Introduction
Missed abortion (MA) is diagnosed when a pregnancy
ceases to develop, but there is a delay in the expulsion of the
products of conception. MA is a complication of early pregnancy
that occurs in ≤15% of all clinically recognized pregnancies
(1). Approximately 90% of all MAs
occur prior to 14 weeks gestation, or in the first trimester. The
etiology of MA is not fully understood. Although MA may occur due
to chromosomal anomalies, hormonal problems, uterine abnormalities,
infections and autoimmune disorders, in certain cases no cause is
identified. Pregnancy is something of a physiological miracle in
which an event that is normally forbidden, the propagation of
foreign tissue, is accommodated for a defined period of time by the
immune system. In order to achieve a successful pregnancy, the
maternal immune system must be immunologically tolerant of the
semi-allograft fetus. Incomplete tolerance may result in a
disturbed pregnancy (2). Evidence
suggests that CD4+CD25+ regulatory T (Treg)
cells participate in the development of maternal tolerance to the
fetus during pregnancy (3,4). CD4+CD25+ Treg
cells are considered be crucial in peripheral tolerance, transplant
tolerance and maternal tolerance to the fetus (5,6). The
expression of forkhead box protein 3 (FoxP3) is regarded as
characteristic of CD4+CD25+ Treg cells and
differentiates them from activated CD4+ T cells
(7). Normal pregnant patients
exhibit an expansion of CD4+CD25+ Treg cells
at the periphery compared with non-pregnant subjects. The
percentage of CD4+CD25+ Treg cells has been
observed to decrease to the levels of those in non-pregnant
patients in the case of miscarriage (8). It seems that immune factors, such as
decreased maternal tolerance, contribute to pathological pregnancy.
Due to these observations, it has been proposed that Treg cells are
crucial to maternal tolerance in humans.
The Th1 subset is defined by the specific production
of interferon-γ (IFN-γ) and interleukin-2 (IL-2), and by the
stimulation of cell-mediated immunity, whereas the Th2 subset
specifically produces IL-4 and IL-10 which stimulate humoral
immunity. Certain cytokines such as IL-4, IL-3 and IL-10 appear to
be favorable to the success of pregnancy, whereas cytokines such as
IL-2 and IFN-γ are reported to have deleterious effects (9). It has been proposed that a normal
pregnancy is associated with a maternal predisposition to Th2-type
immunity and that a preponderance to type 1 reactivity is
associated with pregnancy failure. Numerous studies of unexplained
recurrent spontaneous abortions (10), premature rupture of membranes and
preterm delivery in humans have revealed a close association of
these conditions with the increased production of certain type 1
cytokines by peripheral blood cells. The purpose of this study was
to investigate whether the Th1/Th2 balance was broken in patients
with MA.
Pregnancy induces substantial changes in hormone
levels, initially in hormones produced by the corpus luteum
and trophoblasts followed by complex alterations initiated by the
hypothalamic-pituitary-adrenal (HPA) axis. E2 is a form of estrogen
in the body derived almost entirely from the fetal-placental unit.
Thus, maternal blood or urinary E2 is a good indicator of the
health and well-being of the placenta and fetus. Estrogens have
powerful effects on immune cells and regulate their proliferation,
distribution and function (11).
However, estrogen suppresses the maternal immune response in a
manner that is poorly understood.
The pathogenesis of MA is complicated and multiple
factors are involved in the formation of a clear clinical picture.
We propose that the levels of E2 affect lymphocytes, such as Treg
cells, in addition to the Th1/Th2 imbalance, which may be
responsible for the pathogenic mechanism of development and
progression of MA. To date, to the best of our knowledge, there
have been no data regarding Treg cells and the effect of E2 on the
immune system in patients with MA.
Materials and methods
Patients
In total, 33 MA patients with a median age of
28.4±5.71 years (range, 21–44 years) were included in this study. A
first trimester MA was defined as ultrasound evidence of an intact
gestational sac, no evidence of fetal cardiac activity [6 weeks
from the last menstrual period (LMP)], a closed cervical os, and a
history of no or minimal bleeding (12). The study group is referred to in
the present study as the MA patient group. Patients with
chromosomal anomalies, uterine abnormalities, infections and
autoimmune disorders were not assigned to this group. The two
control groups: one included 33 normal pregnant women in the first
trimester and the other included 27 non-pregnant women. There were
no significant differences in the age and pregnancy duration
between the three groups (Table
I).
 | Table ICharacteristics of missed abortion
patients and control groups in the study. |
Table I
Characteristics of missed abortion
patients and control groups in the study.
| Groups | N | Maternal age
(years) | Gestational age
(days) |
|---|
| Missed abortion | 33 | 28.4±5.71 | 52.75±1.96 |
| Normal pregnancy | 33 | 28.5±5.10 | 52.35±1.63 |
| Non-pregnant
subjects | 27 | 27.0±5.67 | |
This study has the approval of the Ethics Committees
of the Maternity and Child Health Hospital (Zhenjiang, China).
Written consent was obtained from all subjects following a full
explanation of the procedure.
Blood sample preparation
Venous blood ~8ml, was obtained by venipuncture from
early MA (n=33) and healthy non-pregnant (n=27), and early-stage
pregnancy patients (n=33). Of the 8 ml, 6 ml was heparinized for
the isolation of peripheral blood mononuclear cells (PBMCs), while
the remaining 2 ml was used for the preparation of serum. PBMCs
were isolated for analysis by flow cytometry and quantitative
polymerase chain reaction (qPCR) using Ficoll-Hypaque (Lymphoprep™;
Nycomed Pharma, Oslo, Norway) density gradient centrifugation.
Centrifugation was performed at 840 × g for 20 min at 20°C. The
serum was separated from the specimens and stored at −70°C until
required for cytokine determination using an enzyme-linked
immunosorbent assay (ELISA) and a chemiluminescent immunoassay that
was used to examine the serum levels of E2.
Flow cytometry
To each tube, 100 μl prepared PBMCs
(1×106) were added, followed by 20 μl CD4/CD25
[fluorescein isothiocyanate/R-phycoerythrin (FITC/PE); eBioscience,
San Diego, CA, USA]. The mixture was incubated in the dark for 30
min at 4°C and subsequently washed in cold flow cytometry staining
buffer (BD Biosciences, San Jose, CA, USA). After decanting, the
cell pellet was resuspended in the residual buffer and 1 ml freshly
prepared fixation/permeabilization buffer (eBioscience), and
incubated for a further 30–60 min in the dark at 4°C. The cells
were washed with 2 ml permeabilization buffer followed by
centrifugation and decanting of the supernatant. The cells were
washed with 2 ml permeabilization buffer followed by centrifugation
and decanting of the supernatant. The cells were blocked by adding
2 μl normal rat serum in ~100 μl cell suspension, for 15 min.
Following the blocking step, without washing, 20 μl anti-human
FoxP3 [PE-cyanine-5 (cy5); eBioscience] antibody was added and the
cells were incubated at 4°C for at ≥30 min in the dark. The cells
were washed with 2 ml permeabilization buffer (Cytoperm/Cytofix;
Becton Dickinson, San Diego, CA, USA) followed by centrifugation
(500 × g for 5 min at room temperature) and decanting of the
supernatant; this was performed twice. Labeled cells were washed
and analyzed by flow cytometry (Calibrate; Becton Dickinson, Palo
Alto, CA, USA) using CellQuest software (Becton-Dickinson). In each
case, staining was compared with that of the appropriately labeled
isotype control antibody. The isotype control antibodies we used
were Rat IgG2a Isotype Control FITC, Mouse IgG1 Isotype Control PE
and Rat Kappa Isotype Contol PE-Cy5, respectively, and were
purchased from eBioscience.
Flow cytometry was performed with a BD LSK flow
cytometer (BD Biosciences). Data were collected and analyzed using
CellQuest Pro software. Matched isotype controls were used to set
quadrants and regions of positive staining. Cells were gated on
lymphocytes using forward and side light-scattering properties and
a minimum of 10,000 lymphocyte gated events were acquired.
CD4+ T cell lymphocytes were analyzed with bivariate dot
plots of CD25 vs. Foxp3. Treg cells are expressed as a percentage
of CD4+CD25+FoxP3+
lymphocytes.
RNA isolation and qPCR
Total RNA was extracted from individual PBMC
preparations using TRIzol reagent (Invitrogen, Carlsbad, CA, USA)
according to the manufacturer’s instructions. cDNA was prepared by
reverse transcription with oligo(dT) (High-Capacity cDNA Reverse
Transcription kit; Applied Biosystems, Foster city, CA, USA) from
the total RNA extract. cDNA synthesis was performed using the
High-Capacity cDNA Reverse Transcription kit (Applied Biosystems)
according to the manufacturer’s instructions. Real-time PCR was
performed in a 20 μl-system that contained 10 μl of 1X SsoFast
EvaGreen Supermix (Bio-Rad, Hercules, CA, USA), 2 μl of cDNA, 6 μl
of RNase/DNase-free water and 500 nM of each primer. The thermal
cycler conditions were as follows: 30 sec at 95°C, followed by 40
cycles of 5 sec at 95°C and 10 sec at 60°C. A melting curve
analysis was performed for each reaction with a 65–95°C ramp. The
threshold cycle at which the fluorescent signal reached an
arbitrarily set threshold near the middle of the log-linear phase
of the amplification for each reaction was calculated, and the
relative quantity of mRNA were determined. The mRNA levels were
normalized against the mRNA levels of the housekeeping gene,
β-actin. qPCR for FoxP3 and a reference gene (β-actin) was
performed in a Lightcycler Instrument (Roche Molecular Diagnostics,
Mannheim, Germany) with the SYBR-Green Mastermix kit (Takara,
Shiga, Japan). FoxP3 expression data was subsequently normalized
relative to β-actin. The primer sequences for qPCR are shown in
Table II.
 | Table IIPrimers for qRT-PCR. |
Table II
Primers for qRT-PCR.
| Gene | Forward primer | Reverse primer |
|---|
| β-actin |
5′-TTCTGTCAGTCCACTTCACCA-3′ |
5′-CCAGCAGGTCTGAGGCTTG-3′ |
| FoxP3 |
5′-TGAGAAGGACAGGGAGCCAA-3′ |
5′-GAGAAGCTGAGTGCCATGCA-3′ |
Cytokine measurement using ELISA
Serum IL-4 and IFN-γ concentrations were measured by
commercial ELISA according to the manufacturer’s instructions
(Bender MedSystems, Burlingame, CA, USA). All samples were analyzed
in duplicate.
E2 levels
E2 was quantified in the serum of subjects from MA
patients and control groups by chemiluminescence immunoassay
(Architect-i2000; Abbott Laboratories, Green Lakes, IL, USA). The
absorbance value was monitored at 450 nm on a microplate reader
(Safire 2, Tecan, Switzerland).
Statistical analysis
Statistical analysis was performed with GraphPad
Prism version 4.0 (GraphPad software Inc., San Diego, CA, USA).
Data are presented as the means ±SD. P<0.05 was considered to
indicate a statistically significant difference. As determined by
one-way analysis of variance (ANOVA) or the Student’s t-test.
Pearson’s correlation was used to analyze correlations between the
levels of estradiol, Treg cells and IL-4 in MA patients.
Results
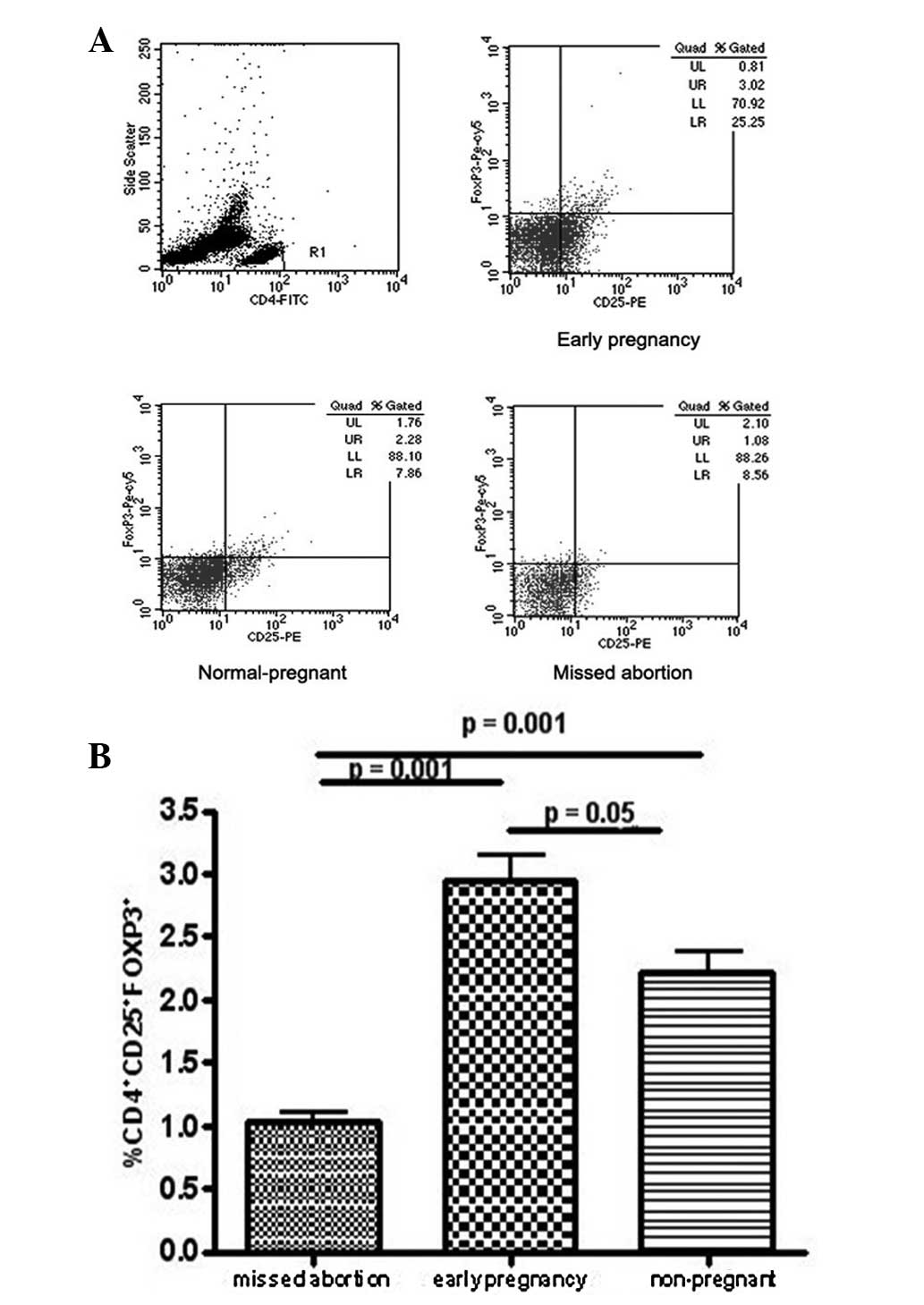
Levels of FoxP3-expressing
CD4+CD25+ T cells in MA patients as
determined by flow cytometry with intracellular staining
Immunostaining for FoxP3 was conducted in order to
detect CD4+CD25+ T cells, the most reliable
markers for CD4+CD25+ Treg cells. The flow
cytometry results demonstrated that the levels of FoxP3+
cells in the peripheral blood of MA patients were lower than those
in normal pregnancy and non-pregnant subjects, suggesting that Treg
cell levels were reduced in the peripheral blood during MA
(Fig. 1).
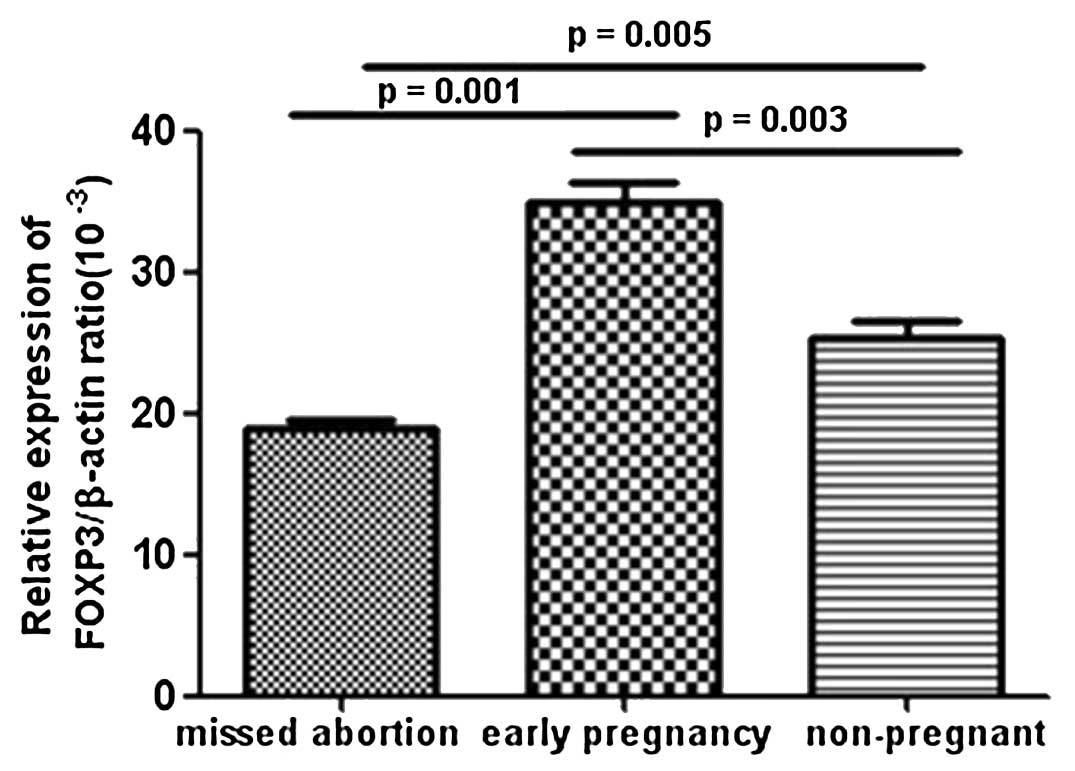
FoxP3 mRNA expression levels in the
peripheral blood of MA patients as determined by qPCR
In order to confirm the previous observations, the
levels of the transcription factor specific for T-cell subsets were
determined by qPCR. As shown in Fig.
2, decreased mRNA expression levels of the Treg cell-specific
transcription factor, FoxP3, were observed in patients with MA
compared with those in normal pregnancy and healthy non-pregnant
females. These results were consistent with the flow cytometric
analysis of Treg cells.
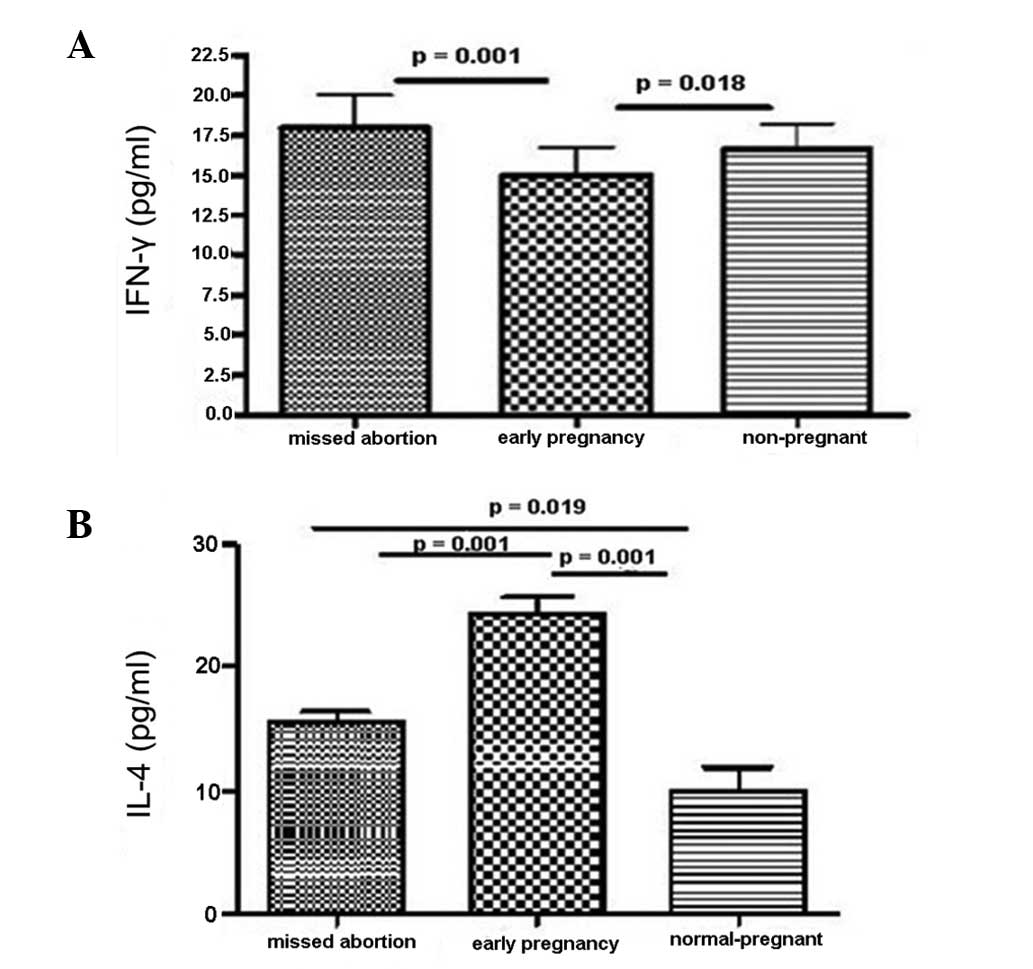
Serum cytokine concentrations as
determined by ELISA
The intracellular expressions of IL-4 and IFN-γ were
determined in the serum by ELISA as an indicator of cytokine
production. As shown in Fig. 3,
the IFN-γ expression levels in MA patients were higher compared
with those in the control groups (Fig.
3A). By contrast, the MA patients demonstrated lower production
levels of the Th2 cytokine IL-4 than those in the control groups
(Fig. 3B). These data suggest an
abnormal immune response in MA patients, characteristic of a shift
to Th1-type immunity.
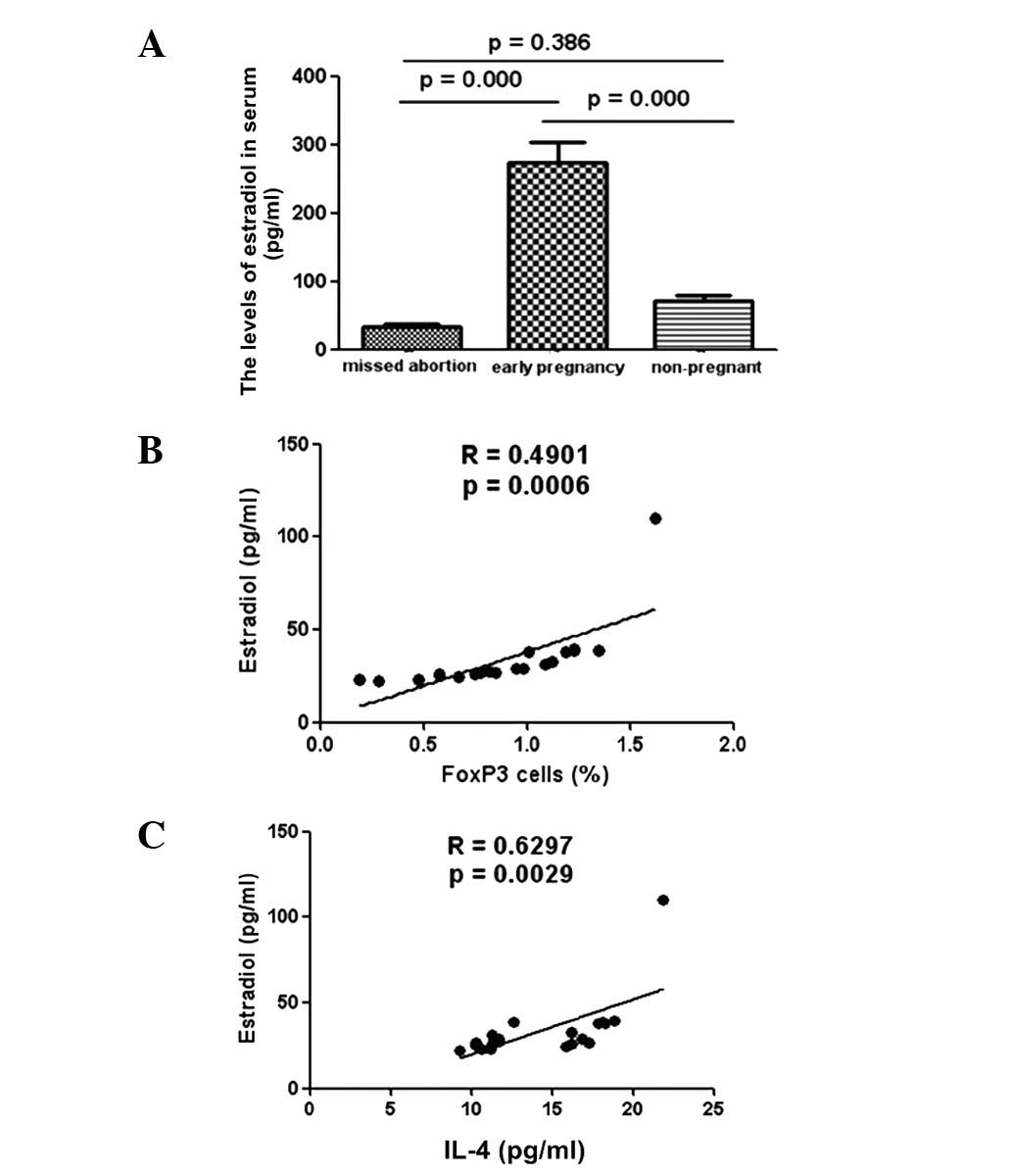
Correlation of E2 with Treg cells and
IL-4 in MA patients
To explore whether E2 expression levels were
affected during the pathogenesis of MA in humans, the serum E2
levels of patients were examined. Lower levels of E2 were detected
in the serum samples of MA patients than in the samples from
healthy pregnant subjects (Fig.
4A). Further analysis revealed a positive correlation between
the low levels of E2 and reductions in the production of Treg cells
in MA patients (Fig. 4B).
Furthermore, low levels of E2 expression in MA patients correlated
positively with the IL-4 levels of these patients (Fig. 4C).
Discussion
The fetus has been viewed as a semi-allograft to the
maternal host. However, pregnant patients do not usually experience
fetus rejection. The acceptance of the semi-allogeneic fetus within
the maternal environment requires mechanisms of tolerance. Evidence
has suggested that CD4+CD25+ Treg cells
participate in the development of maternal tolerance to the fetus
during pregnancy (13). It has
been confirmed that an augmentation in the quantity of Treg cells
during pregnancy and, most importantly, diminished numbers of Treg
cells, are associated with immunological rejection of the fetus
(14). In the present study, a
difference in the frequency of CD4+CD25+ Treg
cells in the peripheral blood of MA patients and normal early
pregnancy and non-pregnant subjects was demonstrated; normal
pregnant patients demonstrated an expansion of
CD4+CD25+ cells at the periphery compared
with non-pregnant subjects. Furthermore, significantly lower
frequencies of Treg were found in MA patients. Analyses have
suggested that decreasing levels of Treg cells indicate that they
have a role in maternal alloantigen tolerance during pregnancy
(15). In humans, low levels of
circulating CD4+CD25+ Treg cells have been
identified to be predictive of a risk of miscarriage in newly
pregnant patients with a history of failure, suggesting that the
level of peripheral Treg cells may serve as a superior pregnancy
marker (16,17). The findings of the present study
imply that CD4+CD25+ Treg cells may also play
a pivotal role in MA. The present study is, to the best of our
knowledge, the first to show that the frequency of
CD4+CD25+FoxP3+ Treg cells in
peripheral lymphocytes is lower in patients with MA than in
controls.
The balance of CD4+Th1 and Th2 cells
plays a role in the pathogenesis of MA patients (18). Based on the analysis of IFN-γ and
IL-4 production, a dominance of Th1 cell activity over Th2 cell
activity has been observed in MA patients. In the present study,
IFN-γ expression levels in MA patients were significantly higher
than those in the control group, whereas the MA patients exhibited
lower levels of the Th2 cytokine, IL-4. These data suggest an
abnormal immune response in MA patients with a characteristic shift
to Th1-type immunity (10).
Sex hormones, such as E2, play a vital and complex
role during pregnancy and interact with each other to mediate
various parts of the pregnancy process. The majority of the E2 in
adult females during reproductive years is derived from the
ovaries, while E2 levels increase markedly only during pregnancy.
During pregnancy E2 is produced by the mother, placenta and fetus.
Furthermore, the blood levels of these hormones change over the
course of pregnancy; in certain cases they correlate with changes
in the maternal immune response (19). In the present study, E2 levels were
identified to be lower in the MA group compared with those in the
normal pregnancy group, while no clear difference was observed
between MA patients and healthy non-pregnant patients. However,
further analysis revealed a positive correlation between low levels
of E2 and decreased levels of Treg cells and IL-4 production in MA
patients. These results indicated that decreased E2 levels may
promote a shift in the Th1/Th2 balance toward Th1 dominant
immunity, leading to low levels of IL-4. Furthermore, E2 may be one
of the regulators of Treg cell proliferation and differentiation
(20,21). The phenomenon illustrates the
intricate working of hormone-immune system interaction in MA.
However, the exact mechanism of action for E2 in various cell types
of the immune system with MA remains unclear.
In conclusion, this study detected low levels of E2
in the sera of MA patients, which correlated with decreased
peripheral blood levels of Treg cells and IL-4 in MA patients.
Thus, these results have revealed the previously unappreciated
association of E2 with Treg cells and cytokines in the pathogenesis
of MA. The findings, which show a positive correlation between low
levels of E2 expression and decreased Treg cell populations and
IL-4 levels in MA patients, may facilitate the potential
development of novel therapies targeting the hormone-immune system
pathway for the treatment of human MA.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (81070423), Jiangsu Province
Clinical Medicine Project (BL 2012059), the Science and Technology
Bureau of Zhenjiang Mandatory Research Projects (FZ2011059) and
Jiangsu University’s Clinical Medicine Project (JLY20120020).
Abbreviations:
|
MA
|
missed abortion
|
|
PBMCs
|
peripheral blood mononuclear cells
|
|
IFN-γ
|
interferon-γ
|
|
IL-4
|
interleukin-4
|
|
E2
|
estradiol
|
|
Treg cells
|
CD4+CD25+FoxP3+ regulatory T
cells
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
References
|
1
|
Le J: Obstetrics and Gynecology. 7th
edition. People’s Medical Publishing House; Beijing: 2008, (In
Chinese).
|
|
2
|
Ata B, Tan SL, Shehata F, Holzer H and
Buckett W: A systematic review of intravenous immunoglobulin for
treatmentof unexplained recurrent miscarriage. Fertil Steril.
95:1080–1085. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mei S, Tan J, Chen H, Chen Y and Zhang J:
Changes of CD4+CD25high regulatory T cells
and FOXP3 expression in unexplained recurrent spontaneous abortion
patients. Fertil Steril. 94:2244–2247. 2010.PubMed/NCBI
|
|
4
|
Bao SH, Wang XP, De Lin Q, Wang WJ, Yin GJ
and Qiu LH: Decidual CD4+CD25+CD127dim/-
regulatory T cells in patients with unexplained recurrent
spontaneous miscarriage. Eur J Obstet Gynecol Reprod Biol.
155:94–98. 2011.
|
|
5
|
Inada K, Shima T, Nakashima A, Aoki K, Ito
M and Saito S: Characterization of regulatory T cells in decidua of
miscarriage cases with abnormal or normal fetal chromosomal
content. J Reprod Immunol. 97:104–111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bansal AS: Joining the immunological dots
in recurrent miscarriage. Am J Reprod Immunol. 64:307–315.
2010.PubMed/NCBI
|
|
7
|
Samstein RM, Josefowicz SZ, Arvey A,
Treuting PM and Rudensky AY: Extrathymic generation of regulatory T
cells in placental mammais mitigates maternal-fetal conflict. Cell.
150:29–38. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zenclussen AC, Gerlof K, Zenclussen ML, et
al: Regulatory T cells induce a privileged tolerant
microenvironment at the fetal-maternal interface. Eur J Immunol.
36:82–94. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Raghupathy R and Kalinka J: Cytokine
imbalance in pregnancy complications and its modulation. Front
Biosci. 13:985–994. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saini V, Arora S, Yadav A and
Bhattacharjee J: Cytokines in recurrent regnancy loss. Clin Chim
Acta. 412:702–708. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tai P, Wang J, Jin H, et al: Induction of
regulatory T cells by physiological level estrogen. J Cell Physiol.
214:456–464. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Daya S: Habitual abortion. Textbook of
Gynecology. Copeland LJ: 2nd edition. WB Saunders; Philadelphia,
PA: pp. 227–271. 2000
|
|
13
|
Guleria M and Sayegh H: Maternal
acceptance of the fetus: true human tolerance. J Immunol.
178:3345–3351. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Arruvito L, Sanz M, Banham AH and Fainboim
L: Expansion of CD4+CD25+ and
FoxP3+ regulatory T cells during the follicular phase of
the menstrual cycle: Implications for human reproduction. J
Immunol. 178:2572–2578. 2007.PubMed/NCBI
|
|
15
|
Rowe JH, Ertelt JM, Xin L and Way SS:
Pregnancy imprints regulatory memory that sustains anergy to fetal
antigen. Nature. 490:102–106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kahn DA and Baltimore D: Pregnancy induces
a fetal antigen-specific maternal T regulatory cell response that
contributes to tolerance. Proc Natl Acad Sci USA. 107:9299–9304.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Winger EE and Reed JL: Low circulating
CD4+CD25+ regulatory T cells level predict
miscarriage risk in newly pregnant women with a history of failure.
Am J Reprod Immunol. 66:320–328. 2011.PubMed/NCBI
|
|
18
|
Yang KM, Ntrivalas E, Cho HJ, Kim NY,
Beaman K, Gilman-Sachs A and Kwak-Kim J: Women with multiple
implantation failures and recurrent pregnancy losses have increased
peripheral blood T cell activation. Am J Reprod Immunol.
63:370–378. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rana SA, Aavani T and Pittman QJ: Sex
effects on neurodevelopmental outcomes of innate immune activation
during prenatal and neonatal life. Horm Behav. 62:228–236. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Luo CY, Wang L, Sun C and Li DJ: Estrogen
enhances the functions of CD4(+)CD25(+)Foxp3(+) regulatory T cells
that suppress osteoclast differentiation and bone resorption in
vitro. Cell Mol Immunol. 8:50–58. 2010.
|
|
21
|
Valor L, Teijeiro R, Aristimuño C, et al:
Estradiol-dependent perforin expression by human regulatory
T-cells. Eur J Clin Invest. 41:357–364. 2011. View Article : Google Scholar : PubMed/NCBI
|