Introduction
Primary immune thrombocytopenia (ITP) is an acquired
immune-mediated disorder characterized by isolated
thrombocytopenia, which is defined as a peripheral blood platelet
count of <100×109/l and the absence of any clear
initiating and/or underlying cause (1). Antibodies that are autoreactive to
platelet antigens, mainly the platelet glycoprotein IIb/IIIa
complex, are considered responsible for the reduced platelet
production and accelerated destruction of platelets by the
reticuloendothelial system (2,3).
Platelet antigen-specific T cells are activated upon the
recognition of platelet auto-antigens and induce the production of
auto-antibodies by B cells in patients with ITP (4,5). ITP
has been further suggested to be a T helper (Th)-1-polarized
autoimmune disease (6–8). These data are consistent with a loss
of peripheral tolerance and an inflammatory phenotype in patients
with chronic ITP.
CD4+CD25+ regulatory T cells
(Tregs) are critical in the maintenance of peripheral tolerance and
directly and indirectly suppress the activation and proliferation
of numerous cell types, including T, B, dendritic, natural killer
and natural killer T (NKT) cells in vivo and/or in
vitro(9). A number of studies
have been conducted to investigate the roles of Tregs in ITP,
particularly in chronic ITP; however, the results have not always
been consistent (10–12). Liu et al(10) and Sakakura et al(11) observed that the level of Tregs was
significantly decreased in the circulation in ITP. By contrast, Yu
et al(12) demonstrated
that the level of circulating Tregs was comparable between patients
with ITP and the controls; however, the inhibitory activity of the
Tregs isolated from the patients with ITP was two-fold lower than
that of the Tregs from the controls.
NKT cells are another T lymphocyte subset with
regulatory functions involved in peripheral tolerance in humans,
and are characterized by invariant expression of the T cell
receptor (TCR) Vα24 and Vβ11 chains (13). The levels and functional status of
NKT cells are associated with multiple human autoimmune diseases;
however, the mechanisms have yet to be elucidated (14). Johansson et al(15) demonstrated that the levels of
circulating NKT cells decreased in patients with ITP, which
suggested the involvement of NKT cells in ITP pathogenesis.
NKT cells and Tregs interact with each other and
contribute functionally to a sophisticated network of immune
regulation in humans (16).
However, few studies have described the changes in levels of
circulating Tregs and NKT cells in ITP. In this study, the
frequency of peripheral Tregs and NKT cells and the Th1/Th2
cytokine profile were analyzed, and a correlation analysis was
performed between the immune response and the disease phenotypes in
adult chronic ITP.
Materials and methods
Subjects
Sixty-eight patients with chronic ITP, who were
hospitalized in the Department of Hematology, Jinhua Hospital of
Zhejiang University (Jinhua, China) from January 2008 to March
2010, were included in this study. An additional 38 healthy age-
and gender-matched volunteers were used as controls. Gender, mean
age and platelet count from the ITP and control groups are
summarized in Table I. The
diagnosis of chronic ITP was in agreement with the standards
proposed by Zhang et al(17), i.e., a platelet count of
<50×109/l for more than six months, normal or
increased bone marrow, megakaryocytes without any features of
dysplasia and a lack of other known causes, such as systemic lupus
erythematosus. In the two weeks prior to sampling, none of the
patients or healthy volunteers took corticosteroids or other
medications that may have affected platelet metabolism. All
patients were in the active phase and were divided into two groups
according to platelet count: <20×109/l (n=30) and
>20×109/l (n=38). This study was conducted in
accordance with the Declaration of Helsinki and with approval from
the ethics committees of Jinhua Hospital of Zhejiang University and
Wenzhou Medical University (Wenzhou, China). Written informed
consent was obtained from all participants.
 | Table ISummary of the clinical and
laboratory parameters of the study subjects. |
Table I
Summary of the clinical and
laboratory parameters of the study subjects.
| Group | Male/female
(n/n) | Age (years) | PLT
(x109/l) |
|---|
| ITP | 40/28 | 43.82±2.46 | 26.69±3.90 |
| Control | 24/14 | 38.53±1.97 | 230.84±11.29 |
Preparation of peripheral blood
mononuclear cells (PBMCs)
EDTA-K2 anti-coagulated venous blood,
collected from patients and healthy volunteers, was diluted with an
equivalent volume of saline. According to standard procedures,
PBMCs were separated over Lymphoprep™ 1.077 medium (Axis-Shield
PoC, Oslo, Norway), washed three times and suspended in pH 7.4
phosphate-buffered saline (PBS) with an adjusted cell density of
5×106/ml.
Measurement of
CD4+CD25+CD127−/low cells
PBMCs were stained with a cocktail of fluorescein
isothiocyanate (FITC) anti-human CD4, phycoerythrin (PE) anti-human
CD25, CD127-PE-Cy5 and immunoglobulin (Ig) G1-PE-Cy5 (all from
eBioscience, Inc., San Diego, CA, USA) for 15 min at room
temperature in the dark, washed three times with pH 7.4 PBS and
resuspended in PBS. The frequency of
CD4+CD25+CD127−/low cells was
determined by three-color flow cytometry on a Beckman-Coulter Epics
XL flow cytometer (Beckman-Coulter, Inc., Brea, CA, USA).
Flow-Check™ fluorospheres (Beckman-Coulter, Inc.) were used in the
daily alignment and verification of the flow cytometer optics and
fluidics. Data acquisition and analysis were performed using the
Expo32 ADC software package (Applied Cytometry, Dinnington, UK).
Tregs were identified as
CD4+CD25+CD127−/low and the
frequency of Tregs was expressed as the percentage of
CD4+ cells. A total of ≥70,000 CD4+ events
were analyzed in each experiment.
Measurement of
TCRVα24+Vβ11+ T cells
PBMCs were stained with CD3-PE-Cy5, Vα24-FITC and
Vβ11-PE (all from Beckman-Coulter, Inc.) for 15 min at room
temperature in the dark, washed three times with pH 7.4 PBS and
resuspended in PBS. Simultaneously, isotype controls were set with
IgG1-FITC and IgG2a-PE (Beckman-Coulter, Inc.). NKT cells were
identified as CD3+Vα24+Vβ11+ and
the frequency of NKT cells was expressed as the percentage of
CD3+ cells. A total of ≥100,000 CD3+ events
were analyzed in each experiment.
Th1/Th2 cytokine profiling
Serum was collected from each patient and healthy
volunteer and stored in a −76°C ultra-low temperature freezer
(Thermo Fisher Scientific, Inc., Middletown, VA, USA) until
analysis. Serum Th1/Th2 cytokine profiles were determined using a
cytometric bead array (CBA) Human Th1/Th2 Cytokine 11-plex kit
(eBioscience, Campus Vienna-Biocenter 2, Vienna, Austria) and 11
cytokines, interleukin (IL)-12p70, IL-10, IL-2, IL-8, IL-6, IL-5,
IL-4, IL-1β, interferon (IFN)-γ, tumor necrosis factor (TNF)-α and
TNF-β, were analyzed. The experimental procedures were in strict
accordance with the manufacturer’s instructions. Data were acquired
through the Expo32 ADC software package (Applied Cytometry) on a
Beckman-Coulter Epics XL flow cytometer (Beckman-Coulter, Inc.).
Data analysis was performed using FlowCytomix™ Pro 2.3
(eBioscience, Campus Vienna-Biocenter 2). The concentration of
serum cytokines was expressed in pg/ml.
Statistical analysis
Data were processed statistically using SPSS 16.0
(SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 4.0 (GraphPad
Software, Inc., La Jolla, CA, USA) software. A Student’s t-test was
used to compare two independent samples, while one-way analysis of
variance (ANOVA) was used for multiple comparisons. A least
significant difference (LSD) test was performed for comparisons in
which equal variances were assumed and Dunnett’s T3 test was used
for comparisons in which equal variances were not assumed.
Non-parametric comparisons were conducted using Pearson’s
χ2 test and a linear regression model was used for
correlation analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
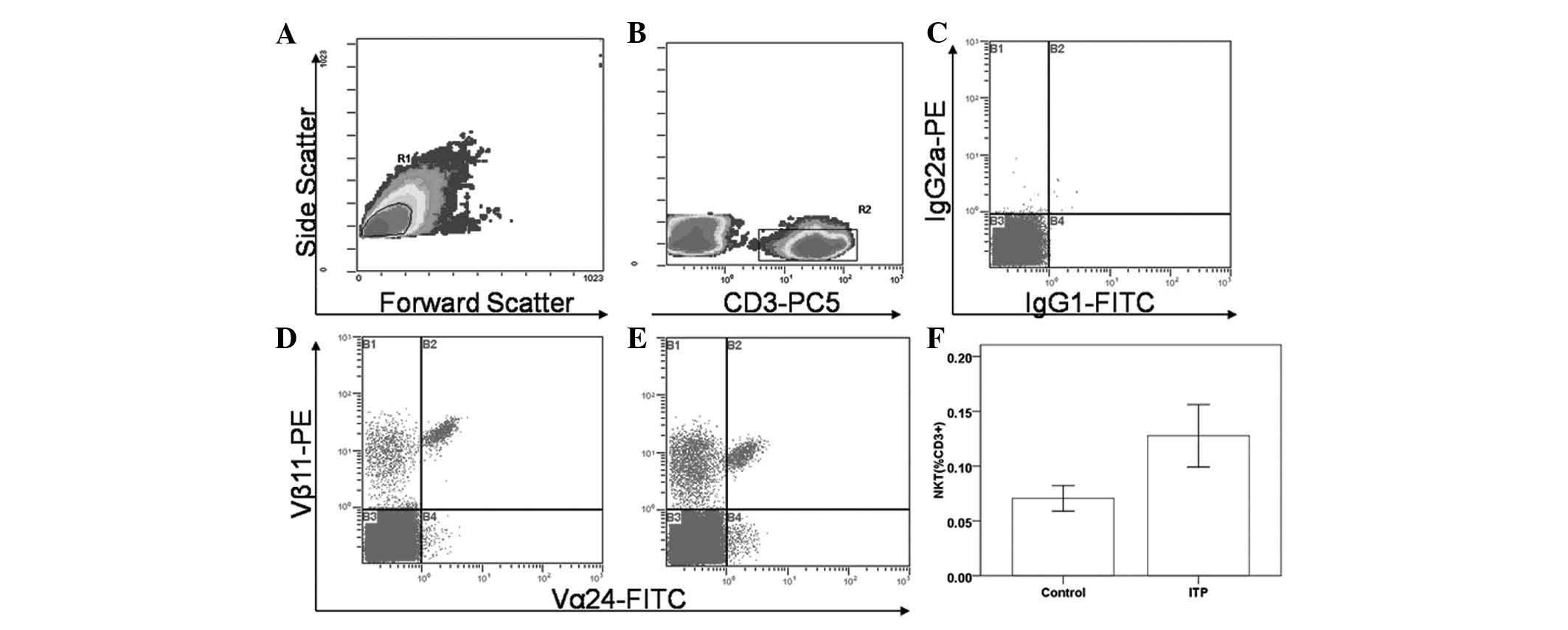
Measurement of NKT cells
The level of NKT cells (Fig. 1) in patients with chronic ITP was
higher than that in the controls (0.13±0.03 versus 0.07±0.01% of
CD3+); however, the difference was not statistically
significant (P>0.05). The results showed that the frequency of
NKT cells was significantly elevated in patients with platelet
counts ≤20×109/l (0.22±0.05%) compared with the
frequency of NKT cells in patients with platelet counts
>20×109/l (0.05±0.01%; P<0.05) and in controls
(0.07±0.01%; P<0.05); however, no significant difference was
observed between the latter two groups.
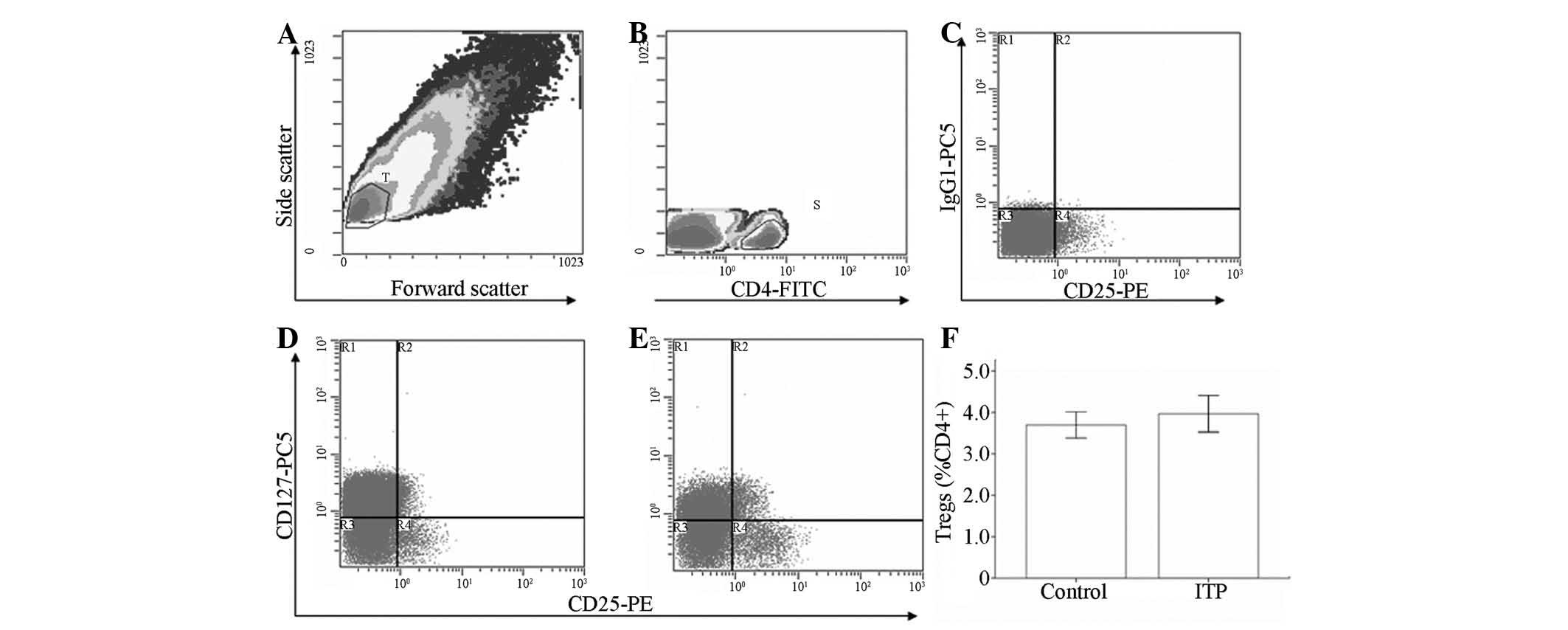
Frequency of Tregs
The frequency of circulating Tregs (Fig. 2) in patients with chronic ITP was
3.97±0.44% of CD4+, which was comparable to 3.69±0.31%
in the control group (P>0.05). Compared with Treg level in the
patients with platelet counts >20×109/l (3.78±0.59%),
the level of Tregs was elevated in patients with chronic ITP with
platelet counts ≤20×109/l (4.21±0.67%); however, the
difference was not statistically significant (P>0.05).
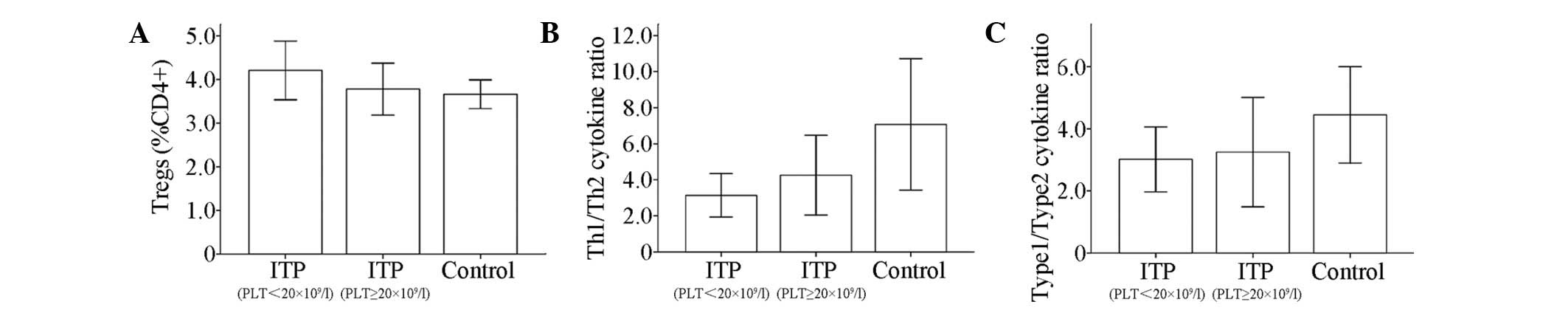
Th1/Th2 cytokine ratio
No significant differences were observed in the
serum levels of IL-12p70, IL-10, IL-2, IL-8, IL-6, IL-5, IL-4,
IL-1β, IFN-γ, TNF-α or TNF-β between the patients with ITP and the
controls (Table II). The Th1
cytokine (IFN-γ, IL-2)/Th2 cytokine (IL-4, IL-5) ratio was
calculated, which was used to predict the disease-specific Th cell
polarization. The type 1 cytokine (IFN-γ, IL-2, IL-12p70 and
TNF-β)/type 2 cytokine (IL-4, IL-5, IL-10 and IL-6) ratio was also
calculated, which was used to evaluate the host’s overall immune
response. The results showed that the Th1/Th2 ratios in patients
with ITP and the controls were 3.77±1.34 and 6.67±3.45,
respectively, which indicated a relative Th2 polarization in
patients with ITP compared with the controls. However, the
difference did not reach statistical significance (P>0.05). In
addition, a similar trend was observed in the type 1/type 2 ratio
between patients with ITP and controls, with ratios of 3.14±1.07
and 4.23±1.48, respectively (P>0.05; Table II, Fig. 3). Furthermore, no significant
difference was observed in either the Th1/Th2 ratio or the type
1/type 2 ratio between patients with ITP with platelet counts
>20×109/l and patients with platelet counts
≤20×109/l or the controls (Table III, Fig. 4).
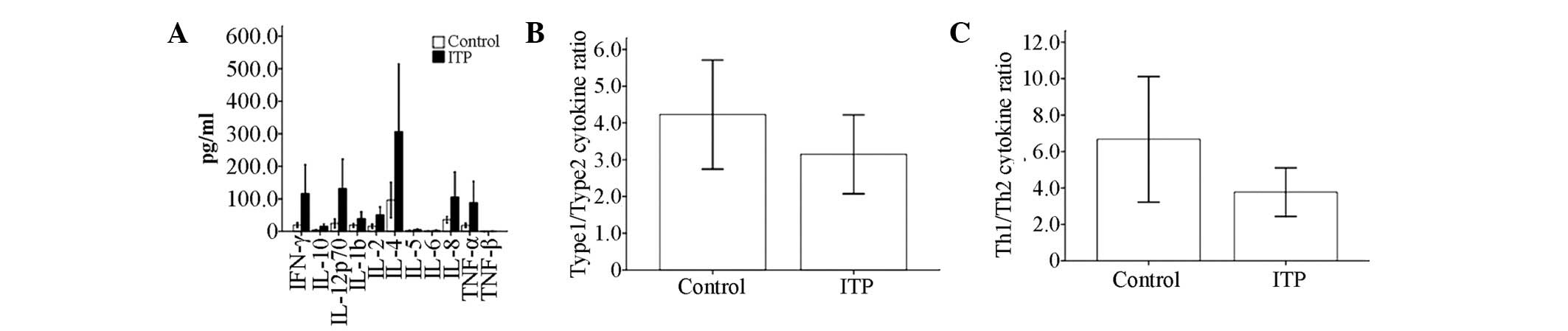 | Figure 3T helper (Th)-1/Th2 cytokine profile
in serum from patients with immune thrombocytopenia (ITP) and
healthy controls, as determined by cytometric bead array (CBA). (A)
Serum levels of interleukin (IL)-12p70, IL-10, IL-2, IL-8, IL-6,
IL-5, IL-4, IL-1β, interferon (IFN)-γ, tumor necrosis factor
(TNF)-α and TNF-β were compared between adults with chronic ITP and
healthy controls. (B) Relative expression of type 1 cytokines
(IFN-γ, IL-2, IL-12p70 and TNF-β) versus type 2 cytokines (IL-4,
IL-5, IL-6 and IL-10). (C) Th1 (IFN-γ and IL-2) and Th2 cytokines
(IL-4 and IL-5) were compared. |
 | Table IIResults of serum cytokine profiling
in patients with ITP and controls. |
Table II
Results of serum cytokine profiling
in patients with ITP and controls.
| Cytokine level
(pg/ml) | |
|---|
|
| |
|---|
| Cytokine | ITP | Control | P-value |
|---|
| IFN-γ | 116.38±88.79 | 20.03±7.35 | >0.05 |
| IL-10 | 16.05±7.03 | 4.20±0.95 | >0.05 |
| IL-12p70 | 131.92±90.74 | 24.59±13.96 | >0.05 |
| IL-1β | 39.47±20.96 | 18.94±5.67 | >0.05 |
| IL-2 | 51.12±24.04 | 16.13±6.54 | >0.05 |
| IL-4 | 306.84±207.59 | 96.27±54.69 | >0.05 |
| IL-5 | 5.52±2.26 | 2.19±0.73 | >0.05 |
| IL-6 | 2.66±1.75 | 1.36±1.00 | >0.05 |
| IL-8 | 105.59±77.24 | 36.08±9.92 | >0.05 |
| TNF-α | 88.68±65.01 | 18.65±6.73 | >0.05 |
| TNF-β | 0.60±0.60 | 0.00±0.00 | >0.05 |
 | Table IIIResults of serum cytokine profiling
in patients with ITP with severe (PLT ≤20×109/l) and
moderate (PLT >20×109/l) thrombocytopenia and
controls. |
Table III
Results of serum cytokine profiling
in patients with ITP with severe (PLT ≤20×109/l) and
moderate (PLT >20×109/l) thrombocytopenia and
controls.
| Cytokine level
(pg/ml) | |
|---|
|
| |
|---|
| Cytokine | ITP (PLT
≤20×109/l) | ITP (PLT
>20×109/l) | Control | P-value |
|---|
| IFN-γ | 17.64±9.29 | 194.34±158.25 | 20.03±7.35 | >0.05 |
| IL-10 | 16.67±11.94 | 15.56±8.64 | 4.20±0.95 | >0.05 |
| IL-12p70 | 22.01±12.39 | 218.68±161.15 | 24.59±13.96 | >0.05 |
| IL-1β | 27.02±21.62 | 49.29±33.79 | 18.94±5.67 | >0.05 |
| IL-2 | 39.59±19.66 | 60.23±40.59 | 16.13±6.54 | >0.05 |
| IL-4 | 64.34±48.44 | 498.29±367.87 | 96.27±54.69 | >0.05 |
| IL-5 | 6.52±4.43 | 4.72±2.14 | 2.19±0.73 | >0.05 |
| IL-6 | 0.94±0.66 | 4.02±3.09 | 1.36±1.00 | >0.05 |
| IL-8 | 45.02±31.92 | 153.42±136.59 | 36.08±9.92 | >0.05 |
| TNF-α | 20.32±9.29 | 142.64±115.96 | 18.65±6.73 | >0.05 |
| TNF-β | 1.37±1.37 | 0.00±0.00 | 0.00±0.00 | >0.05 |
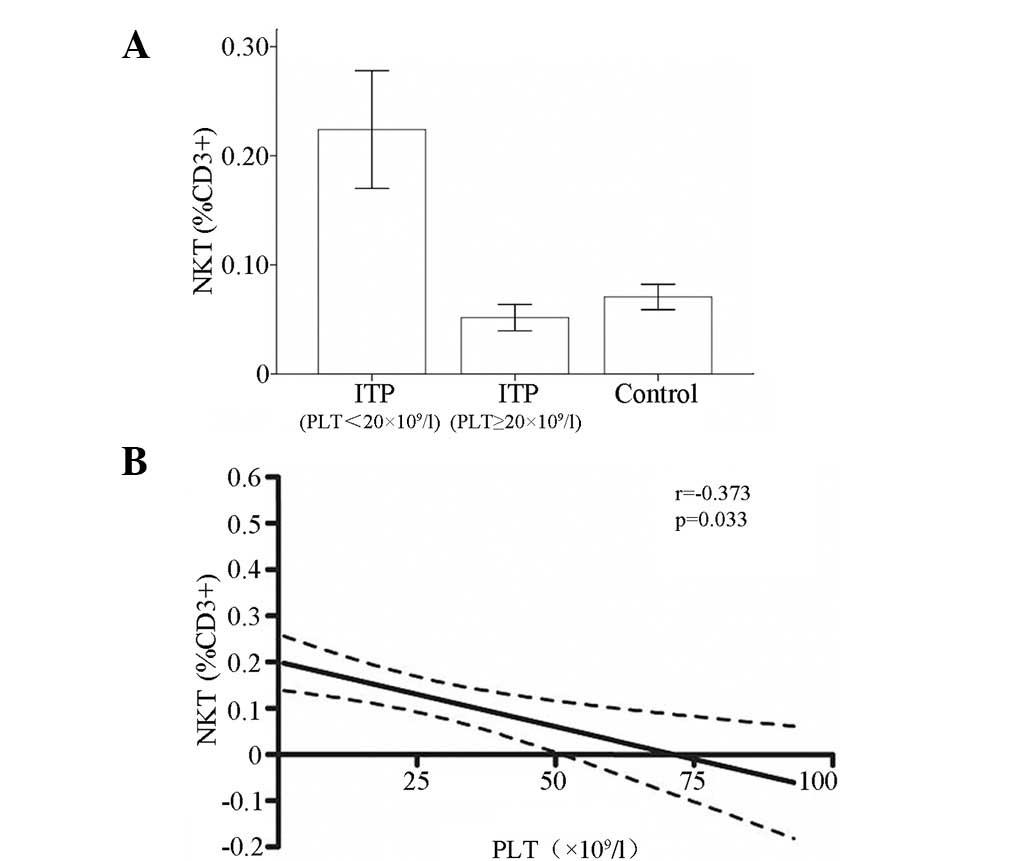
Negative correlation between circulating
NKT cells and platelet count
Although the difference in the frequency of
circulating NKT cells between the patients with ITP and the
controls was marginal, the level of NKT cells in patients with
chronic ITP and severe thrombocytopenia (≤20×109/l) was
significantly elevated compared with that in either the controls or
the patients with moderate thrombocytopenia
(>20×109/l). Thus, a correlation analysis was
performed between the level of circulating NKT cells and the
platelet count in patients with ITP. A negative correlation between
platelet count and NKT cell circulation level was revealed by a
linear regression analysis in adult patients with chronic ITP
(r=−0.373; P=0.033; Fig. 5). In
addition, a positive correlation between the frequency of Tregs and
the Th1/Th2 ratio was detected in adults with chronic ITP (r=0.451;
P = 0.011). Also, the platelet count was positively correlated with
serum levels of IL-12p70 (r=0.354; P=0.044), IFN-γ (r=0.365;
P=0.037), IL-4 (r=0.354; P=0.044) and TNF-α (r=0.366; P=0.036) in
patients with ITP (data not shown). However, the results did not
reveal a correlation between circulating Tregs and peripheral NKT
cells in adult chronic ITP.
Discussion
NKT cells and Tregs are important in the maintenance
of peripheral tolerance in humans. Abnormalities in the levels or
quality of NKT cells and Tregs have been implicated in numerous
autoimmune diseases. The loss of peripheral tolerance of a host
immune system to platelet auto-antigens leads to premature platelet
destruction and a variety of clinical presentations in patients
with ITP. Liu et al(10)
and Sakakura et al(11)
observed that levels of circulating Tregs decreased significantly
in patients with ITP. However, Yu et al(12) demonstrated that the inhibitory
activity, and not the number, of Tregs in the peripheral blood of
patients with ITP contributed to the loss of peripheral tolerance
in ITP. By contrast, our results showed that levels of circulating
Tregs were not decreased significantly in adult chronic ITP, unlike
in the controls. This discrepancy may have been due to the
different protocols for the identification of Tregs in different
studies. Transcription factor FoxP3 has been indicated to be the
most efficacious marker for Treg identification to date (18). However, FoxP3 expression was also
detected in CD4+ cells with low or negative CD25 antigen
and CD8+ cells (18),
thereby leading to an inaccurate measurement of Tregs. Liu et
al(19) revealed that a
phenotype of CD4+CD25+CD127+/− was
able to be used reliably as a marker for functional Tregs in
humans. Different markers for the identification of Tregs may, to
some extent, explain the discrepancy in the measurements of Tregs
between different studies, including the present study.
Johansson et al(15) identified that the proliferative
potential of peripheral NKT cells was markedly decreased in
patients with ITP compared with the control group. Furthermore, the
decreased proliferative potential of NKT cells worsened following
corticosteroid therapy, which indicated that NKT cells are involved
in ITP pathogenesis. Levels of NKT cells were noted to be elevated
in the peripheral blood of a female with ITP; these elevated NKT
cells inhibited the in vitro proliferation of autologous
CD4+ T cells, which indicated the protective role of NKT
cells in ITP (20). This
observation was further supported by the results of Ho et
al(21), which indicated that
activated NKT cells inhibited the in vitro proliferation of
CD8+ cells, and that CD8+ NKT cells
suppressed T cell activation through the killing mechanism of
antigen-presenting cells. In the present study, the levels of
circulating NKT cells increased in patients with ITP; however, the
difference between the patients with ITP and the controls was not
statistically significant. Further analysis revealed that NKT cell
levels were markedly elevated in adult patients with ITP with
severe thrombocytopenia, which suggested the importance of NKT
cells in ITP, particularly with severe thrombocytopenia. In
addition, our results showed a negative correlation between
platelet count and peripheral NKT cells in ITP. Our results and
data from other studies further indicate that NKT cells are
important in the pathogenesis of ITP. Cao et al(22) observed that plasma levels of IL-22
were significantly increased in patients with active ITP, and
high-dose dexamethasone administration reduced IL-22 production and
corrected the imbalance between Th1 and Th22 subsets. The NKT cell
is an IL-22-secreting cell in vivo(23). Thus, IL-22 and NKT cells in chronic
ITP appear to be correlated.
Azuma et al(24) reported that Tregs inhibited the
proliferation, cytokine secretion (including IFN-γ, IL-4, IL-13 and
IL-10) and cytotoxicity of CD4+ and
CD4−CD8− NKT cells through a cell-cell
contact mechanism. NKT cells, particularly CD4+ NKT
cells, promoted Treg proliferation through IL-2 synthesis and
secretion (25). However, in the
present study, no correlation between NKT cells and Tregs was
identified. Additional studies are required to delineate the
correlation between these two regulatory T cell subsets in chronic
ITP.
In this study, no significant difference was
observed in the serum cytokine profiles and the Th1/Th2 and type
1/type 2 ratios between the patients with ITP and the controls. Our
data contradicted the results of other studies on serum cytokine
profiles in ITP (6,8). However, linear regression analysis
showed that the platelet count correlated positively with the serum
levels of IL-12p70, IFN-γ, IL-4 and TNF-α in patients with ITP,
which implied the involvement of cytokines in ITP.
At present, a direct comparison between different
studies is difficult due to the exclusive nature of the ITP
diagnosis (26) and the diversity
of the disease phenotype. Zehnder et al(27) proposed that a specific labeling and
reliable enumeration for platelet antigen-specific T and B cells
was necessary in the future. Accordingly, investigations into ITP
may be expanded considerably when platelet antigen-specific T and B
cell data are combined with the analysis of NKT cells, Tregs and
serum cytokine profiles in adult chronic ITP.
Acknowledgements
This study was supported grants from Jinhua
Municipal Bureau of Science and Technology (no. 2007-3-040) and the
Department of Science & Technology of Zhejiang Province (no.
2010C13011).
References
|
1
|
Rodeghiero F, Stasi R, Gernsheimer T, et
al: Standardization of terminology, definitions and outcome
criteria in immune thrombocytopenic purpura of adults and children:
report from an international working group. Blood. 113:2386–2393.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gernsheimer T: Chronic idiopathic
thrombocytopenic purpura: mechanisms of pathogenesis. Oncologist.
14:12–21. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou B, Zhao H, Yang RC and Han ZC:
Multi-dysfunctional pathophysiology in ITP. Crit Rev Oncol Hematol.
54:107–116. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Semple JW: Immune pathophysiology of
autoimmune thrombocytopenic purpura. Blood Rev. 16:9–12. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Coopamah MD, Garvey MB, Freedman J and
Semple JW: Cellular immune mechanisms in autoimmune
thrombocytopenic purpura: An update. Transfus Med Rev. 17:69–80.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Panitsas FP, Theodoropoulou M, Kouraklis
A, et al: Adult chronic idiopathic thrombocytopenic purpura (ITP)
is the manifestation of a type-1 polarized immune response. Blood.
103:2645–2647. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang T, Zhao H, Ren H, et al: Type 1 and
type 2 T-cell profiles in idiopathic thrombocytopenic purpura.
Haematologica. 90:914–923. 2005.PubMed/NCBI
|
|
8
|
Zhang J, Ma D, Zhu X, Qu X, Ji C and Hou
M: Elevated profile of Th17, Th1 and Tc1 cells in patients with
immune thrombocytopenic purpura. Haematologica. 94:1326–1329. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
von Boehmer H: Mechanisms of suppression
by suppressor T cells. Nat Immunol. 6:338–344. 2005.PubMed/NCBI
|
|
10
|
Liu B, Zhao H, Poon MC, et al: Abnormality
of CD4+CD25+ regulatory T cells in idiopathic
thrombocytopenic purpura. Eur J Haematol. 78:139–143. 2007.
|
|
11
|
Sakakura M, Wada H, Tawara I, et al:
Reduced Cd4+Cd25+ T cells in patients with
idiopathic thrombocytopenic purpura. Thromb Res. 120:187–193.
2007.
|
|
12
|
Yu J, Heck S, Patel V, et al: Defective
circulating CD25 regulatory T cells in patients with chronic immune
thrombocytopenic purpura. Blood. 112:1325–1328. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kronenberg M and Gapin L: The
unconventional lifestyle of NKT cells. Nat Rev Immunol. 2:557–568.
2002.PubMed/NCBI
|
|
14
|
Wilson SB and Delovitch TL: Janus-like
role of regulatory iNKT cells in autoimmune disease and tumor
immunity. Nat Rev Immunol. 3:211–222. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Johansson U, Macey MG, Kenny D, Provan D
and Newland AC: Alpha-galactosylceramide-driven expansion of human
natural killer T cells is inhibited by prednisolone treatment. Br J
Haematol. 125:400–404. 2004. View Article : Google Scholar
|
|
16
|
La Cava A, Van Kaer L and Fu DS:
CD4+CD25+ Tregs and NKT cells: regulators
regulating regulators. Trends Immunol. 27:322–327. 2006.
|
|
17
|
Zhang L, Li H, Zhao H, Ji L and Yang R:
Hepatitis C virus-related adult chronic idiopathic thrombocytopenic
purpura: experience from a single Chinese center. Eur J Haematol.
70:196–197. 2003. View Article : Google Scholar
|
|
18
|
Ziegler SF: FOXP3: of mice and men. Annu
Rev Immunol. 24:209–226. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu W, Putnam AL, Xu-Yu Z, et al: CD127
expression inversely correlates with FoxP3 and suppressive function
of human CD4+ T reg cells. J Exp Med. 203:1701–1711.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Johansson U, Macey MG, Kenny D, Provan AB
and Newland AC: The role of natural killer T (NKT) cells in immune
thrombocytopenia: is strong in vitro NKT cell activity related to
the development of remission? Br J Haematol. 129:564–565. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ho LP, Urban BC, Jones L, Ogg GS and
McMichael AJ: CD4−CD8αα subset of CD1d-restricted NKT
cells controls T cell expansion. J Immunol. 172:7350–7358.
2004.
|
|
22
|
Cao J, Chen C, Li L, et al: Effects of
high-dose dexamethasone on regulating interleukin-22 production and
correcting Th1 and Th22 polarization in immune thrombocytopenia. J
Clin Immunol. 32:523–529. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wolk K, Witte E, Witte K, Warszawska K and
Sabat R: Biology of interleukin-22. Semin Immunopathol. 32:17–31.
2010. View Article : Google Scholar
|
|
24
|
Azuma T, Takahashi T, Kunisato A, Kitamura
T and Hirai H: Human CD4+ CD25+ regulatory T
cells suppress NKT cell functions. Cancer Res. 63:4516–4520.
2003.PubMed/NCBI
|
|
25
|
Jiang S, Game DS, Davies D, Lombardi G and
Lechler RI: Activated CD1d-restricted natural killer T cells
secrete IL-2: innate help for CD4+CD25+
regulatory T cells? Eur J Immunol. 35:1193–1200. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Provan D, Stasi R, Newland AC, et al:
International consensus report on the investigation and management
of primary immune thrombocytopenia. Blood. 115:168–186. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zehnder JL, Semple JW, Imbach P, Neufeld
EJ, Buchanan GR and Cines DB: Future research in ITP: an ICIS
consensus. Ann Hematol. 89(Suppl 1): S19–S23. 2010. View Article : Google Scholar
|