Introduction
Pseudomyxoma peritonei (PMP) is a rare disease with
an incidence of ~2/10,000 (1). The
majority of PMP cases are reported to originate from the ovaries
and appendix. Females present more frequently with PMP and the male
to female ratio is 1:3.4 (2). The
patient usually presents with abdominal pain, bloating, abdominal
mass, progressive increase of abdominal circumference, weight loss
and fatigue; abdominal percussion dullness and signs of ascites are
not obvious. Ascites is not easily removed from the mucinous
liquid. This disease shows no marked specificity and misdiagnosis
is easy. The course of the disease is long and relapse is common.
The present study collected clinical data in The General Hospital
of PLA (Beijing, China) between 2002 and 2011. The preoperative
evaluation included thoracic and abdominal computed tomography (CT)
scans or ultrasound, as well as complete blood tests. Cytoreductive
surgery (CRS) and hyperthermic intraperitoneal chemoperfusion
(HIPEC) have been the common treatments for PMP since the 1980s. A
5-year survival rate of 86% was reported in a study by Sugarbaker
(3). The purpose of the present
study was to evaluate the effects of treatment and the prognostic
factors influencing the recurrence and overall survival (OS) times
for PMP.
Materials and methods
Patients
A total of 39 patients were admitted to The General
Hospital of PLA between 2002 and 2011. Retrospective analysis of
the clinical data of 39 cases of PMP included age, gender, cardinal
symptom, image analysis, treatment mode, pathological diagnosis,
tumor markers (carcinoembryonic antigen, CEA; alpha-fetoprotein,
AFP; cancer antigen 125, CA125) and postoperative adjuvant therapy.
The patients met the clinical and pathological diagnostic criteria.
The clinicopathological features of the 39 patients with PMP are
shown in Table I. Peritoneal
lesions were classified into three groups, consisting of
disseminated peritoneal adenomucinosis (DPAM), peritoneal mucinous
carcinomatosis (PMCA) and peritoneal mucinous carcinomatosis with
intermediate or discordant features (PMCA-I/D). The common clinical
symptoms and signs were abdominal bulge, abdominal distension,
abdominal pain and massive ascites. Four patients were admitted for
an abdominal mass. The patients underwent tumor marker
measurements. Abdominal ultrasound and CT scans revealed the
following: ascites; inward gathering of the intestinal canal;
thickening of the peritoneum; widely distributed fluid sonolucent
area in the peritoneal cavity, the surrounding of the liver and
stomach and the interspaces of the intestines; and thickening and
calcification of the peritoneum with widespread and multiple small
nodular foci. The informed consent was obtained from the patients
or patient’s family.
 | Table IClinicopathological features of 39
cases of pseudomyxoma peritonei. |
Table I
Clinicopathological features of 39
cases of pseudomyxoma peritonei.
| Number | Gender | Age (years) | Pathology type | Tumor marker | Treatment | Recurrence time
(months) | Survival time
(months) |
|---|
|
|---|
| CEA | CA125 | CA19-9 | CA724 |
|---|
| 1 | M | 61 | DPAM | H | H | H | H | HIPEC | 0 | 3+ |
| 2 | F | 69 | DPAM | H | H | H | - | IC | 32 | 37+ |
| 3 | F | 69 | DPAM | H | - | H | H | CS | 53 | 58+ |
| 4 | M | 61 | DPAM | H | H | H | H | PIC | 0 | 7+ |
| 5 | M | 58 | DPAM | - | - | - | - | CS | 0 | 8+ |
| 6 | M | 50 | DPAM | H | H | H | H | HIPEC | 1 | 16+ |
| 7 | F | 68 | DPAM | - | - | - | - | CS | 0 | 8+ |
| 8 | M | 65 | DPAM | - | - | - | - | HIPEC | 27 | 37 |
| 9 | F | 48 | DPAM | - | H | H | - | CS | 0 | 10+ |
| 10 | F | 65 | DPAM | - | - | - | - | CS | 0 | 11+ |
| 11 | F | 70 | PMCA | - | - | - | - | CS | 1 | 3+ |
| 12 | F | 74 | PMCA-I/D | H | H | - | H | CS | 0 | 2+ |
| 13 | F | 43 | DPAM | - | H | H | H | CS | 0 | 2+ |
| 14 | F | 67 | DPAM | - | - | - | - | CS | 55 | 18+ |
| 15 | F | 59 | DPAM | - | H | H | H | CS | 32 | 50+ |
| 16 | M | 62 | DPAM | - | - | - | - | CS | 48 | 13+ |
| 17 | F | 56 | DPAM | H | H | H | - | CS | 24 | 117 |
| 18 | F | 45 | DPAM | H | H | - | - | HIPEC | 0 | 30+ |
| 19 | M | 67 | DPAM | H | H | H | - | HIPEC | 15 | 32+ |
| 20 | F | 62 | DPAM | H | - | - | - | HIPEC | 0 | 31+ |
| 21 | F | 48 | PMCA-I/D | H | - | H | H | IC | 27 | 73+ |
| 22 | F | 70 | DPAM | - | - | - | - | CS | 6 | 25+ |
| 23 | F | 40 | DPAM | H | - | - | H | HIPEC | 0 | 25+ |
| 24 | F | 74 | DPAM | - | - | - | - | PIC | 4 | 27 |
| 25 | M | 45 | PMCA | H | H | H | H | IC | 4 | 55+ |
| 26 | F | 41 | DPAM | - | - | - | - | CS | 12 | 58+ |
| 27 | F | 76 | DPAM | - | - | - | - | CS | 1 | 144 |
| 28 | F | 34 | DPAM | - | - | - | - | CS | 10 | 58+ |
| 29 | F | 43 | DPAM | - | - | H | H | PIC | 0 | 60+ |
| 30 | M | 60 | DPAM | - | - | - | - | PIC | 0 | 50+ |
| 31 | F | 57 | DPAM | - | - | - | - | CS | 21 | 91+ |
| 32 | M | 43 | DPAM | - | - | - | - | CS | 6 | 105 |
| 33 | M | 47 | DPAM | - | - | - | - | PIC | 65 | 73+ |
| 34 | F | 80 | DPAM | - | - | - | - | CS | 36 | 75 |
| 35 | M | 67 | PMCA | - | - | - | - | CS | 4 | 90+ |
| 36 | F | 75 | DPAM | - | H | - | - | CS | 0 | 92 |
| 37 | M | 42 | DPAM | H | - | H | H | PIC | 24 | 93+ |
| 38 | M | 26 | DPAM | - | - | - | - | CS | 12 | 118+ |
| 39 | M | 70 | DPAM | H | - | - | - | PIC | 235 | 362 |
Treatment
Surgical resection in combination with perioperative
intraperitoneal chemotherapy (PIC) and HIPEC was the major
treatment approach for PMP. Intraoperative chemotherapy medication
included fluorouracil (5-FU), mitomycin and cisplatin, while HIPEC
comprised 5-FU, cisplatin and heating to 43ºC for 60 min. A total
of 39 patients with PMP were treated with CRS. HIPEC was performed
in seven patients (18%) at a temperature of 43ºC, with cisplatin
plus 5-FU. Twenty-two patients received PIC with cisplatin plus
5-FU.
Statistical analysis
OS and recurrence times were calculated from surgery
to the time of mortality or post-surgical disease recurrence.
Survival estimates were calculated using the Kaplan-Meier method.
The log-rank test was used to assess the significance of the
survival distribution. On the basis of univariate analysis,
significant variables were included in a Cox proportional hazard
model for multivariate analysis. All analyses were performed using
SPSS 13.0 statistical software (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Clinicopathological features
Among the 39 patients, 15 were male (38%) and 24
were female (62%). The median age was 61 years (range 26–80 years).
Thirty-four patients were diagnosed with DPAM (67%), three with
PMCA (8%) and two with PMCA-I/D (5%). The baseline marker levels
were elevated in 20 patients prior to the surgery (51%), and 15
patients with an elevated marker level demonstrated recurrence
following surgery (52%). All patients received surgery and 25 cases
recurred. Thirteen patients underwent further surgery. Seven
patients received HIPEC following surgery and three cases recurred.
The evaluation methods included physical examination, CT scan and
blood test.
Prognosis
With a mean follow-up of 40 months, the overall 5-
and 10-year survival rates were 89 and 35%, respectively. The
median OS and recurrence times were 37 and 4 months, respectively.
The recurrence-free survival rates were 56 and 23% at 1 and 3
years, respectively. Table II
shows a univariate model of the association between
clinicopathological factors and survival. The log-rank test
revealed that pathological type exerted an impact on recurrence
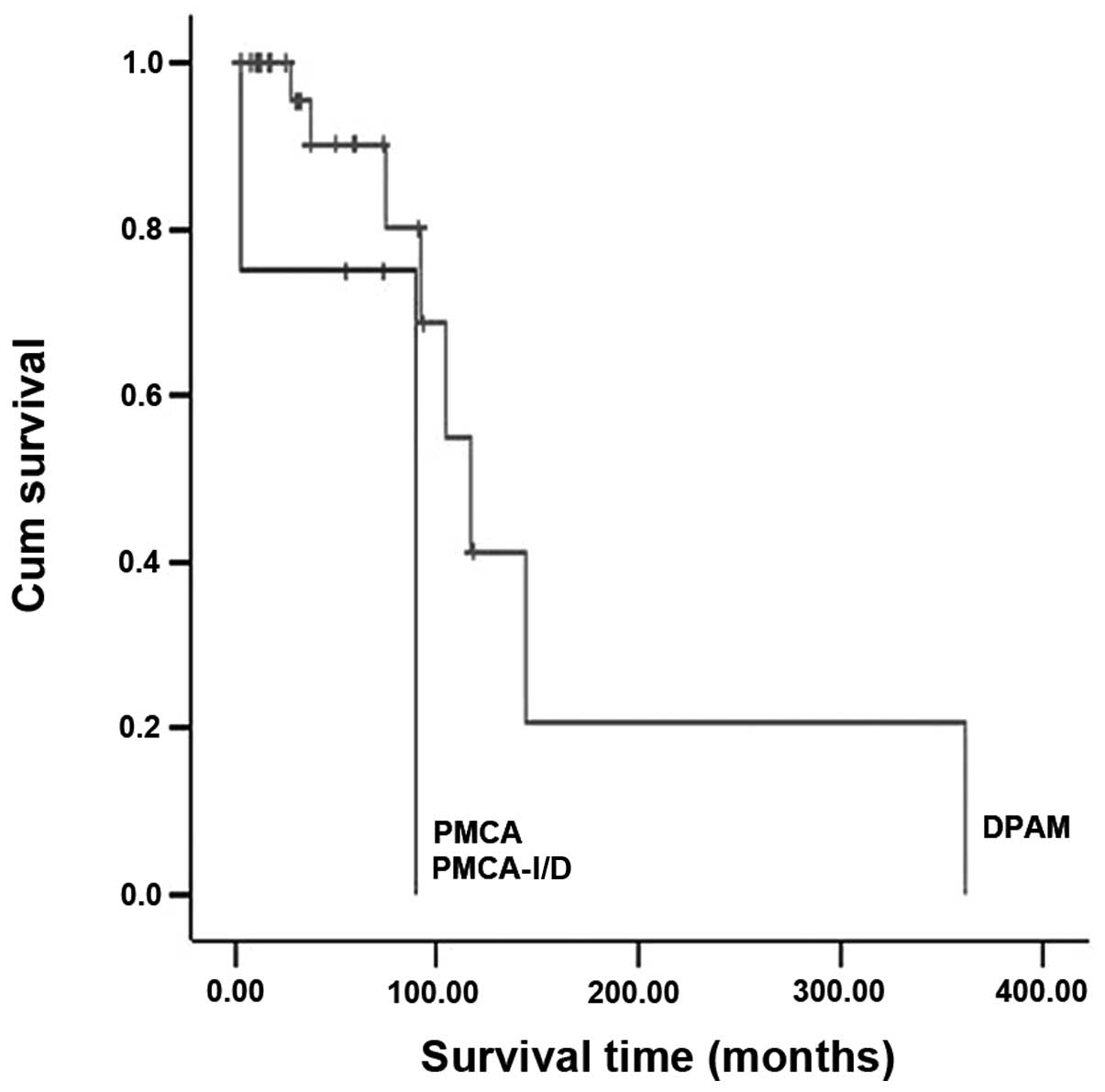
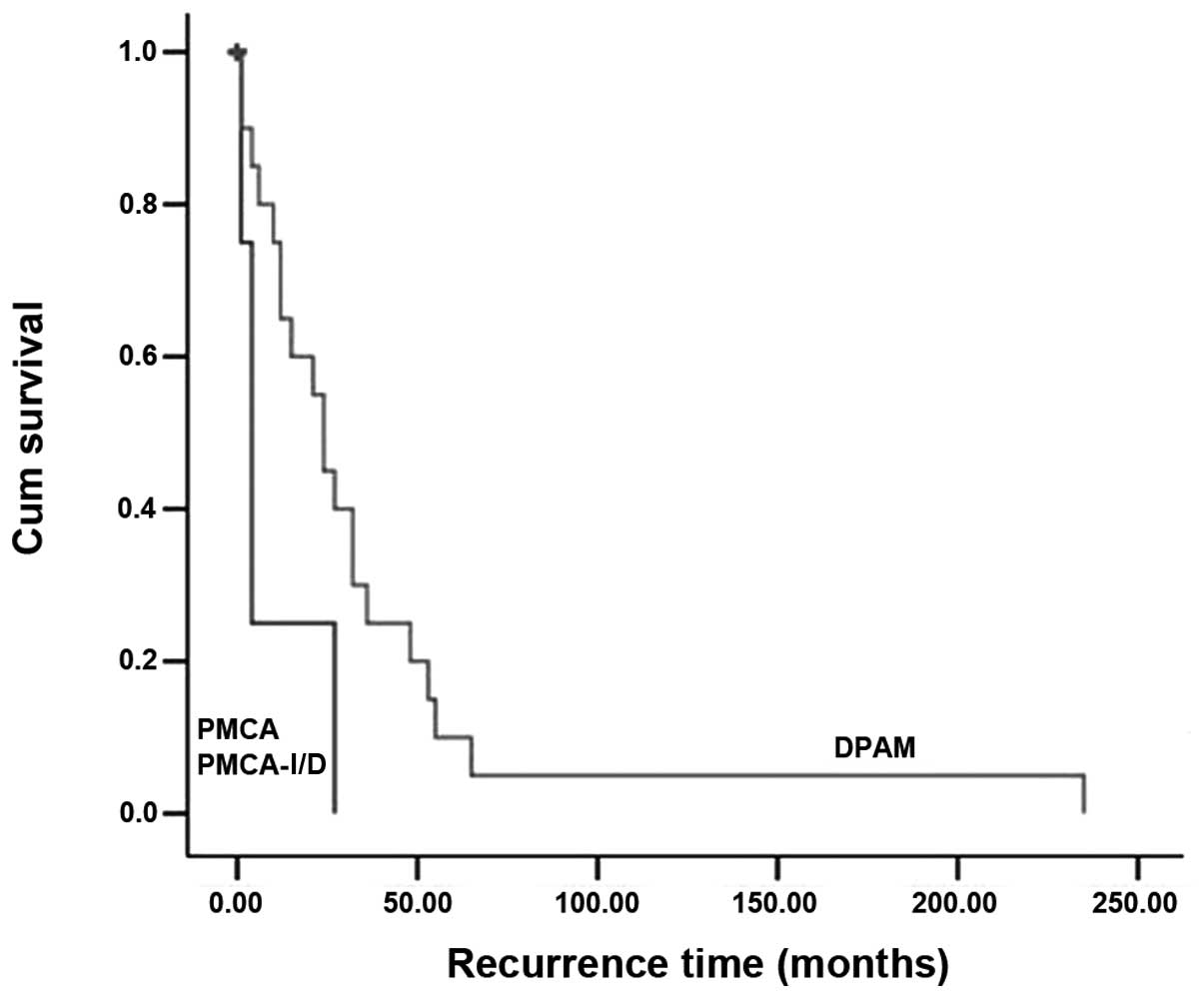
time (P=0.047; Fig. 1) and OS
(P=0.048; Fig. 2). The log-rank
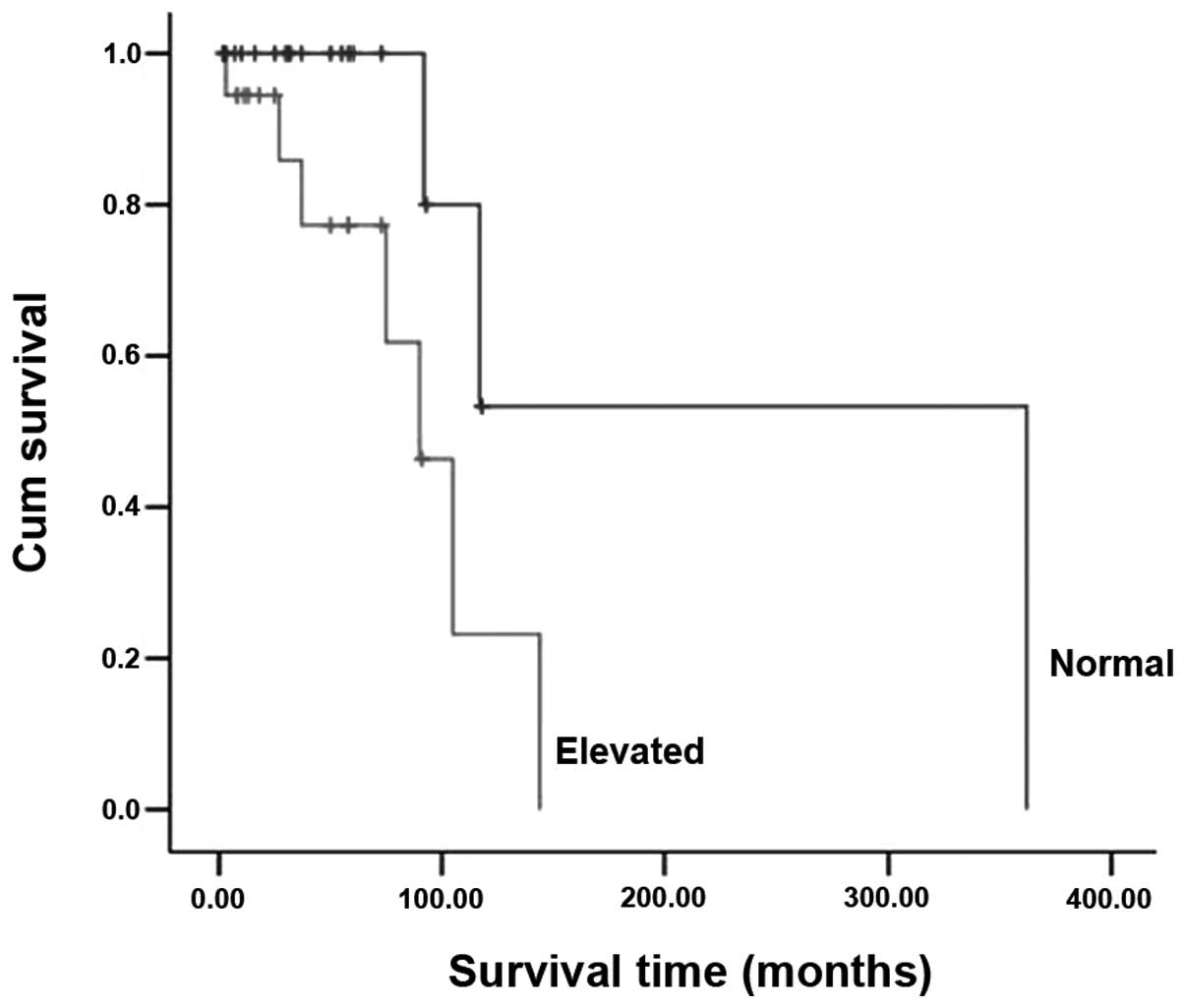
test also revealed preoperative levels of tumor markers (P=0.027;
Fig. 3) to be a prognostic factor
for survival time. Treatment did not improve patient prognosis,
unlike pathological type.
 | Table IIUnivariate analysis of
clinicopathological factors for survival. |
Table II
Univariate analysis of
clinicopathological factors for survival.
| | | P-value |
|---|
| | |
|
|---|
| Variable | Total (n) | Recurrence (n) | Recurrence-free
survival | Overall
survival |
|---|
| Gender | | | 0.656 | 0.325 |
| Male | 15 | 11 | | |
| Female | 24 | 14 | | |
| Age | | | 0.268 | 0.155 |
| ≤61 | 21 | 12 | | |
| >61 | 18 | 13 | | |
| Pathological
type | | | 0.047 | 0.048 |
| DPAM | 34 | 20 | | |
| PMCA/PMCA-I/D | 5 | 3 | | |
| Tumor maker
(preoperative) | | | 0.572 | 0.027 |
| Elevated | 20 | 15 | | |
| Normal | 19 | 10 | | |
| Type of
intraperitoneal chemotherapy | | | 0.287 | 0.296 |
| PIC | 32 | 21 | | |
| HIPEC | 7 | 3 | | |
The multivariate analysis identified pathological
type as an independent prognostic influence on survival (P=0.033;
Table III). The survival
characteristics were significantly different between the DPAM and
PMCA-I/D or PMCA groups. The prognosis was the most favorable for
DPAM. No significant difference in survival was observed with
regard to treatment method (PIC or HIPEC), gender, age and the
baseline level of tumor markers. Multivariate analysis of
recurrence-free survival identified two significant factors: the
pathological subtype (P=0.042) and the use of HIPEC (P=0.017;
Table IV).
 | Table IIIMultivariate analysis of prognostic
factors for overall survival. |
Table III
Multivariate analysis of prognostic
factors for overall survival.
| Variable | B | SE | Wald statistic | P-value | Exp (B) | 95.0% CI |
|---|
| Gender | 1.537 | 1.031 | 2.223 | 0.136 | 4.653 | 0.616–35.114 |
| Age | −0.367 | 1.004 | 0.134 | 0.715 | 0.693 | 0.097–4.955 |
| Pathology | 2.530 | 1.189 | 4.523 | 0.033 | 12.549 | 1.219–129.152 |
| Marker | −0.940 | 0.983 | 0.914 | 0.339 | 0.391 | 0.057–2.684 |
| Treatment
method | −9.954 | 491.896 | 0.000 | 0.984 | 0.000 | 0.000–0.000 |
 | Table IVMultivariate analysis of prognostic
factors for recurrence-free survival. |
Table IV
Multivariate analysis of prognostic
factors for recurrence-free survival.
| Variable | B | SE | Wald statistic | P-value | Exp (B) | 95.0% CI |
|---|
| Gender | 0.550 | 0.525 | 1.100 | 0.294 | 1.734 | 0.620–4.849 |
| Age | 0.597 | 0.479 | 1.556 | 0.212 | 1.817 | 0.711–4.640 |
| Pathology | 1.344 | 0.663 | 4.116 | 0.042 | 3.836 | 1.047–14.059 |
| Marker | −0.740 | 0.527 | 1.974 | 0.160 | 0.477 | 0.170–1.340 |
| Treatment
method | 2.452 | 1.029 | 5.675 | 0.017 | 11.610 | 1.544–87.276 |
Discussion
PMP is a low-grade malignant tumor. It is considered
to be a secondary disease that originates from the appendix or
ovaries (4,5). PMP generally exhibits no metastasis
and rarely affects neighboring organs. The likely pathogenesis
includes dissemination occurring by the rupture of appendicular
adenocarcinoma or ovarian tumors with the release of neoplastic
cells of mucus into the abdominal cavity. This is known as the
redistribution phenomenon (6). The
production of copious amounts of mucinous fluid results in a ‘jelly
belly’, where the mucinous fluid accumulates in the abdomen.
PMP is a slowly progressive disease, and the
pathological morphology is benign or of low-grade malignancy in the
majority of cases; however, its biological behavior is malignant,
and it is not able to be removed completely during surgery. Relapse
may occur easily. Postoperative recurrence occurs in 60–76% of
patients and the patients may have to undergo secondary surgical
resections. In this study, 25 cases of PMP recurred, of which 13
cases received the second debulking surgery. The median recurrence
time was four months. The recurrence-free survival rates were 56
and 23% at one and three years, respectively.
It has previously been reported that the 5- and
10-year survival rates were 50.0–81.0 and 18.2–32.0%, respectively
(7). In France, a large
multicentric retrospective study of 301 patients reported that the
5-year overall and disease-free survival rates were 73 and 56%,
respectively (8). Furthermore, a
total of 103 patients were treated at The Netherlands Cancer
Institute (Amsterdam, The Netherlands), and the 3- and 5-year
disease-free survival probabilities were revealed to be 43.6 and
37.4%, respectively (9). In the
present study, the median OS was 37 months. With a mean follow-up
of 40 months, the overall 5- and 10-year survival rates were 89 and
35%, respectively. Taking the small number of cases for the
analysis into account, the statistical results may have been
influenced. The 5-year survival rate was higher than that reported
in the aforementioned studies.
The elevated baseline levels of CEA, AFP and CA125
were useful for diagnosing this disease. Hsieh et
al(10) suggested that
preoperatively positive CEA and CA19.9 may normalize following
surgery. An increasing level of CEA and CA19–9 prognosticates early
recurrence. In a study by Carmignani et al(11), survival was associated with
preoperative CEA and CA19.9 determination. Among 532 patients, the
increasing CEA level was 58% and CA19.9 level was 67.1% higher than
the baseline level within one week. The elevated levels of CEA and
CA19.9 were not correlated with prognosis; however, poor prognosis
was associated with an elevated CEA level prior to the second
surgery. Short progression-free survival was associated with the
preoperative baseline CA19.9 level and favorable prognosis was
associated with a normal baseline level of CA125, as shown by
Baratti et al(12). The
present study revealed that preoperative elevated baseline levels
of tumor markers prolonged the survival time in univariate analyses
(P=0.027). Patients with normal baseline levels of tumor markers
appeared to have a more favorable survival result. The reported
prognostic factors of PMP are age (13), histology, residual tumor volume
(14) and intraperitoneal
chemotherapy (15). Ronnett et
a1(16) classified PMP into
three histological subtypes: DPAM, PMCA and PMCA-I/D. According to
the study by Ronnett et a1, the 5-year survival
postoperatively was 75% and the 10-year survival was 68% with DPAM;
this was compared with 14 and 3%, respectively, with PMCA and 50
and 21%, respectively, with PMCA-I/D. The data indicated that
histological type was a significant factor for treatment and
prognosis. The multivariate analysis in the present study also
demonstrated that histological type was able to act as an
independent prognostic factor (P=0.033). Complete CRS was able to
reduce the negative prognostic impact of the pathological grade, as
previously demonstrated by Elias et al(17). It appeared that for patients with
the DPAM type, treatment supplemented by complete CRS may prolong
long-term survival.
At present, it is generally accepted that aggressive
CRS and HIPEC is a novel option to treat PMP. Surgical
cytoreduction as a traditional treatment had a high recurrence
rate, as reported by Sugarbaker (18), with a 5-year survival rate of only
20%. Furthermore, in the majority of the patients with PMP,
recurrence occurred within 18 months. Repeated surgery was also
likely to have increased the severity of the disease (19). According to the synergistic effect
of thermochemotherapy and the different tolerances of tumors and
normal tissue to temperature, Spratt et al(20) designed a novel treatment technique
known as HIPEC. HIPEC was performed with a closed abdomen cavity
for 60 min, at a temperature of 42–43ºC, using perfusate mixed with
a cytotoxic drug (fluorouracil or cisplatin). The hyperthermia
potentiated tumor cells on the peritoneal surface to absorb high
doses of cytotoxic drugs and increased local tissue drug
concentration. The aim was to eliminate microscopic and minimal
residual disease remaining in the abdominal cavity following
surgical resection (21).
Sugarbaker (22) used the
combination of CRS with PIC to treat PMP. In a cohort of 205
patients, who were classified into two groups, the 5-year survival
rate was 86% for patients with HIPEC and 20% for the patients with
CRS only (23). Deraco et
al(24,25) reported a 96% survival rate at five
years in 22 patients with PMP treated with HIPEC, and recurrence
occurred in only one patient within a year. This demonstrated that
applying HIPEC to treat PMP, in order to reduce the postoperative
recurrence rate, improved quality of life and the survival rate. A
large multicentric retrospective study by Elias et
al(8) indicated that CRS
combined with HIPEC should be considered as the gold standard
treatment of PMP. The study underlined the prognostic impact of the
extent of peritoneal seeding, particularly in patients treated by
complete CRS. This prognostic impact appeared to be greater than
that of the pathological grade. In our study, hyperthermic
intraperitoneal chemotherapy did not have a more favorable OS rate
than intraoperative intraperitoneal chemotherapy or no
chemotherapy. In the present study, the recurrence time subsequent
to surgery was reported from the data of the CRS and HIPEC
treatment. It was suggested that this was a more accurate
representation of the impact of treatment on the course of the
disease. In a report from the Mayo Clinic on a subgroup analysis of
31 patients with incomplete surgical removal of mucus,
intra-abdominal chemotherapy significantly reduced the recurrence
(26). In the present study, HIPEC
with CRS was correlated with recurrence-free survival, as shown by
multivariate analysis (Table IV).
As long as PMP recurred, the treatment of surgical debulking was
repeated, as necessary, to alleviate pressure (26). However, repeated surgeries became
more difficult due to progressively thickened intra-abdominal
adhesions (27); therefore, a
longer recurrence time following initial surgery was necessary to
mitigate symptoms and improve the quality of life.
There were few clinical data to support how to
prolong postoperative recurrence time. In the present study,
stepwise multivariate Cox proportional-hazard regression analysis
indicated that pathological classification and HIPEC were
independent predictors of recurrence-free survival. However, due to
the small sample size and the retrospective nature of the study,
larger multicenter studies are required in the future.
In conclusion, the pathological subtype remained the
dominant factor for survival. The efficacy of CRS with HIPEC was
demonstrated in the treatment of PMP by prolonging the
postoperative recurrence time. Patients with normal baseline levels
of tumor markers appeared to have an improved survival result.
References
|
1
|
Wertheim I, Fleischhacker D, McLachlin CM,
Rice LW, Berkowitz RS and Goff BA: Pseudomyxoma Peritonei: a review
of 23 cases. Obstet Cynecol. 84:17–21. 1994.PubMed/NCBI
|
|
2
|
Guo AT, Song X, Wei LX and Zhao P:
Histological origin of pseudomyxoma peritonei in Chinese women:
clinicopathology and immunohistochemistry. World J Gastroenterol.
17:3531–3537. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sugarbaker PH: Cytoreductive surgery and
peri-operative intraperitoneal chemotherapy as a curative approach
to pseudomyxoma peritonei syndrome. Eur J Surg Oncol. 27:239–243.
2001. View Article : Google Scholar
|
|
4
|
Young RH, Gilks CB and Scully RE: Mucinous
tumors of the appendix associated with mucinous tumors of the ovary
and pseudomyxoma peritonei. A clinicopathological analysis of 22
cases supporting an origin in the appendix. Am J Surg Pathol.
15:415–429. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Szych C, Staebler A, Connolly DC, et al:
Molecular genetic evidence supporting the clonality and appendiceal
origin of Pseudomyxoma peritonei in women. Am J Pathol.
154:1849–1855. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou Z, Mo CC, Zhuang JY and Deng ZH:
Pseudomixoma peritonei associated with ovary and omentum majus: 1
case report. Journal of Guiyang Medical College. 28:186–192.
2003.(In Chinese).
|
|
7
|
Miner TJ, Shia J, Jaques DP, Klimstra DS,
Brennan MF and Coit DG: Long-term survival following treatment of
pseudomyxoma peritonei: an analysis of surgical therapy. Ann Surg.
241:300–308. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Elias D, Gilly F, Quenet F, Bereder JM, et
al: Pseudomyxoma peritonei: A French multicentric study of 301
patients treated with cytoreductive surgery and intraperitoneal
chemotherapy. Eur J Surg Oncol. 36:456–462. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Smeenk RM, Verwaal VJ, Antonini N and
Zoetmulder FA: Survival analysis of pseudomyxoma peritonei patients
treated by cytoreductive surgery and hyperthermic intraperitoneal
chemotherapy. Ann Surg. 245:104–109. 2007. View Article : Google Scholar
|
|
10
|
Hsieh SY, Chiu CT, Sheen IS, Lin DY and Wu
CS: A clinical study on pseudomyxoma peritonei. J Gastroenterol
Hepatol. 10:86–91. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Carmignani CP, Hampton R, Sugarbaker CE,
Chang D and Sugarbaker PH: Utility of CEA and CA19–9 tumor markers
in diagnosis and prognostic assessment of mucinous epithelial
cancers of the appendix. J Surg Oncol. 87:162–166. 2004.
|
|
12
|
Baratti D, Kusamura S, Martinetti A,
Seregni E, Laterza B, Oliva DG and Deraco M: Prognostic value of
circulating tumor markers in patients with pseudomyxoma peritonei
treated with cytoreductive surgery and hyperthermic intraperitoneal
chemotherapy. Ann Surg Oncol. 14:2300–2308. 2007. View Article : Google Scholar
|
|
13
|
Lee JK, Song SH, Kim I, Lee KH, Kim BG,
Kim JW, Kim YT, Park SY, Cha MS and Kang SB: Retrospective
multicenter study of a clinicopathologic analysis of pseudomixoma
peritonei associated with ovarian tumors (KGOG 3005). Int J Gynecol
Cancer. 18:916–920. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Miner TJ, Shia J, Jaques DP, et al:
Long-term survival following treatment of pseudomixoma peritonei:
an analysis of surgical therapy. Ann Surg. 241:300–308. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baratti D, Kusamura S, Nonaka D, et al:
Pseudomixoma Peritonei: clinical pathological and biological
prognostic factors in patients treated with cytoreductive surgery
and hyperthermic intraperitoneal chemotherapy (HIPEC). Ann Surg
Oncol. 15:526–534. 2008. View Article : Google Scholar
|
|
16
|
Ronnett BM, Yan H, Kurman RJ, Shmookler
BM, Wu L and Sugarbaker PH: Patients with pseudomyxoma peritonei
associated with disseminated peritoneal adenomucinosis have a
significantly more favorable prognosis than patients with
peritoneal mucinous carcinomatosis. Cancer. 92:85–91. 2001.
View Article : Google Scholar
|
|
17
|
Elias D, Honoré C, Ciuchendéa R, et al:
Peritoneal pseudomyxoma: results of a systematic policy of complete
cytoreductive surgery and hyperthermic intraperitoneal
chemotherapy. Br J Surg. 95:1164–1171. 2008. View Article : Google Scholar
|
|
18
|
Sugarbaker PH: Pseudomyxoma peritonei: a
cancer whose biology is characterized by a redistribution
phenomenon. Ann Surg. 219:109–111. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu B and Wu HP: Dignosis and treatment of
Pseudomyxoma peritonei: 5 cases report. Journal of Abdominal
Surgery. 10:228–229. 1997.(In Chinese).
|
|
20
|
Spratt JS, Adcock RA, Sherrill W and
Travathen S: Hyperthermic peritoneal perfusion system in canines.
Cancer Res. 40:253–255. 1980.PubMed/NCBI
|
|
21
|
Zhang SH and Liu ZL: Pseudomyxoma
peritonei: the treatment experience of 9 cases. Chinese Journal of
Clinical Oncology. 26:1–12. 1999.(In Chinese).
|
|
22
|
Sugarbaker PH: Pseudomyxoma peritonei. A
cancer whose biology is characterized by a redistribution
phenomenon. Ann Surg. 219:109–111. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sugarbaker PH: Cytoreductive surgery and
peri-operative intraperitoneal chemotherapy as a curative approach
to pseudomyxoma peritonei syndrome. Eur J surg Oncol. 27:239–243.
2001. View Article : Google Scholar
|
|
24
|
Deraco M, Kusamura S and Gronchi A:
Cytoreductive surgery (peritonectomy) and intraperitoneal
hyperthermic chemotherapy: an innovative and effective approach to
the treatment of pseudomyxoma peritonei. Tumori. 89:54–55. 2003.(In
Italian).
|
|
25
|
Deraco M, Gronchi A, Mazzaferro V, et al:
Feasibility of peritonectomy associated with intraperitoneal
hyperthermic perfusion in patients with pseudomyxoma peritonei.
Tumori. 88:370–375. 2002.PubMed/NCBI
|
|
26
|
Gough DB, Donohue JH, Schutt AJ, et al:
Pseudomyxoma peritonei. Long tern patient survival with an
aggressive regional approach. Ann Surg. 219:112–119. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yan TD, Black D, Savady R and Sugarbaker
PH: A systematic review of the efficacy of cytoreductive surgery
and perioperative intraperitoneal chemotherapy for pseudomyxoma
peritonei. Ann Surg Oncol. 14:484–492. 2007. View Article : Google Scholar : PubMed/NCBI
|