Introduction
Mechanical loading is considered essential for
maintaining skeletal integrity and bone mass. Previous studies have
shown that mechanical stimulation regulates signaling, gene
expression, proliferation and differentiation in osteoblasts
(1–4). The application of mechanical loading
to osteoblasts induces the activation of mitogen-activated protein
kinases (MAPKs), such as extracellular signal-regulated kinase
(ERK), c-jun-NH2-terminal kinase (JNK) and p38 MAPK, and
expression of numerous osteogenic genes, including cyclooxygenase-2
(Cox-2), early growth response-1 (Egr-1) and transcription factor
c-fos (5,6).
Type 1 diabetes mellitus, which is characterized by
a lack of insulin production, is associated with decreased skeletal
mass (7–9). Studies have shown that insulin is
important in the regulation of bone metabolism (10,11).
Insulin is capable of promoting osteoblast proliferation and
differentiation, collagen synthesis and alkaline phosphatase
production (12–14). Insulin deficiency accelerates bone
loss and is potentially the main cause of osteoporosis in patients
with type 1 diabetes mellitus (15). Following the binding of insulin to
its receptor, which is present on osteoblasts (10,11,13),
downstream signaling cascades, including the phosphatidylinositol
3-kinase (PI3K) and MAPK pathways, are activated (16). It has been shown that insulin
stimulates osteoblast proliferation and differentiation through the
ERK and PI3K pathways (13), which
are also involved in osteoblast response to mechanical stimulation
(5,17). Although insulin and mechanical
forces activate similar signaling pathways in osteoblasts, whether
there is convergence of these activated signaling pathways has yet
to be elucidated.
Integrins, the main receptors that connect the
cytoskeleton and extracellular matrix (ECM), have been shown to
have key roles in linking ECM components with various intracellular
signaling mechanisms (18),
including the transmission of mechanical stresses into chemical
signals in a wide variety of cells seeded on the ECM (19). Human osteoblasts express several
types of integrins. Integrins mediate the expression of bone
formation-associated genes in osteoblasts in response to mechanical
stimulation via the ERK, JNK and p38 MAPK pathways (5). A previous study demonstrated the
crucial role of integrin β1 signaling events in mediating
cross-talk of insulin action (20). Although integrins have been
recognized as mechanosensors in osteoblasts in response to
mechanical stimuli and regulators of insulin signaling, whether
insulin regulates the mechanical responsiveness of signaling and
gene expression in osteoblasts through integrins has yet to be
elucidated.
The aim of the present study was to investigate the
role of insulin in regulating the response of osteoblasts to
mechanical stimulation by examining changes in the activation of
the ERK pathway and the expression of Cox-2.
Materials and methods
Materials
MG63 cells were obtained from the American Type
Culture Collection (Manassas, VA, USA). Fetal bovine serum (FBS)
was obtained from HyClone (Logan, UT, USA) and Dulbecco's modified
Eagle medium (DMEM) was purchased from Gibco-BRL (Grand Island, NY,
USA). Mouse monoclonal antibodies against ERK2 (sc-1647) and
phospho-ERK1/2 (sc-7383), and the ERK inhibitor, PD98059, were
obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Insulin and echistatin were purchased from Sigma (St. Louis, MO,
USA). A First Strand cDNA Synthesis kit was obtained from Fermentas
(Fermentas UAB, Vilnius, Lithuania) and a LightCycler®
480 SYBR Green I Master was purchased from Roche Applied Science
(Indianapolis, IN, USA). All additional chemicals of reagent grade
were obtained from Sigma unless otherwise noted. The study protocol
conforms to the ethical guidelines of the World Medical
Association, Declaration of Helsinki. The experimental protocols
were approved by the Ethics Committee of Xiangya Hospital.
Cell culture
Cells were cultured in DMEM supplemented with 10%
FBS and 1% penicillin/streptomycin at 37°C in a humidified
atmosphere of 95% air and 5% CO2. Upon reaching
confluence, cells were trypsinized and seeded into the
force-loading plate at a density of 1×104
cells/cm2. The force-loading plates were made using the
method described previously (3).
The cells were incubated in DMEM supplemented with 10% FBS
(pretreated with charcoal to remove endogenous insulin in the
serum) and 1% penicillin/streptomycin for 48 h. The medium was then
changed to FBS-free medium for 24 h to equalize cell growth prior
to the experiments.
Experimental groups
The cells were divided into three groups. Group 1:
the cells were maintained as a control or pretreated with varied
doses of insulin (0, 10 and 100 nm) for 4 h, then exposed to
tensile stress for 10 min. Group 2A: the cells were maintained as a
control or pretreated with varied doses of insulin (0, 10 and 100
nm) for 4 h, then exposed to tensile stress for 1 h. Group 2B: the
cells were pretreated with DMSO or PD98059 for 1 h, stimulated with
insulin (10 nm) for 4 h, then followed by exposure to tensile
stress for 10 min. Group 3: the cells were maintained as a control
or pretreated with echistatin for 24 h, stimulated with insulin (10
nm) for 4 h, then exposed to tensile stress for 10 min or 1 h.
Mechanical stress application
The plates were subjected to cyclic uniaxial tensile
strain by the four-point bending system at a frequency of 0.5 Hz,
and cells were loaded with tensile stress at 2,000 μ strain, using
a method that has been previously characterized and described in
detail (3). In the group without
the mechanical stimulus, cells were seeded into similar plates and
incubated in the same incubator without mechanical stress loading.
For certain experiments, MG63 cells were incubated with 30 μM
PD98059 (the specific inhibitor for ERK) for 1 h and 50 nM
echistatin (integrin antagonist) for 24 h prior to stimulation with
insulin or exposure to mechanical stress. The treatments were
repeated three times.
RNA isolation and quantitative polymerase
chain reaction (qPCR)
Following the harvesting of the cells, total RNA was
extracted using the guanidium isothiocyanate/phenochloroform
method. The samples were lysed in 1 ml TRI reagent. The homogenate
was stored at room temperature for 5 min to complete the
dissociation of nucleoprotein complexes, at which point 0.2 ml
chloroform was added to the homogenate, followed by centrifugation
at 12,000 × g for 15 min. After centrifugation, RNA was
precipitated from the upper aqueous phase by adding 0.5 ml
isopropanol to the tubes and then centrifuging at 12,000 × g for 10
min. After this centrifugation step, the RNA pellet was washed with
75% ethanol and centrifuged at 7,000 × g for 5 min. The RNA pellet
was air dried and dissolved in 75 μl H2O at 60°C for 15
min. Total RNA was quantified using spectrophotometric analysis of
the absorbance at 260 nm (ultraviolet spectrophotometer UV-752;
Shanghai optical instrument factory, Shanghai, China). RNA was
reverse-transcribed using the First Strand cDNA Synthesis kit
(Fermentas) in accordance with the manufacturer's instruction. cDNA
was amplified by qPCR using the LightCycler instrument (Roche
Applied Science). Sequences of all PCR primers are shown in
Table I. Data were analyzed using
the 2−ΔΔCT method by Livak and Schmittgen (21), using the housekeeping gene β-actin
to calculate the ΔCT. The control was used at each time point to
calculate the −ΔΔCT.
 | Table IPrimer sequences used for qPCR. |
Table I
Primer sequences used for qPCR.
| Gene name | Primer sequence | Size (bp) | Annealing Temperature
(°C) |
|---|
| Cox-2 |
TCACGCATCAGTTTTTCAAGATC
ACCGTAAATATGATTTAAGTCCAC | 94 | 60 |
| β-actin |
AAATCGTCCGTGACATCAAG
GGAAGGAAGGCTGG AAGA GA | 180 | 60 |
Western blot analysis
Following mechanical stress loading, the treated
cells and corresponding controls were immediately washed twice with
ice-cold phosphate-buffered saline (PBS) and lysed with buffer
containing 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS and a
protease inhibitor mixture (phenylmethylsulfonyl fluoride,
aprotinin and sodium orthovanadate). Protein concentration was
measured with the bicinchoninic acid (BCA) protein assay. Briefly,
2 μg protein in 500 μl was prepared, then 500 μl working solution
was added to each 500 μl sample and mixed. The samples were
incubated for 60 min at 60°C, cooled and analyzed at 562 nm.
Protein extracts (10 μg) were separated on 10% SDS-polyacrylamide
gels and transblotted to polyvinylidene difluoride membranes for
western blot analysis. The target bands were determined according
to the molecular weight of the target proteins [phosphorylated ERK1
(p-ERK1), p-ERK2 and ERK2, weighing 44, 42 and 42 kDa,
respectively]. The band intensity ratio of p-ERK1/2 and ERK2
(p-ERK/ERK) was analyzed to assess ERK1/2 activation (normalized to
β-actin).
Statistical analyses
Results are shown as the mean ± standard deviation.
Statistical analysis was performed using an independent Student's
t-test for two groups of data and analysis of variance followed by
Scheffe's test for multiple comparisons. P<0.05 was considered
to indicate a statistically significant difference.
Results
Insulin augments tensile stress-induced
ERK phosphorylation in a dose-dependent manner in MG63 cells
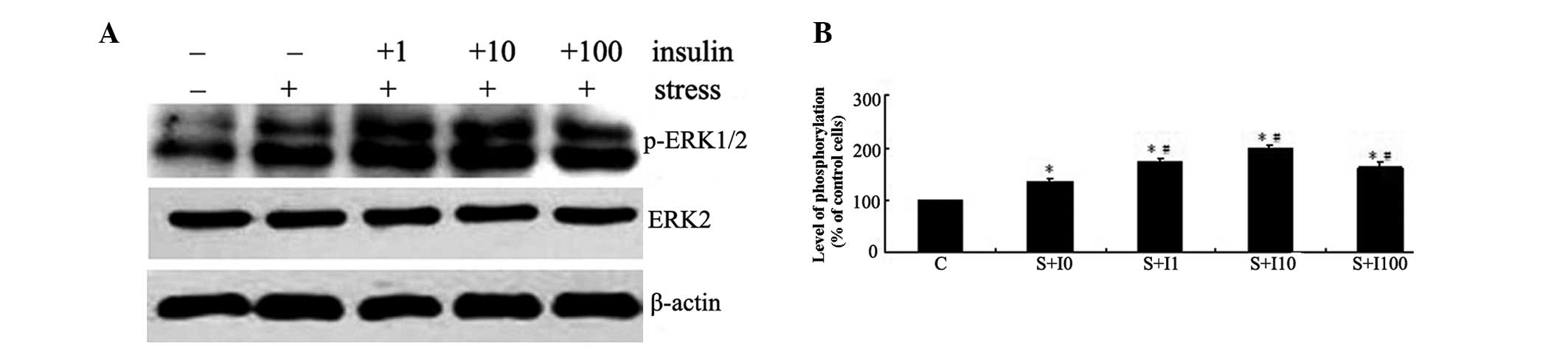
The initial experiment examined the effects of
insulin on mechanical strain-induced ERK phosphorylation. The
phosphorylation of ERK was determined by western blot analysis
(Fig. 1A). MG63 cells were
pretreated with varied doses of insulin (0, 1, 10 and 100 nM) for 4
h and then exposed to tensile stress for 10 min. The activation of
ERK in these cells was compared with that in the cells not
pretreated with insulin or exposed to tensile stress. As shown in
Fig. 1, mechanical strain resulted
in increased ERK phosphorylation following tensile stress
stimulation in MG63 cells (P<0.05). Pretreatment of MG63 cells
with insulin prior to exposure to tensile stress resulted in a
dose-dependent increase in ERK phosphorylation in these cells
compared with the cells exposed to tensile stress alone
(P<0.05). The highest levels of ERK phosphorylation were
observed in the group pretreated with 10 nM insulin (P<0.05;
Fig. 1B).
Insulin augments tensile stress-induced
Cox-2 expression levels via the ERK pathway in MG63 cells
To investigate the enhancing effect of insulin on
mechanical strain-induced Cox-2 expression levels, MG63 cells were
treated with varied doses of insulin (0, 1, 10 and 100 nM) for 4 h
and then exposed to tensile stress for 1 h. Cox-2 mRNA expression
levels in these cells was measured using qPCR and compared with
those in the cells not pretreated with insulin or exposed to
tensile stress. Mechanical strain resulted in increased Cox-2 mRNA
expression levels in MG63 cells. Insulin augmented mechanical
strain-induced Cox-2 mRNA expression in a dose-dependent manner and
the most notable effect was observed in the group pretreated with
10 nM insulin (P<0.05; Fig.
2A). Pretreatment of MG63 cells with PD98059 inhibited the
insulin-augmented mechanical strain-induced Cox-2 expression
levels, suggesting that the effect of insulin on tensile
stress-induced Cox-2 expression levels in MG63 cells was mediated
by the ERK pathway (P<0.05; Fig.
2B).
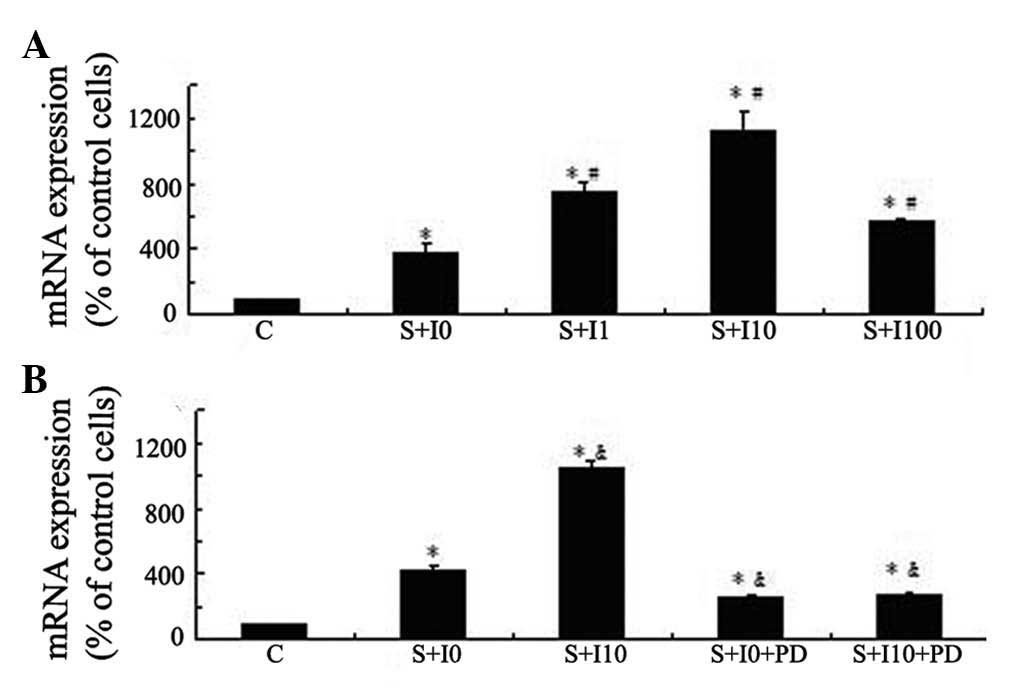 | Figure 2Insulin augments tensile
stress-induced Cox-2 expression levels via the ERK pathway in MG63
cells. (A) MG63 cells were kept as controls or pretreated with
varied doses of insulin (0, 1, 10 and 100 nM) for 4 h and then
exposed to tensile stress for 1 h. (B) MG63 cells were pretreated
with vehicle control DMSO or PD98059 (30 μM) for 1 h then
stimulated with insulin (10 nM) for 4 h, followed by exposure to
tensile stress for 1 h. Cox-2 expression levels were determined
using qPCR. The results are expressed as the mean ± standard
deviation. *P<0.05 vs. unstimulated control cells;
#P<0.05 vs. stressed cells without insulin
pretreatment; &P<0.05 vs. stressed cells without
insulin and PD pretreatment. Cox-2, cyclooxygenase-2; ERK,
extracellular signal-regulated kinase; DMSO, dimethylsulfoxide;
qPCR, quantitative polymerase chain reaction; C, control; I,
insulin; S, tensile stress; PD, PD98059. |
Insulin augmentation of tensile
stress-induced ERK phosphorylation and Cox-2 expression levels in
MG63 cells is mediated by integrins
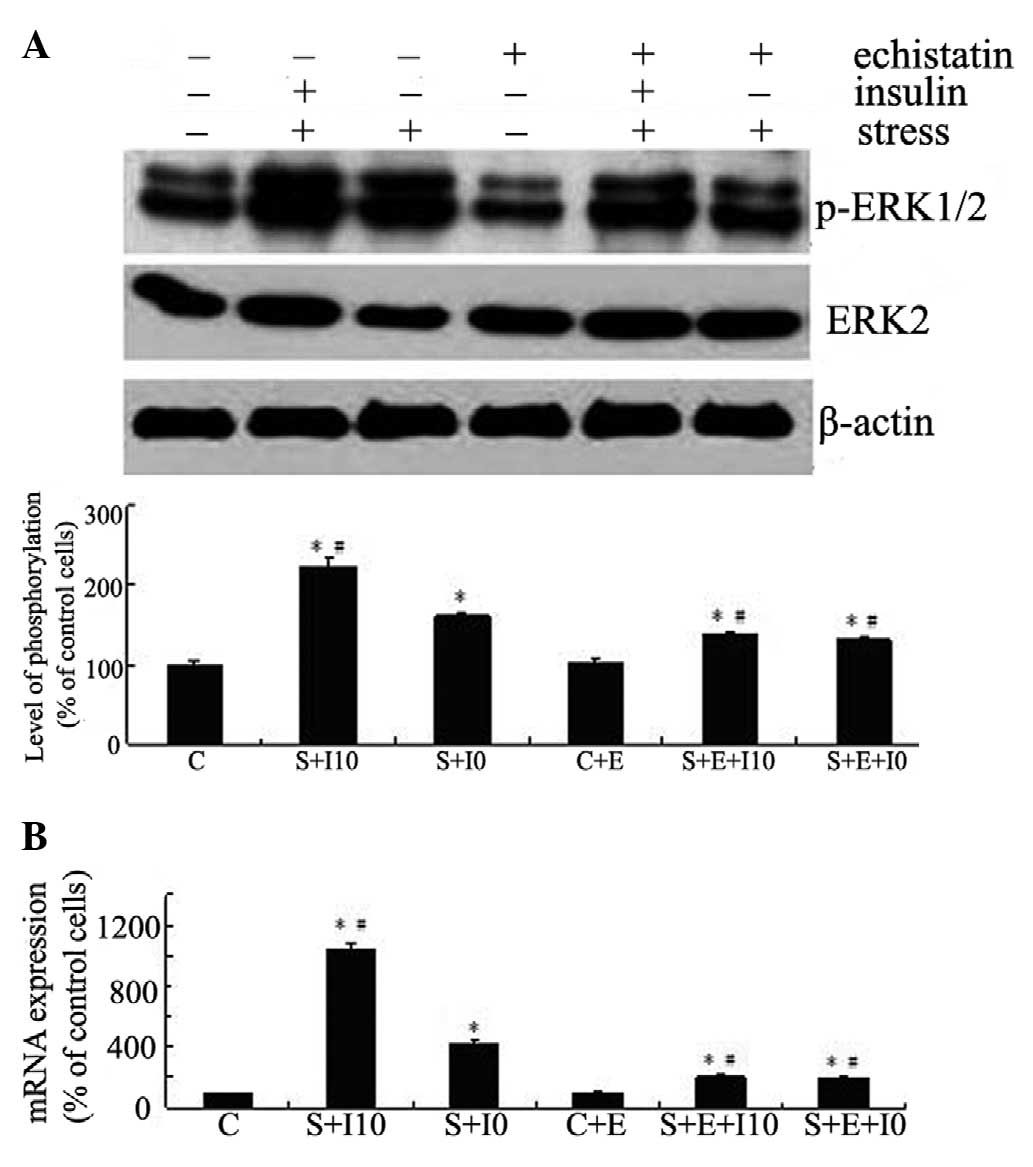
MG63 cells were kept as controls or pretreated with
echistatin for 24 h, stimulated with insulin for 4 h, and exposed
to tensile stress for 10 min (for ERK) or 1 h (for Cox-2). The ERK
phosphorylation and Cox-2 expression levels were examined by
western blotting and qPCR, respectively. As shown in Fig. 3, suppression of integrin function
inhibited not only the tensile stress-induced ERK phosphorylation
(Fig. 3A) and Cox-2 expression
(Fig. 3B), but also the effects of
insulin on the tensile stress-induced activation of this signaling
pathway and expression of this gene (P<0.05).
Discussion
Osteoblasts may exhibit distinct responses to
mechanical loading under various in vivo conditions, as
defective cellular responses to mechanical loading have been
observed in patients with musculoskeletal diseases, including
disuse osteoporosis, senile osteoporosis and osteoarthritis
(22,23). The concentration of insulin is much
lower in patients with type 1 diabetes mellitus than in healthy
individuals, which may impact the skeletal response to mechanical
loading in these patients. Therefore, this investigation explored
the potential effects of insulin on the response of osteoblasts to
mechanical stimulation by examining changes in the activation of
the ERK pathway and the expression of Cox-2.
ERK is regarded as a key point of upstream signal
transduction pathway in the cellular response to extracellular
signals (24), including
mechanical signals (1,5). ERK is rapidly activated by mechanical
stimuli (5). It is involved in the
increased expression of bone formation-associated genes (Egr-1,
c-fos and Cox-2) in osteoblasts induced by mechanical stimulation
(5), and in osteoblast
proliferation and differentiation induced by insulin (13). In the present study we demonstrated
for the first time, to the best of our knowledge, that insulin
augments tensile stress-induced ERK phosphorylation in a
dose-dependent manner in MG63 cells. The results indicated that
insulin upregulates the mechanosensitivity of osteoblasts via the
ERK pathway, which suggests that the mechanical sensitivity of bone
may be reduced in patients with type 1 diabetes mellitus.
Variations in insulin concentration may also affect the
mechanosensitivity of osteoblasts.
Cox-2 is a rate-limiting enzyme in the regulation of
prostaglandin (PG) synthesis in bone with Cox-1. This enzyme
appears to be important for the osteogenic response of bone to
exogenous mechanical loading. Cox-2 is immediately upregulated in
response to mechanical stimulation in osteoblasts (25), and has been shown to be important
in bone formation in vivo(26). Inhibition of Cox-2 expression
significantly decreased bone formation rates induced by mechanical
stimulation in rats (26). Through
a series of systematic studies, we demonstrated for the first time
that insulin augments tensile stress-induced Cox-2 expression
levels in a dose-dependent manner in MG63 cells. Furthermore, the
increases in Cox-2 expression levels were inhibited by blockade of
the ERK pathway. These results indicate that insulin modulates the
tensile stress-induced Cox-2 expression levels in osteoblasts
through the ERK pathway, which suggests that insulin may affect the
signaling and function of bone cells in response to mechanical
forces and, consequently, affect the mechanoresponsiveness of bone
in the process of bone formation. Insulin deficiency may decrease
Cox-2 expression levels in patients with type 1 diabetes mellitus
and subsequently decrease bone formation.
A previous study demonstrated that adhesive ability
and integrin-mediated signaling activation were lower in
osteoblasts derived from patients with osteoporosis than those from
healthy individuals (27), which
indicates that integrins may be involved in the development of
osteoporosis in patients with type 1 diabetes mellitus. Previous
studies have also suggested an important role of integrins in
regulating insulin signaling (28). For example, engagement of the β1
subunit containing integrin receptors was observed to increase
insulin-stimulated insulin receptor substrate (IRS) phosphorylation
and activate downstream signaling cascades, such as IRS-associated
PI3K and protein kinase B/Akt (28). In addition, previous studies have
shown that insulin and insulin-like growth factor (IGF)-I are
capable of binding to each other's receptors, and both receptors
phosphorylate IRS proteins on the same tyrosine residues to recruit
and activate downstream signaling cascades, such as PI3K and MAPK
pathways (10,11,16,29).
Furthermore, IGF-I regulates the mechanical responsiveness of
signaling and proliferation in osteoblasts via integrins (30). Consequently, it was hypothesized
that insulin and IGF-I may share a common signaling pathway when
they act on cells, and integrins may be partly involved in insulin
regulation of mechanical responsiveness in osteoblasts. The results
of the present study showed that suppression of integrin functions
inhibited the enhancing effects of insulin on mechanical
strain-induced ERK phosphorylation and Cox-2 expression. These
results suggested that integrins are important in the
insulin-modulated responsiveness of signaling and gene expression
in osteoblasts in response to mechanical stimulation, which
consequently may influence the formation and remodeling of
bone.
In conclusion, this study demonstrated for the first
time, to the best of our knowledge, that insulin upregulates
mechanical strain-induced ERK signaling and Cox-2 expression in
MG63 cells. The effect of insulin on signaling and gene expression
is mediated by integrins. These findings show that insulin may
influence the mechanical response of osteoblasts and provide
mechanistic insights into the cross-talk between the integrin and
insulin signaling pathways. In addition, the results of this study
may aid the future development of pharmacological therapies for
type 1 diabetes mellitus. Further validation of these results
requires studies focusing on normal, primary osteoblasts.
References
|
1
|
Thompson WR, Rubin CT and Rubin J:
Mechanical regulation of signaling pathways in bone. Gene.
503:179–193. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Qi MC, Zou SJ, Han LC, Zhou HX and Hu J:
Expression of bone-related genes in bone marrow MSCs after cyclic
mechanical strain: implications for distraction osteogenesis. Int J
Oral Sci. 1:143–150. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang X, Gong P, Lin Y, et al: Cyclic
tensile stretch modulates osteogenic differentiation of
adipose-derived stem cells via the BMP-2 pathway. Arch Med Sci.
6:152–159. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yan YX, Gong YW, Guo Y, et al: Mechanical
strain regulates osteoblast proliferation through integrin-mediated
ERK activation. PLoS One. 7:e357092012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee DY, Yeh CR, Chang SF, et al:
Integrin-mediated expression of bone formation-related genes in
osteoblast-like cells in response to fluid shear stress: roles of
extracellular matrix, Shc, and mitogen-activated protein kinase. J
Bone Miner Res. 23:1140–1149. 2008. View Article : Google Scholar
|
|
6
|
Yeh CR, Chiu JJ, Lee CI, et al: Estrogen
augments shear stress-induced signaling and gene expression in
osteoblast-like cells via estrogen receptor-mediated expression of
beta1-integrin. J Bone Miner Res. 25:627–639. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Soto N, Pruzzo R, Eyzaguirre F, et al:
Bone mass and sex steroids in postmenarcheal adolescents and adult
women with Type 1 diabetes mellitus. J Diabetes Complications.
25:19–24. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pater A and Odrowąż-Sypniewska G:
Alterations of bone metabolism in children and adolescents with
diabetes mellitus type 1. Pediatr Endocrinol Diabetes Metab.
17:158–161. 2011.(In Polish).
|
|
9
|
Saha MT, Sievänen H, Salo MK, Tulokas S
and Saha HH: Bone mass and structure in adolescents with type 1
diabetes compared to healthy peers. Osteoporos Int. 20:1401–1406.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fulzele K, Riddle RC, DiGirolamo DJ, et
al: Insulin receptor signaling in osteoblasts regulates postnatal
bone acquisition and body composition. Cell. 142:309–319. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ferron M, Wei J, Yoshizawa T, et al:
Insulin signaling in osteoblasts integrates bone remodeling and
energy metabolism. Cell. 142:296–308. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hie M, Iitsuka N, Otsuka T and Tsukamoto
I: Insulin-dependent diabetes mellitus decreases osteoblastogenesis
associated with the inhibition of Wnt signaling through increased
expression of Sost and Dkk1 and inhibition of Akt activation. Int J
Mol Med. 28:455–462. 2011.
|
|
13
|
Yang J, Zhang X, Wang W and Liu J: Insulin
stimulates osteoblast proliferation and differentiation through ERK
and PI3K in MG-63 cells. Cell Biochem Funct. 28:334–341. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Capilla E, Teles-García A, Acerete L,
Navarro I and Gutiérrez J: Insulin and IGF-I effects on the
proliferation of an osteoblast primary culture from sea bream
(Sparus aurata). Gen Comp Endocrinol. 172:107–114. 2011.
View Article : Google Scholar
|
|
15
|
Valkusz Z: Diabetes and osteoporosis. Orv
Hetil. 152:1161–1166. 2011.(In Hungarian).
|
|
16
|
Taniguchi CM, Emanuelli B and Kahn CR:
Critical nodes in signaling pathways: insights into insulin action.
Nat Rev Mol Cell Biol. 7:85–96. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee DY, Li YS, Chang SF, et al:
Oscillatory flow-induced proliferation of osteoblast-like cells is
mediated by alphavbeta3 and beta1 integrins through synergistic
interactions of focal adhesion kinase and Shc with
phosphatidylinositol 3-kinase and the Akt/mTOR/p70S6K pathway. J
Biol Chem. 285:30–42. 2010. View Article : Google Scholar
|
|
18
|
Li J, Zhao Z, Wang J, et al: The role of
extracellular matrix, integrins, and cytoskeleton in
mechanotransduction of centrifugal loading. Mol Cell Biochem.
309:41–48. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liedert A, Kaspar D, Blakytny R, Claes L
and Ignatius A: Signal transduction pathways involved in
mechanotransduction in bone cells. Biochem Biophys Res Commun.
349:1–5. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zong H, Bastie CC, Xu J, et al: Insulin
resistance in striated muscle-specific integrin receptor
beta1-deficient mice. J Biol Chem. 284:4679–4688. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
|
|
22
|
Mulvihill BM and Prendergast PJ:
Mechanobiological regulation of the remodelling cycle in trabecular
bone and possible biomechanical pathways for osteoporosis. Clin
Biomech (Bristol, Avon). 25:491–498. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Carter DR, Beaupré GS, Wong M, et al: The
mechanobiology of articular cartilage development and degeneration.
Clin Orthop Relat Res. 427(Suppl): S69–S77. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lai CF, Chaudhary L, Fausto A, et al: Erk
is essential for growth, differentiation, integrin expression, and
cell function in human osteoblastic cells. J Biol Chem.
276:14443–14450. 2001.PubMed/NCBI
|
|
25
|
Rumney RM, Sunters A, Reilly GC and
Gartland A: Application of multiple forms of mechanical loading to
human osteoblasts reveals increased ATP release in response to
fluid flow in 3D cultures and differential regulation of immediate
early genes. J Biomech. 45:549–554. 2012. View Article : Google Scholar
|
|
26
|
Forwood MR: Inducible cyclo-oxygenase
(COX-2) mediates the induction of bone formation by mechanical
loading in vivo. J Bone Miner Res. 11:1688–1693. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Perinpanayagam H, Zaharias R, Stanford C,
Brand R, Keller J and Schneider G: Early cell adhesion events
differ between osteoporotic and non-osteoporotic osteoblasts. J
Orthop Res. 19:993–1000. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guilherme A and Czech MP: Stimulation of
IRS-1-associated phosphatidylinositol 3-kinase and Akt/protein
kinase B but not glucose transport by beta1-integrin signaling in
rat adipocytes. J Biol Chem. 273:33119–33122. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bailyes EM, Navé BT, Soos MA, Orr SR,
Hayward AC and Siddle K: Insulin receptor/IGF-I receptor hybrids
are widely distributed in mammalian tissues: quantification of
individual receptor species by selective immunoprecipitation and
immunoblotting. Biochem J. 327:209–215. 1997.
|
|
30
|
Kapur S, Mohan S, Baylink DJ and Lau KH:
Fluid shear stress synergizes with insulin-like growth factor-I
(IGF-I) on osteoblast proliferation through integrin-dependent
activation of IGF-I mitogenic signaling pathway. J Biol Chem.
280:20163–20170. 2005. View Article : Google Scholar
|