Introduction
Echinococcus granulosus (E.
granulosus) causes cystic echinococcosis (CE), which seriously
injures human health and delays the development of the animal
breeding industry. China is one of the countries with a high rate
of E. granulosus disease (1,2),
which is mainly common in the west pastoral and semi-pastoral areas
of China, including Xinjiang, Qinghai and Gansu (3). The main treatment of hydatid disease
is surgery supplemented by drug treatment (4). However, the surgery is harmful to the
human body, whereas a molecular vaccine is an ideal method to
prevent alveolar echinococcosis (5,6).
Since E. granulosus is a multicellular parasite and the
antigen structure is highly complex, it is necessary to study the
E. granulosus protoscolex antigen and the adult worm antigen
to identify the protein for a polyvalent vaccine.
The EgA31 antigen was currently studied as the
dominant antigen of the adult worm. If the rostellum of the
protoscolex entering the host intestine is not removed or absorbed
by the intestinal mucosa, the worms are excreted out of the host
(7). Fraize et al(8) built a model in vitro to
evaluate the T cell response of the final host to the protoscolex
and Eg antigen. The study observed that the protoscolex did not
cause changes in cytokine production, which was consistent with the
Eg metacestode showing no or little immunogenicity within the final
host (9). This was consistent with
Vuitton’s (10) theory, i.e. that
the parasites are able to shed the surface antigen and interfere
with antigen presentation mechanisms to reduce immunogenicity and
evade the immune system of the host. EgA31 antigen may increase
interleukin-10 (IL-10) and IL-12 production. IL-12 may promote the
production of interferon-γ (IFN-γ) and inhibit protective immunity,
resulting in chronic infection (11).
Eg95 protein is an ideal protective antigen and is
one of the most extensively studied antigenic components. The Eg95
recombinant protein vaccine immunized the intermediate host
(sheep), and 86% complete immune protection was obtained (6). Furthermore, Ding et
al(12) and Liu et
al(13) demonstrated that the
pcDNA3-Eg95 gene vaccine and the Eg95 recombinant protein were able
to produce specific humeral and cellular immune responses in mice.
Alvite and Esteves (14) observed
that the expression of the EgA31 antigen may be correlated with the
sucking function of the scolex in each growth stage of the adult.
This antigen is located at multiple sites on the parasite
protoscolex and adult worms. Saboulard et al(15) observed that EgA31 protein exhibited
a high level of antigenicity and immunogenicity. EgA31 was also
demonstrated to show the strongest immunoreactivity in a different
study (16). It is possible to
develop a protein composition vaccine; thus, the Eg95 and EgA31
antigens were selected as vaccine candidates and recombinant
antigens, and were predicted to enhance the immune response and
play a significant role in immunogenicity. The aim was to provide
further experimental foundation for a multivalent EgA31-Eg95
vaccine.
Materials and methods
E. granulosus protoscolex and adult
specimens, serum, plasmids and strains
The E. granulosus protoscoleces were obtained
from the slaughterhouse (Urumqi, China) from E. granulosus
infection to liver cysts, while the adult specimens were obtained
from infected canine intestine provided by the Veterinary Research
Institute of the Xinjiang Academy of Animal Science and (Urumqi,
China). E. granulosus-infected dog serum and healthy serum
were also provided by the Veterinary Research Institute of the
Xinjiang Academy of Animal Science. The mice infected with
Echinococcus granulosus were supplied by Animal Center of
Xinjiang Medical University. The cloning plasmid pUCm-T was
purchased from MBI, Inc. (Pomona, CA, USA). The prokaryotic
expression plasmid pET30a and the recombinant plasmids pET28a-Eg95
and pET30a-EgA31 were obtained from Xinjiang Laboratory of Hydatid
Fundamental Medicine, First Affiliated Hospital of Xinjiang Medical
University (Urumqi, China), while the Escherichia coli
(E. coli) DH5-α was obtained from Invitrogen Life
Technologies (Carlsbad, CA, USA). The E. coli strain BL21
(DE3) (Panvera, Madison, WI, USA) was used to amplify the
recombinant vector. All patients and healthy controls signed the
informed consent, the experimental design was approved by the
ethical committee (Approval Number: 20120220-126). All experiments
using mice were performed in accordance with protocols approved by
Xinjiang Medical University Animal Ethics Committee according to
China Guidelines on Animals Care (No. A-20100920002).
Main reagents and formula
TRIzol®, DL2000 DNA Marker,
λHindIII digest, the restriction enzymes BamHI,
SacI and NotI, and T4 DNA ligase were purchased from
Takara Bio, Inc. (Shiga, Japan). A cDNA synthesis kit (MBI, Inc.)
was used to amplify the AMV reverse transcriptase, while Taq DNA
polymerase and pUCm-T (MBI, Inc.) were used to amplify the genes.
Prestained protein marker and a Bicinchoninic acid (BCA) Protein
Quantitation kit from BioTeke Corp. (Beijing, China) were used in
the western blotting. Goat anti-rabbit immunoglobulin G
(IgG)-horseradish peroxidase (HRP) and goat anti-human IgG-HRP were
obtained from Sigma (St. Louis, MO, USA). In addition, a UNIQ-10
Column Mini Plasmid kit and UNIQ-10 Column DNA Gel Extraction kit
(Bio-Rad Laboratories (Shanghai) Co., Ltd., Shanghai, China) were
used.
Primer design and synthesis
According to the Echinococcus EgA31 gene sequence
(GenBank Accession: AF067807), Eg95 gene sequence (GenBank
Accession: X90928) and Prokaryotic expression plasmid pET30a
restriction map, we designed two pairs of primers with the
software. Two pairs of primers were designed with the DNAman
software (Lynnon Corp., Pointe-Claire, QC, Canada), and synthesized
by Sangon (Shanghai, China). The primers were as follows: EgA31
forward primer P1, 5′-GGA TCC CGT CTA AGA ATA TCT GCA GCT
GA-3′ (bold sequence was the restriction site of BamHI) and
reverse primer P2, 5′-GAG CTC AGT CTC AGC CCT TGT TTC AAG
CA-3′ (bold for the restriction site of SacI); Eg95 upstream
primer P3, 5′-GAG CTC ATG GCA TTC CAG TTA TGT CT-3′ (bold
nucleotides represent the SacI restriction site) and reverse
primer P4, 5′-GCG GCC GCC AGT GCT TTC CTT CTT-3′ (bold
nucleotides represent the NotI restriction site).
Transcription and reverse extraction of
the total RNA of E. granulosus protoscolex and adult specimens
The total RNA was extracted from the liver of mice
using TRIzol reagent. A transcription kit was used to amplify all
mRNA into complementary DNA (cDNA).
Cloning and identification of the EgA31-
and Eg95-encoding genes
The cDNA in adult specimens was used as a template
to clone the EgA31 target fragment using reverse
transcription-polymerase chain reaction (RT-PCR). The cDNA in the
protoscolex specimens was used as a template for the Eg95-encoding
gene to clone the Eg95 target fragment with an RT-PCR kit,
according to the manufacturer’s instructions (Invitrogen Life
Technologies).
Construction of the prokaryotic
expression plasmid pET30a-EgA31-Eg95
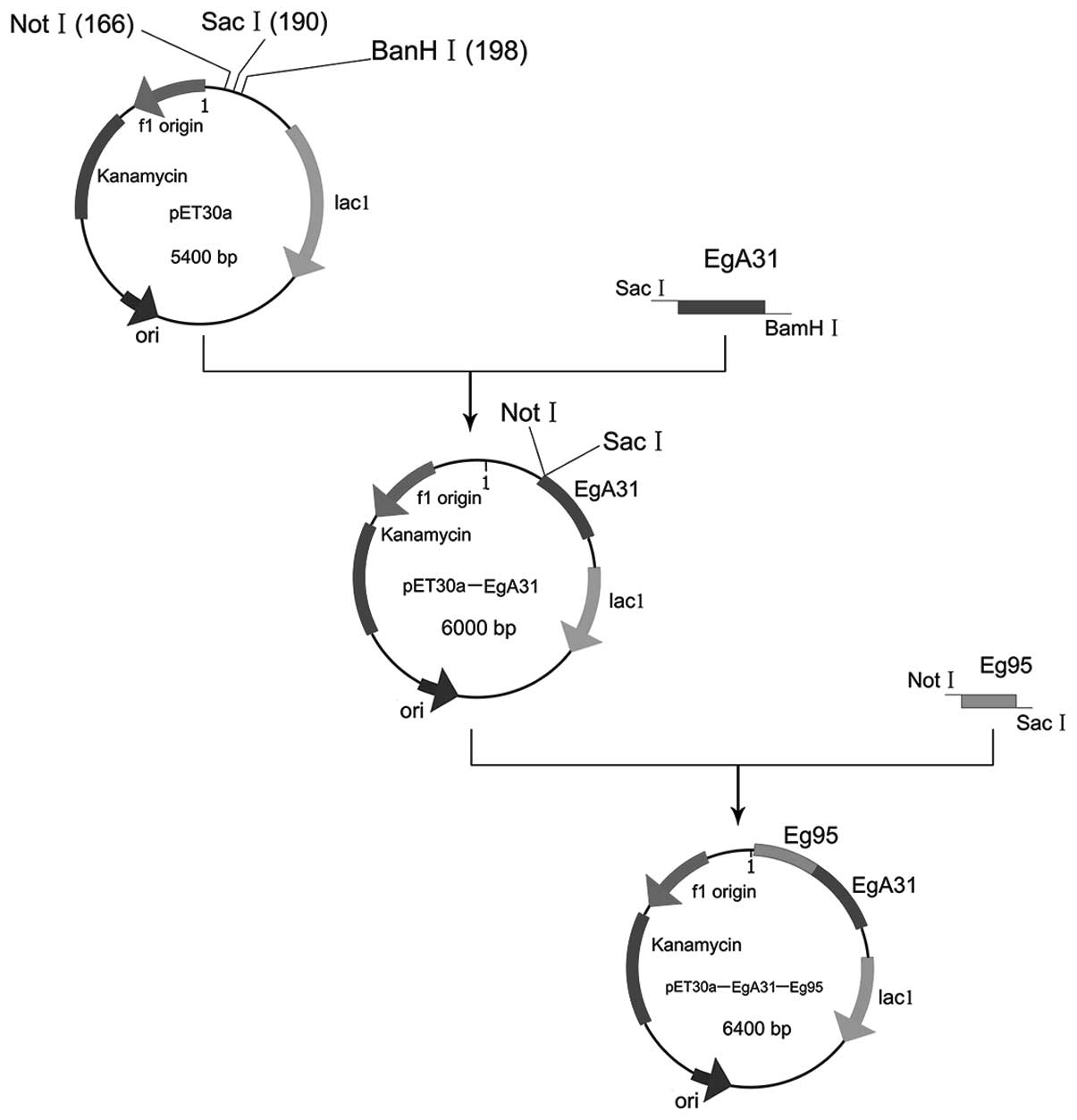
Plasmid construction is shown schematically in
Fig. 1. Genetic engineering and
cloning were used to channel the Eg95 antigen gene into the shuttle
plasmid pET30a-EgA31 to build the recombinant plasmid
pET30a-EgA31-Eg95 with two sections of the target gene. The
recombinant plasmids pET30a-EgA31 and pUCm-T/Eg95 were extracted.
The double digestion of the recombinant plasmid pET30a-EgA31
consisted of a total reaction volume of 160 μl, which included the
following: Plasmid 16 μl, 8 U/μl SacI 5 μl, 10 U/μl
NotI 4 μl, 10X K buffer 8 μl, 0.1% bovine serum albumin
(BSA) 16 μl and ddH2O 111 μl. The double digestion of
the recombinant plasmid pUCm-T/Eg95 consisted of a total reaction
volume of 200 μl, which included the following: Plasmid 20 μl, 8
U/μl SacI 6 μl, NotI 5 μl 10 U/μl, 10X K buffer 10
μl, 0.1% BSA 20 μl and ddH2O 139 μl. The digestions were
performed at 37°C for 12 h. Following separation by 1.2% agarose
gel, the corresponding fragment of target gene Eg95 and a large
fragment of linear pET30a were recycled by a DNA gel extraction
kit. The DNA was then dissolved in ddH2O.
The two digested plasmids were linked. Subsequently,
the recombinant plasmid pET30a-EgA31-Eg95 was identified via
sequencing: P1 and P4 were amplified (annealing temperature 55°C).
The restriction enzyme digestion was used to confirm the amplified
DNA and affirm that the sites were correct (BamHI,
NotI digestion). The recombinant plasmid pET30a-EgA31-Eg95
was sequenced to confirm its identity. The measurement analysis was
performed with a kit purchased from Sangon.
Expression and purification of the
recombinant protein
Recombinant Eg95 protein
The prokaryotic expression plasmid pET28a-Eg95 was
transformed into E. coli BL21 (DE3), and 2% of the
inoculation amount of the overnight culture of a single bacterium
was transferred to liquid LB medium containing kanamycin. The A600
absorbance value was ~0.6. Protein expression was induced with the
final concentration of 0.1 mmol/l isopropylthio-β-galactoside
(IPTG) at 28 and 37°C. The samples were collected at different
induction times (0, 1, 2, 3, 4, 5 and 6 h) and bacteria were
obtained. The samples were then placed into a boiling water bath
for 5 min and 12% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) was performed to assess expression.
Recombinant EgA31 and EgA31-Eg95
protein
The recombinant plasmid pET30a-EgA31-Eg95 1 μl and
E. coli BL21 (DE3) competent cells transformed by
pET30a-EgA31-Eg95 1 μl were taken for transformation into E.
coli BL21 (DE3)-competent cells, and PCR was used to screen the
recombinants. Cultured and expression-induced thalli were collected
at 0, 2, 4 and 6 h using SDS-PAGE (pET30a-EgA31-Eg95 recombinant
protein was detected using SDS-PAGE Mini Protein IH using a
miniature protein electrophoresis system). The recombinant protein
was purified by a His column using chromatographic purification of
the target protein. Protease inhibitor (Ben 15 μg/ml, Leu 2 μg/ml,
PMSF 1 mmol/l, Pep 1 μg/ml) was added to 200 ml of bacterial
culture following induction for 3 h. The cells were lysed by ice
bath sonication and centrifugation, and the purified recombinant
pET28a-Eg95 protein was obtained by His-Bind Resin, SDS-PAGE
electrophoresis analysis.
Recombinant protein detection
The separation gel was retained for transformation
to a membrane, with a constant current of 120 mA at 4°C overnight.
The gel was sealed and agitated at 37°C for 2 h. The primary
antibody was added and incubated at 37°C for 2 h. The
nitrocellulose membrane was placed in the diluted secondary
antibody, with stable shaking at 37°C for an hour.
3,3′-Diaminobenzidine (DAB) staining was performed prior to rinsing
with water. Dog serum infected with E. granulosus (1:100
dilution) was used as the primary antibody to EgA31 recombinant
protein and recombinant EgA31-Eg95 protein, and the secondary
antibody was HRP-labeled rabbit anti-dog IgG (1:400 dilution with
phosphate-buffered saline with Tween 20).
Results
Total RNA extraction from E. granulosus
protoscolex and adult
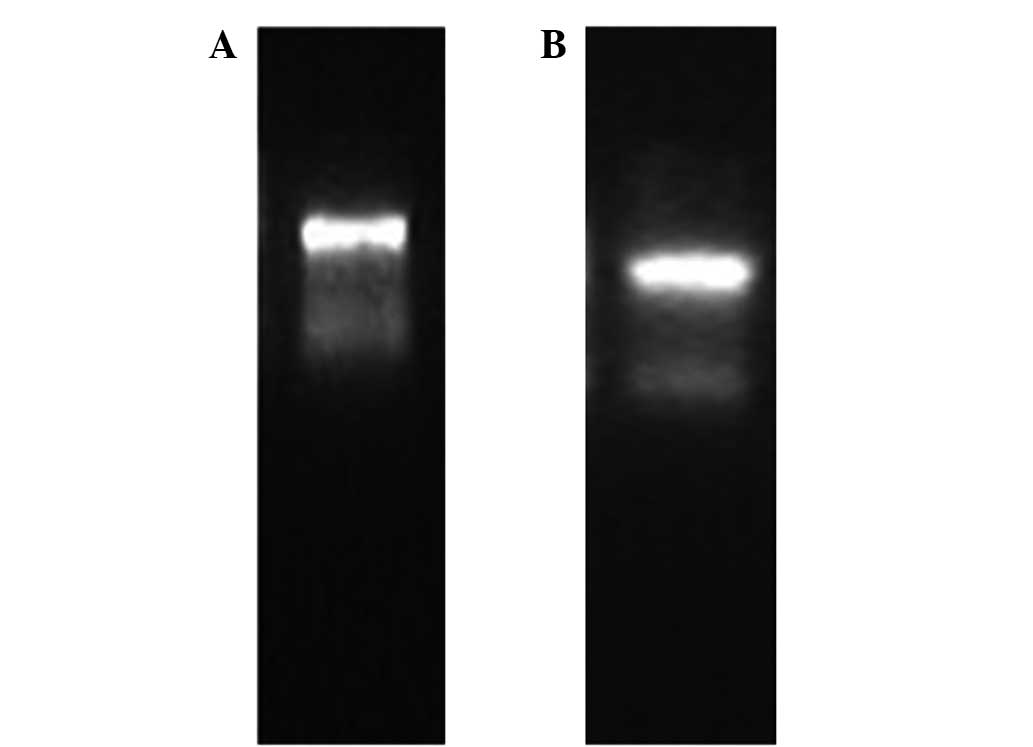
The total RNA was run in 1.2%
3-(N-morpholino)propanesulfonic acid (MOPS)-formaldehyde denaturing
gel electrophoresis (Fig. 2). The
density of the RNA bands was measured by the absorption at
wavelengths of 260 and 280 nm with a nucleic acid and protein
valuating machine (NanoDrop 2000; Thermo Scientific, Waltham, MA,
USA). The A260/A280 ratio for protoscolex RNA was 1.81 and 1.85 for
adults. The total RNA extraction was successful and the purity and
concentration of RNA were high.
Cloning of EgA31 and Eg95 antigen
genes
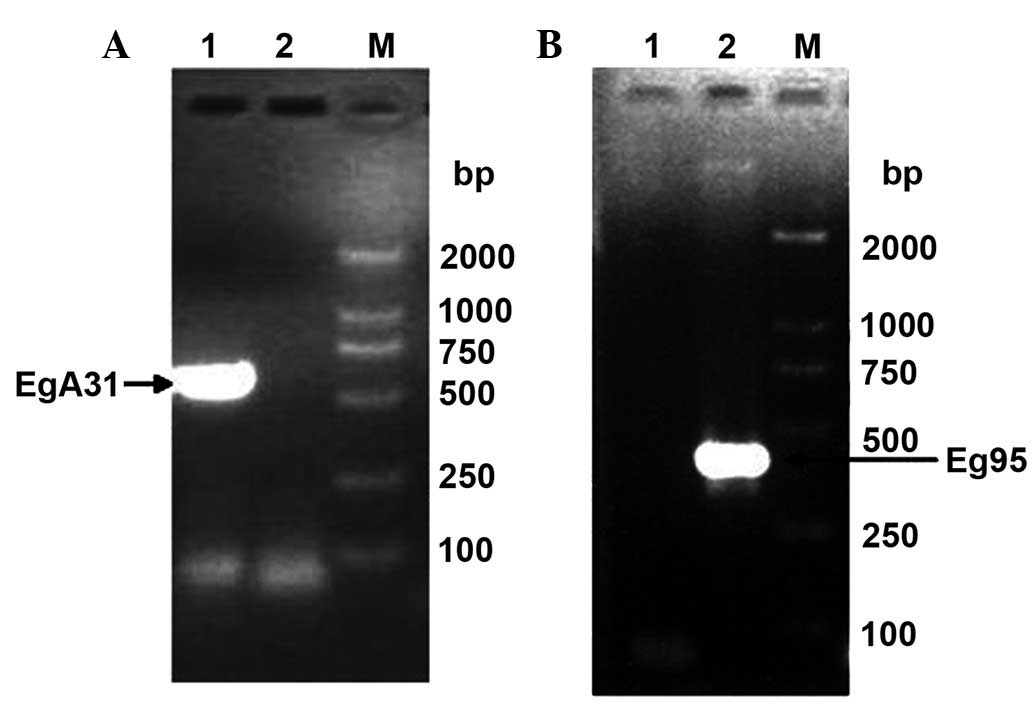
Using adult E. granulosus cDNA and E.
granulosus protoscolex cDNA as templates, respectively, EgA31
primers and Eg95 primers were used for RT-PCR amplification. The
PCR products were analyzed using 1.2% agarose gel electrophoresis,
and specific bands showed at 636 and 402 bp (Fig. 3), which were consistent with the
expected results. The negative control without template showed no
specific band, demonstrating that the amplification of the EgA31
and Eg95 gene fragments was successful.
EgA31 and Eg95 antigen gene
sequences
A comparison between the recorded or registered
EgA31 antigen gene sequence in GenBank (accession no. AF067807) and
the cloning EgA31 antigen-specific sequence showed that the two
sequences were identical. The cloned Eg95 antigen gene sequence was
also consistent with the Eg95 antigen gene sequence in GenBank
(accession no. X90928).
Construction of the prokaryotic
expression plasmid pET30a-EgA31-Eg95
Identification of enzyme digestion and
amplification
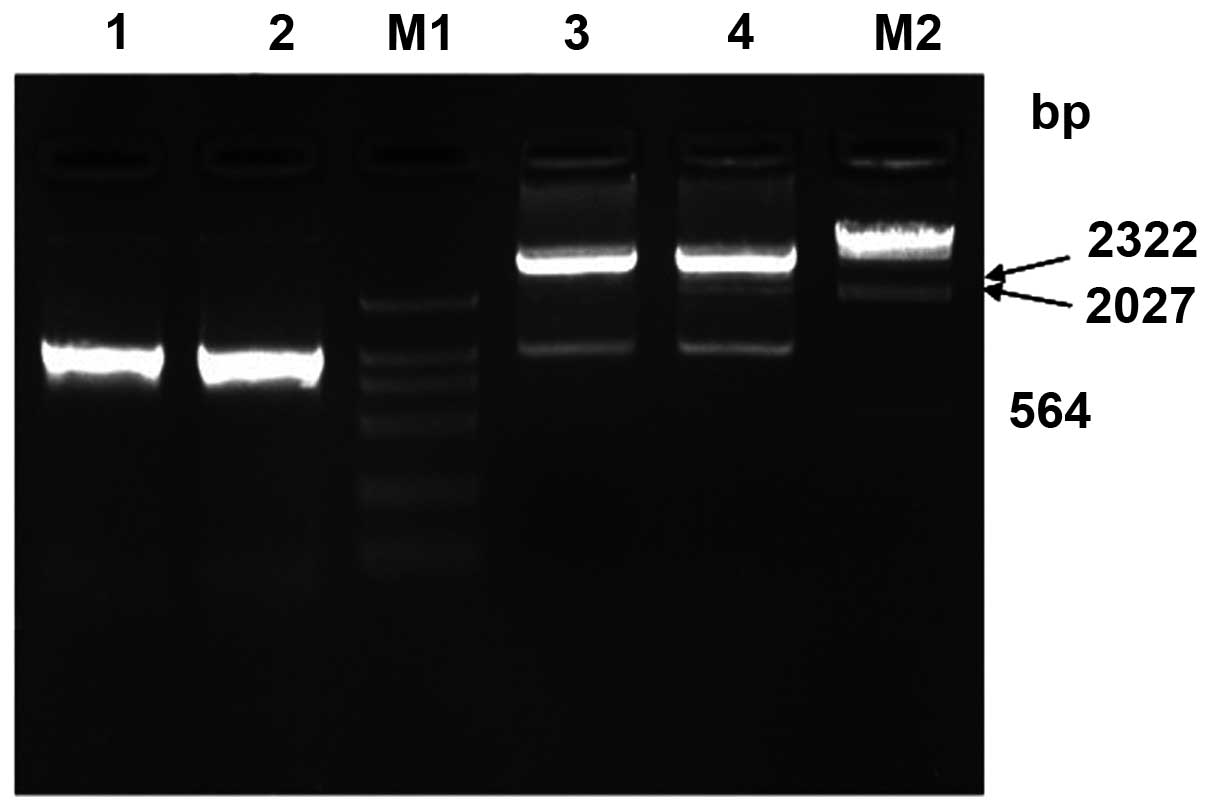
Eg95 antigen-targeted gene fragments were obtained
at 402 bp and a pET30a-EgA31 fragment at 5.5 kbp. Eg95 fragment and
pET30a-EgA31 fragment were recycled by electrophoresis and
connected by T4 DNA ligase, directionally cloned pET30a-EgA31-Eg95
vector through prokaryotic expression, following the process of
transformation, culturing and extraction of small amount of
plasmid. The 1038 bp EgA31-Eg95 DNA fragment was obtained by either
PCR method or recombinant plasmid pET30a-EgA31-Eg95 digestion with
BamHI and NotI, consistent with expected products
(Fig. 4).
Sequencing analysis
The digested identified recombinant plasmid broth (1
ml) was sent to Sangon for sequencing. Due to the connected
fragment length of 1,038 bp, bidirectional sequencing was used. The
cloned EgA31-Eg95 antigen gene was identical to the EgA31 and Eg95
cDNA sequences from the gene library, encoding 346 amino acids. The
molecular weight of the recombinant protein was 31 kDa.
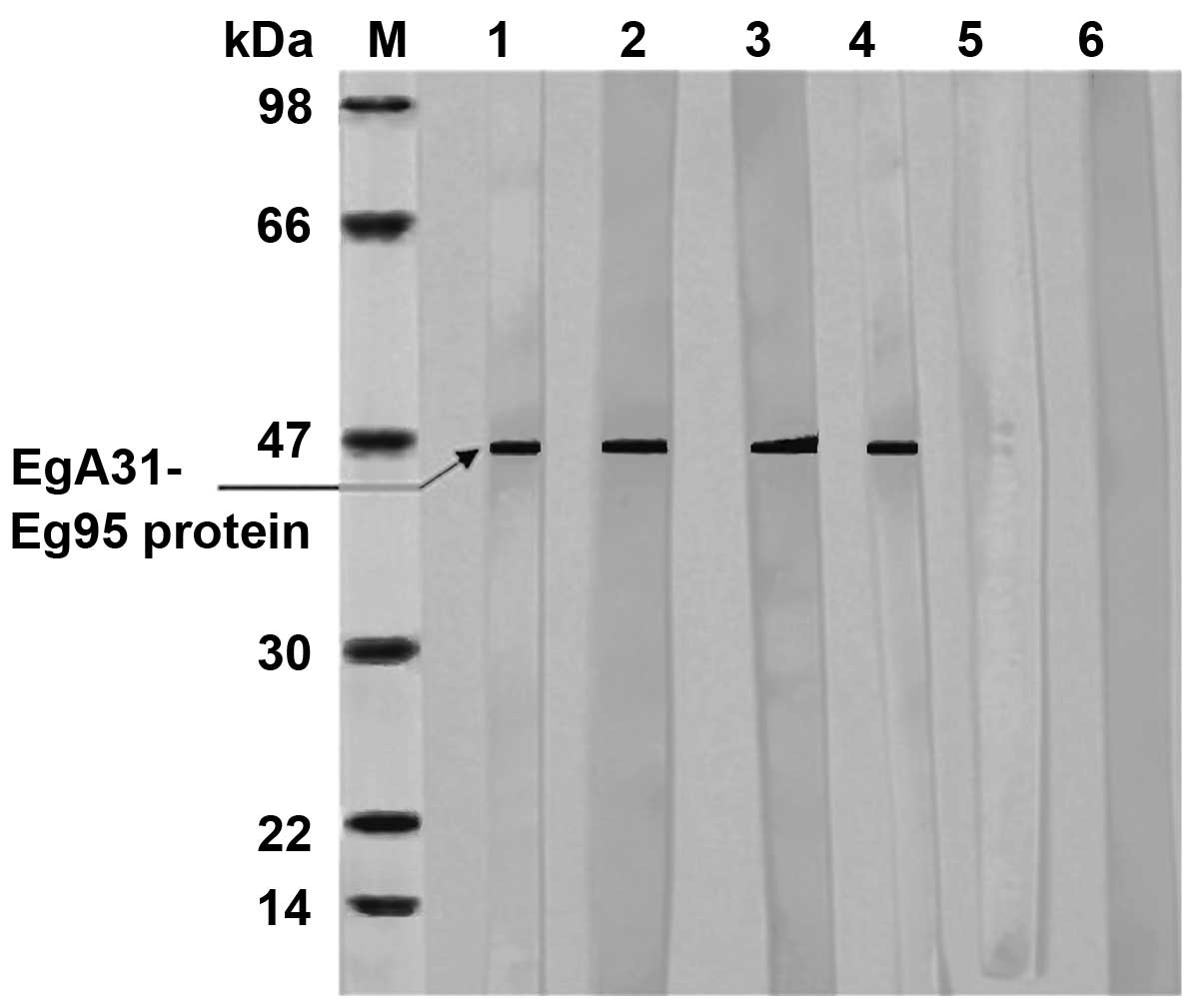
Western blotting results of EgA31-Eg95
recombinant protein
The correctly identified recombinant expression
vector pET30a-EgA31-Eg95 was detected using 12% SDS-PAGE, which
showed that its size was consistent with the expected protein size.
The induced recombinant protein EgA31-Eg95 was transferred to a
nitrocellulose membrane to combine with the corresponding antibody
(the primary antibody) in the serum of dogs infected with E.
granulosus, to form antigen-antibody complexes. The primary
antibody then combined with the HRP-labeled antibody (the secondary
antibody), resulting in the complex also being labeled with HRP.
Following a chromogenic reaction, the recombinant antigen proteins
EgA31-Eg95 were colored. The results are shown in Fig. 5.
Discussion
The immune response caused by hydatid infection is
complex and diverse, including humeral and cellular immunity, and
the involvement of other cells and the complement system. Different
mechanisms of protective immunity are induced by different
antigens, and the protective antigen specificity is also different
at various stages. During the long-term co-evolution of hydatid and
host, a variety of immune evasion mechanisms were induced (17). This is the reason that the ideal
effect of the hydatid monovalent vaccine is difficult to achieve. A
combined immunization program was used, aiming at different
developmental stages or different parts of Schistosoma
japonicum and selecting antigens from different sources, with
the aim of producing a synergistic effect by different immune
mechanisms (18). This was
performed in order to overcome the problems of the low-level immune
protection induced by single antigen molecules and to enhance the
immune effect of the vaccine. This is likely to represent a novel
direction in the development of an anti-hydatid vaccine (19).
The amino acid sequence analysis of EgA31 antigen
gene showed a 20–30% homology with flat phylum worm paramyosin and
myosin, containing epitopes recognized by specific IgE (20,21).
In this study, according to the screening for the kanamycin
resistance marker gene of the pET30a vector, positive clones were
sent for sequencing following PCR amplification and correct
restriction enzyme digestion. The sequencing results showed that
the selected positive clones were positive connection recombinants.
The cloned EgA31 antigen gene was identical to the EgA31 cDNA
sequence in the gene library, located between 493 and 1386 bp of
the full-length sequences and encoding 212 amino acids. Sequence
analysis showed that pET30a, which expresses in the form of the 6X
His-EgA31 fusion protein, contained 280 amino acids, and that the
molecular weight of the recombinant protein should be 31 kDa. This
was consistent with the SDS-PAGE results. The data showed that the
mixed EgA31 antigens [EgA31, EgTrp and fatty acid-binding protein 1
(FABP1)] caused higher levels of cytokines than the use of
mono-EgA31 antigen. EgA31 antigen belongs to a protein family that
confers protective immunity against numerous worm infections
(22).
The nucleotide sequencing showed that the cloned 5′
end in Eg95 was nine nucleotides longer than in Eg48. The Eg48
recombinant protein was used to immune sheep, the larva may be
reduced of 83%, average amount of cysts was 26.6 (23), which suggests that there are more
likely protective immunodominant epitopes within the nine
nucleotides. However, the response between Eg95 recombinant protein
and the serum of patients with CE has been rarely reported in
China. The positive response of Eg95 protein and patient sera has
an important role in this vaccine development. If the positive rate
of Eg95 in the patient serum is high, then it may be considered as
a postoperative treatment in addition to clinical services.
Therefore, this study selected to combine the protective antigens
Eg95 and EgA31 against Echinococcus infection.
The western blotting results showed that there were
positive reactions to the EgA31 and EgA31-Eg95 antigens in the sera
of infected dogs, whereas this reaction was not observed in normal
serum. This showed that the obtained EgA31 and the EgA31-Eg95
fusion protein had good antigenicity and were antigen molecules
with immunological activity. Induced EgA31 and EgA31-Eg95 protein
may be used as heterologous antigen to immune animals (including
rat and rabbit), to test their immune characteristics and evaluate
whether EgA31-Eg95 protein has a high level of immunological
protection as a candidate vaccine against Echinococcosis.
Polyclonal antiserum or anti-EgA31 and anti-EgA31-Eg95 monoclonal
antibodies (McAbs) were obtained by a hybridoma technique. These
were able to be identified using western blotting to analyze the
induced expression of the corresponding proteins produced by the
transformed bacteria or cells. The anti-EgA31-Eg95 antibody in
Eg-infected dog serum in the western blot analysis was able to be
used as the positive control serum. The most basic role of the
EgA31-Eg95 protein is as an antigen.
The results of this study showed that three
recombinant plasmids pET30a-EgA31, pET30a-EgA31-Eg95 and
pET28a-Eg95 were stably expressed in E. coli. Among the
three factors of induction temperature, induction time and
concentration, the effect of temperature and time on the expression
of the fusion protein was not notable. The expression of
pET30a-EgA31-Eg95 increased with the extension of induction time.
We selected Eg95 antigen, which is expressed in the protoscolex and
may protect the host against Schistosoma mansoni infection,
and EgA31 antigen, which is expressed in the scolex, skin and
subcutaneous muscle layer, as candidate vaccine molecules. Eg95 and
EgA31 antigen genes were constructed into a multivalent vaccine, to
stimulate the immune response of the host immune system against
E. granulosus protoscolex and the adult worm, enabling the
host to obtain effective immunological protection. This may provide
a wide application prospect for the design and development of an
Echinococcosis vaccine.
Acknowledgements
This study was supported by the National Nature
Science Foundation of China (grant nos. 81160200, 81060135,
81160378 and 30860263) and the University Research Projects of
Xinjiang Autonomous Region (grant no. XJEDU2010S25).
Abbreviations:
|
E. granulosus
|
Echinococcus granulosus
|
|
qPCR
|
quantitative real-time polymerase
chain reaction
|
|
CE
|
cystic echinococcosis
|
|
E. coli
|
Escherichia coli
|
|
cDNA
|
complementary DNA
|
References
|
1
|
Cardona GA and Carmena D: A review of the
global prevalence, molecular epidemiology and economics of cystic
echinococcosis in production animals. Vet Parasitol. 192:10–32.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang W and McManus DP: Recent advances in
the immunology and diagnosis of echinococcosis. FEMS Immunol Med
Microbiol. 47:24–41. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Heath DD, Robinson C, Shakes T, et al:
Vaccination of bovines against Echinococcus granulosus
(cystic echinococcosis). Vaccine. 30:3076–3081. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tomuş C, Zaharie F, Mocan L, et al:
Minimal invasive treatment of abdominal multiorgan echinococcosis.
Int Surg. 98:61–64. 2013.PubMed/NCBI
|
|
5
|
Dalton JP and Mulcahy G: Parasite vaccines
- a reality. Vet Parasitol. 98:149–167. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lightowlers MW, Flisser A, Gauci CG, et
al: Vaccination against cysticercosis and hydatid disease.
Parasitol Today. 16:191–196. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barnes TS, Deplazes P, Gottstein B, et al:
Challenges for diagnosis and control of cystic hydatid disease.
Acta Trop. 123:1–7. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fraize M, Sarciron ME, Saboulard D, et al:
An in vitro model to evaluate the cytokine response in Echinococcus
infections. Parasitol Res. 92:506–512. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fraize M, Sarciron ME, Azzouz S, et al:
Immunogenicity of two Echinococcus granulosus antigens EgA31
and EgTrp in mice. Parasitol Res. 96:113–120. 2005.
|
|
10
|
Vuitton DA: The ambiguous role of immunity
in echinococcosis: protection of the host or of the parasite? Acta
Trop. 85:119–132. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Urban JF Jr, Madden KB, Svetić A, et al:
The importance of Th2 cytokines in protective immunity to
nematodes. Immunol Rev. 127:205–220. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ding JB, Lin RY, Wen H, et al: Cloning and
eukaryotic expression plasmid construct of Echinococcus
granulosus 95 (Eg95) antigen gene. The Chinese people Zoonosis
magazine. 19:42–44. 2003.(In Chinese).
|
|
13
|
Liu XF, Ding JB, Li YJ, et al: The
Echinococcus multilocularis 95 antigen T-B epitope analysis.
Chinese Journal of Parasitic Disease. 7:779–773. 2012.(In
Chinese).
|
|
14
|
Alvite G and Esteves A: Echinococcus
granulosus tropomyosin isoforms: from gene structure to
expression analysis. Gene. 433:40–49. 2009. View Article : Google Scholar
|
|
15
|
Saboulard D, Lahmar S, Petavy AF and
Bosquet G: The Echinococcus granulosus antigen EgA31:
localization during development and immunogenic properties.
Parasite Immunol. 25:489–501. 2003.
|
|
16
|
Fu Y, Martinez C, Chalar C, et al: A new
potent antigen from Echinococcus granulosus associated with
muscles and tegument. Mol Biochem Parasitol. 102:43–52.
1999.PubMed/NCBI
|
|
17
|
Capron A, Capron M and Riveau G: Vaccine
development against schistosomia sis from concepts to clinical
trials. Br Med Bull. 62:139–148. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kalinna BH and McManus DP: A vaccine
against the Asian schistosome, Schistosoma japonicum: an
update on paramyosin as a target of protective immunity. Int J
Parasitol. 27:1213–1219. 1997.PubMed/NCBI
|
|
19
|
Jounai N, Kobiyama K, Takeshita F and
Ishii KJ: Recognition of damage-associated molecular patterns
related to nucleic acids during inflammation and vaccination. Front
Cell Infect Microbiol. 2:1682012.PubMed/NCBI
|
|
20
|
McManus DP and Loukas A: Current status of
vaccines for schistosomiasis. Clin Microbiol Rev. 21:225–242. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nara T, Tanabe K, Mahakunkijcharoen Y, et
al: The B cell epitope of paramyosin recognized by a protective
monoclonal IgE antibody to Schistosoma japonicum. Vaccine.
15:79–84. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Esmaelizad M, Ahmadian G, Aghaiypour K, et
al: Induction of prominent Th1 response in C57Bl/6 mice immunized
with an E. coli-expressed multi T-cell epitope EgA31 antigen
against Echinococcus granulosus. Folia Parasitol (Praha).
60:28–34. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Heath DD, Robinson C and Lightowlers MW:
Maternal antibody parameters of cattle and calves receiving EG95
vaccine to protect against Echinococcus granulosus. Vaccine.
30:7321–7326. 2012. View Article : Google Scholar : PubMed/NCBI
|