was
significantly reduced, while the expression of activation markers,
CD25 and CD69, was unchanged in anti-CD3/CD28-stimulated T cells
(31). These results suggest that
the suppressive effects of ASCs on the activated spleen cells did
not interfere with the signaling loop mediated by T cell
receptors.
The results of the Transwell assay in the present
study demonstrated that in the absence of cell contact, ASCs
partially suppressed IFN-γ production, possibly via one or more
secreted products. Several factors have been implicated in the
immunomodulatory effects of MSCs, including PGE2,
TGF-β1, IDO (15,16) and nitric oxide (NO) (30). However, in the present study, the
results obtained with the Transwell system demonstrated that ASCs
require direct cell-to-cell contact to maximally suppress the
activated spleen cells.
NO is known as one of the major mediators of T-cell
suppression. T-cell-MSC contact is also critical for the efficient
production of NO from MSCs (31),
suggesting a dynamic cross talk, including direct cell-cell contact
between MSCs and lymphocytes, is required for these
immunosuppressive effects. It has been reported that BM-derived
MSCs express HLA-G protein, a non-classical HLA class I molecule,
and the molecule has an immunosuppressive function by reducing
lymphocyte proliferation (29).
Stable expression of HLA-G1 has been shown to enhance the
immunosuppressive effects of human ASCs (32). Furthermore, inhibitors of
mevalonate synthesis have demonstrated the ability to downregulate
the expression levels of adhesion molecules, including HLA class I,
resulting in the prevention of immunosuppressive effects of
BM-derived MSCs (33). Therefore,
we have attempted to knockdown the endogenous expression of mouse
MHC class I molecules, including H2-D1, H2-K1, H2-Ke and H2-Ke6 in
mouse ASCs using siRNAs; however, the immunosuppressive effects of
the ASCs were unaffected (data not shown). Following this, in the
present study, the expression of endogenous β2M was knocked down in
ASCs because this molecule is a universal component of MHC class I
complexes necessary for cell surface expression and stability of
MHC class I molecules (33). The
results revealed that the immunosuppressive effects of ASCs on
activated spleen cells were significantly, but only partially
alleviated in β2M siRNA-transfected ASCs. In addition, in the
contactless co-culture using a Transwell, the immunosuppressive
effects of ASCs were unaffected regardless of the level of
endogenous β2M, which was significantly reduced by siRNA,
suggesting that the effects of β2M are not mediated merely by
soluble factors. These results suggest that one or more additional
MHC class I molecules may be responsible for the immunomodulatory
functions of mouse ASCs, or that a combination of a number of
adhesion molecules is essential to confer the function to the
cells.
In conclusion, in the direct co-culture, the
suppressive function of mouse ASCs on spleen cells is partially
mediated by an MHC class I complex. The results of the present
study may provide a novel insight for further analysis of the
immunomodulatory mechanisms in MSCs.
Acknowledgements
This study was supported by a grant from the
Grant-in-Aid for Scientific Research (C) (grant no. 23592976)
provided by the Ministry of Education, Culture, Sports, Science and
Technology of Japan.
References
|
1
|
Friedenstein AJ, Gorskaja JF and Kulagina
NN: Fibroblast precursors in normal and irradiated mouse
hematopoietic organs. Exp Hematol. 4:267–274. 1976.PubMed/NCBI
|
|
2
|
Prockop DJ: Marrow stromal cells as stem
cells for nonhematopoietic tissues. Science. 276:71–74. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell
JW, Katz AJ, Benhaim P, Lorenz HP and Hedrick MH: Multilineage
cells from human adipose tissue: implications for cell-based
therapies. Tissue Eng. 7:211–228. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
In ‘t Anker PS, Scherjon SA, Kleijburg-van
der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE and Kanhai HH:
Isolation of mesenchymal stem cells of fetal or maternal origin
from human placenta. Stem Cells. 22:1338–1345. 2004.
|
|
5
|
In ‘t Anker PS, Scherjon SA, Kleijburg-van
der Keur C, Noort WA, Claas FH, Willemze R, Fibbe WE and Kanhai HH:
Amniotic fluid as a novel source of mesenchymal stem cells for
therapeutic transplantation. Blood. 102:1548–1549. 2003.PubMed/NCBI
|
|
6
|
In ‘t Anker PS, Noort WA, Scherjon SA,
Kleijburg-van der Keur C, Kruisselbrink AB, van Bezooijen RL,
Beekhuizen W, Willemze R, Kanhai HH and Fibbe WE: Mesenchymal stem
cells in human second-trimester bone marrow, liver, lung, and
spleen exhibit a similar immunophenotype but a heterogeneous
multilineage differentiation potential. Haematologica. 88:845–852.
2003.
|
|
7
|
Fraser JK, Schreiber R, Strem B, Zhu M,
Alfonso Z, Wulur I and Hedrick MH: Plasticity of human adipose stem
cells toward endothelial cells and cardiomyocytes. Nat Clin Pract
Cardiovasc Med. 3(Suppl 1): S33–S37. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Le Blanc K, Tammik L, Sundberg B,
Haynesworth SE and Ringdén O: Mesenchymal stem cells inhibit and
stimulate mixed lymphocyte cultures and mitogenic responses
independently of the major histocompatibility complex. Scand J
Immunol. 57:11–20. 2003.PubMed/NCBI
|
|
9
|
Potian JA, Aviv H, Ponzio NM, Harrison JS
and Rameshwar P: Veto-like activity of mesenchymal stem cells:
functional discrimination between cellular responses to
alloantigens and recall antigens. J Immunol. 171:3426–3434. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tse WT, Pendleton JD, Beyer WM, Egalka MC
and Guinan EC: Suppression of allogeneic T-cell proliferation by
human marrow stromal cells: implications in transplantation.
Transplantation. 75:389–397. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Di Nicola M, Carlo-Stella C, Magni M,
Milanesi M, Longoni PD, Matteucci P, Grisanti S and Gianni AM:
Human bone marrow stromal cells suppress T- lymphocyte
proliferation induced by cellular or nonspecific mitogenic stimuli.
Blood. 99:3838–3843. 2002.
|
|
12
|
Plumas J, Chaperot L, Richard MJ, Molens
JP, Bensa JC and Favrot MC: Mesenchymal stem cells induce apoptosis
of activated T cells. Leukemia. 19:1597–1604. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Djouad F, Plence P, Bony C, Tropel P,
Apparailly F, Sany J, Noël D and Jorgensen C: Immunosuppressive
effect of mesenchymal stem cells favors tumor growth in allogeneic
animals. Blood. 102:3837–3844. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Corcione A, Benvenuto F, Ferretti E,
Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi
GL, Pistoia V and Uccelli A: Human mesenchymal stem cells modulate
B-cell functions. Blood. 107:367–372. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Spaggiari GM, Capobianco A, Abdelrazik H,
Becchetti F, Mingari MC and Moretta L: Mesenchymal stem cells
inhibit natural killer-cell proliferation, cytotoxicity, and
cytokine production: role of indoleamine 2,3-dioxygenase and
prostaglandin E2. Blood. 111:1327–1333. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
English K, Ryan JM, Tobin L, Murphy MJ,
Barry FP and Mahon BP: Cell contact, prostaglandin E(2) and
transforming growth factor beta 1 play non-redundant roles in human
mesenchymal stem cell induction of
CD4+CD25High forkhead box P3+
regulatory T cells. Clin Exp Immunol. 156:149–160. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Le Blanc K, Rasmusson I, Sundberg B,
Götherström C, Hassan M, Uzunel M and Ringdén O: Treatment of
severe acute graft-versus-host disease with third party
haploidentical mesenchymal stem cells. Lancet. 363:1439–1441.
2004.PubMed/NCBI
|
|
18
|
Hoogduijn MJ, Popp FC, Grohnert A, Crop
MJ, van Rhijn M, Rowshani AT, Eggenhofer E, Renner P, Reinders ME,
Rabelink TJ, van der Laan LJ, Dor FJ, Ijzermans JN, Genever PG,
Lange C, Durrbach A, Houtgraaf JH, Christ B, Seifert M, Shagidulin
M, Donckier V, Deans R, Ringden O, Perico N, Remuzzi G, Bartholomew
A, Schlitt HJ, Weimar W, Baan CC and Dahlke MH; MISOT Study Group.
Advancement of mesenchymal stem cell therapy in solid organ
transplantation (MISOT). Transplantation. 90:124–126. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Le Blanc K, Frassoni F, Ball L, Locatelli
F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger
M, Dini G, Egeler RM, Bacigalupo A and Fibbe W; Ringdén O;
Development Committee of the Eurpoean Group for Blood and Marrow
Transplantation. Mesenchymal stem cells for treatment of
steroid-resistant, severe, acute graft-versus-host disease: a phase
II study. Lancet. 371:1579–1586. 2008.
|
|
20
|
Ciccocioppo R, Bernardo ME, Sgarella A,
Maccario R, Avanzini MA, Ubezio C, Minelli A, Alvisi C, Vanoli A,
Calliada F, Dionigi P, Perotti C, Locatelli F and Corazza GR:
Autologous bone marrow-derived mesenchymal stromal cells in the
treatment of fistulising Crohn’s disease. Gut. 60:788–798.
2011.PubMed/NCBI
|
|
21
|
Tse WT, Pendleton JD, Beyer WM, Egalka MC
and Guinan EC: Suppression of allogeneic T-cell proliferation by
human marrow stromal cells: implications in transplantation.
Transplantation. 75:389–397. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Melief SM, Zwaginga JJ, Fibbe WE and
Roelofs H: Adipose tissue-derived multipotent stromal cells have a
higher immunomodulatory capacity than their bone marrow-derived
counterparts. Stem Cells Transl Med. 2:455–463. 2013. View Article : Google Scholar
|
|
23
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kondoh N, Ishikawa T, Ohkura S, Arai M,
Hada A, Yamazaki Y, Kitagawa Y, Shindoh M, Takahashi M, Ando T,
Sato Y, Izumo T, Hitomi K and Yamamoto M: Gene expression
signatures that classify the mode of invasion of primary oral
squamous cell carcinomas. Mol Carcinog. 47:744–756. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lim JY, Park MJ, Im KI, Kim N, Jeon EJ,
Kim EJ, Cho ML and Cho SG: Combination cell therapy using
mesenchymal stem cells and regulatory T cells provides a
synergistic immunomodulatory effect associated with reciprocal
regulation of Th1/Th2 and Th17/Treg cells in a murine acute
graft-versus-host disease model. Cell Transplant. Feb 26–2013.(Epub
ahead of print).
|
|
26
|
Schroder K, Hertzog PJ, Ravasi T and Hume
DA: Interferon-gamma: an overview of signals, mechanisms and
functions. J Leukoc Biol. 75:163–189. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cambiaggi C, Scupoli MT, Cestari T, Gerosa
F, Carra G, Tridente G and Accolla RS: Constitutive expression of
CD69 in interspecies T-cell hybrids and locus assignment to human
chromosome 12. Immunogenetics. 36:117–120. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang HM, Sung JH, Choi YS, Lee HJ, Roh CR,
Kim J, Shin M, Song S, Kwon CH, Joh JW and Kim SJ: Enhancement of
the immunosuppressive effect of human adipose tissue-derived
mesenchymal stromal cells through HLA-G1 expression. Cytotherapy.
14:70–79. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nasef A, Mathieu N, Chapel A, Frick J,
François S, Mazurier C, Boutarfa A, Bouchet S, Gorin NC, Thierry D
and Fouillard L: Immunosuppressive effects of mesenchymal stem
cells: involvement of HLA-G. Transplantation. 84:231–237. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Alberts B, Johnson A, Lewis J, Raff M,
Roberts K and Walter P: T cells and MHC proteins. Molecular biology
of the cell. 5th edition. Garland Science; New York, NY: pp.
1569–1588. 2008
|
|
31
|
Sato K, Ozaki K, Oh I, Meguro A, Hatanaka
K, Nagai T, Muroi K and Ozawa K: Nitric oxide plays a critical role
in suppression of T-cell proliferation by mesenchymal stem cells.
Blood. 109:228–234. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang HM, Sung JH, Choi YS, Lee HJ, Roh CR,
Kim J, Shin M, Song S, Kwon CH, Joh JW and Kim SJ: Enhancement of
the immunosuppressive effect of human adipose tissue-derived
mesenchymal stromal cells through HLA-G1 expression. Cytotherapy.
14:70–79. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Musso A, Zocchi MR and Poggi A: Relevance
of the mevalonate biosynthetic pathway in the regulation of bone
marrow mesenchymal stromal cell-mediated effects on T-cell
proliferation and B-cell survival. Haematologica. 96:16–23. 2011.
View Article : Google Scholar : PubMed/NCBI
|