Introduction
The fibula is a rare anatomical location for
malignant primary bone sarcomas and metastatic lesions (1). The proximal fibula is the most common
area of the fibula to be affected by tumors; and osteosarcoma,
giant cell tumors, chondrosarcoma and aneurysmal bone cysts are the
most common type of tumor to develop at this location. The proximal
fibula osteosarcoma in the Mayo series reported an incidence of 2%
(2). Resection of an aggressive or
malignant tumor of the proximal fibula necessitates an en bloc
extra-articular resection of the proximal fibula [the proximal
tibiofibular joint (PTFJ) transmits loads between the knee and
ankle during weight bearing], as well as the lateral collateral
ligament (LCL) and biceps femoris tendon, which attach to the
proximal fibula, leading to varying degrees of knee instability
(3,4). Studies have revealed that the LCL is
a predominant constraint to primary varus rotation at all positions
of knee flexion (5). Isolated
sectioning of the LCL resulted in a marginal but significant
increase in varus rotation at all angles of knee flexion.
The biceps femoris imparts a posteriorly directed
force to the proximal tibia and the iliotibial band, leading to
anterior stability, thus reducing strain on the anterior cruciate
ligament (6). The method of
lateral-knee reconstruction to improve stability and whether
conducting an early reconstruction leads to an improved functional
outcome, remains controversial (4,7–9).
Therefore, the aim of the present study was to
evaluate the use of suture anchors (used to attach the LCL and
biceps femoris tendon to the lateral tibial metaphysis) and compare
the postoperative lateral knee stability and functional outcomes
with those of patients that had not received reconstruction.
Subjects and methods
Patients
Between January 2006 and December 2009, 29 proximal
fibula tumor resections were performed. Eighteen of these
resections involved reconstruction of the LCL and biceps femoris
tendon to the lateral tibial metaphysis using suture anchors. A
further 11 proximal fibula tumor resections without surgical
reconstruction served as the non-reconstruction group. All tumors
were histopathologically defined from biopsied specimens and the
histological diagnoses are presented in Table I. This study was conducted in
accordance with the declaration of Helsinki. Approval was obtained
from the Ethics Committee and the Institutional Review Board of
Shanghai Sixth People’s Hospital, Shanghai Jiaotong University
(Shanghai, China). Written informed consent was obtained from all
participants.
 | Table IHistological diagnoses of included
subjects. |
Table I
Histological diagnoses of included
subjects.
| Histological
diagnosis | Reconstruction group
(n=18) | Non-reconstruction
group (n=11) |
|---|
| Giant cell tumor | 7 | 4 |
| Aneurysmal bone
cyst | 5 | 2 |
| Chondrosarcoma | 1 | 1 |
| Osteosarcoma | 2 | 2 |
| Ewing’s sarcoma | 0 | 1 |
| Benign fibrous
histiocytoma | 3 | 1 |
Preoperative preparation
Preoperative detailed history, a comprehensive
physical examination and adequate imaging studies, including X-ray,
computed tomography, magnetic resonance imaging, single photon
emission computerized tomography and magnetic resonance
angiography, were required. The imaging studies assisted in
defining tumor staging, the extent of bone destruction,
intramedullary involvement and soft-tissue extension. In addition,
the position of the tumor to the nerves, blood vessels and tibia
was noted. Biopsies were performed with an anterolateral approach
in the safe area formed by the fibular head and the deep peroneal
nerve in the anterior compartment. This was required to protect the
peroneal nerves and LCL from contamination by tumor tissue during
biopsy. This applied to benign lesions and to malignant bone tumors
(10).
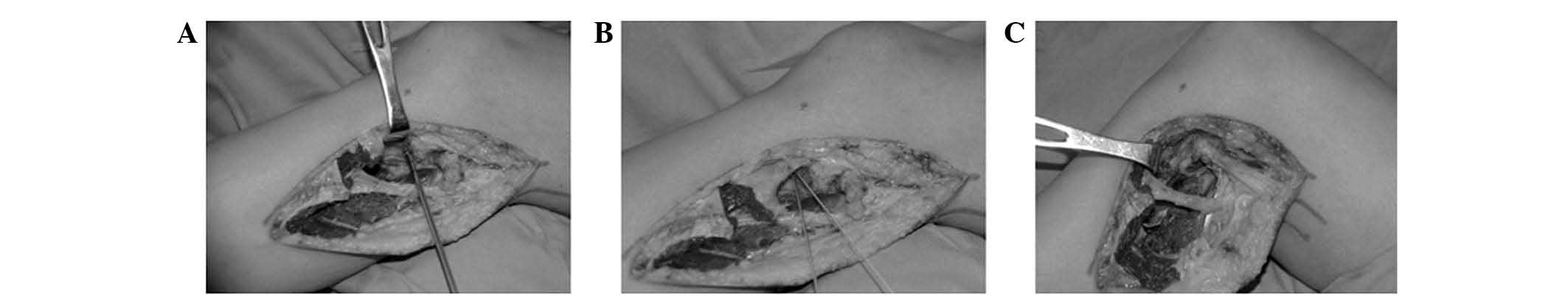
Type of proximal fibula resection
The types of proximal fibula resection have been
previously described by Malawer (11). A type I proximal fibula resection
is reserved for benign aggressive, low-grade malignant tumors and
metastatic tumors (Fig. 1). It
includes intra-articular resection of the proximal fibula with 2–3
cm of normal diaphysis, with a thin muscle cuff in all dimensions
and the LCL attachment site. The anterior tibial artery is
occasionally sacrificed. The peroneal nerve and its motor branches
may be preserved. A type II resection is reserved for high-grade
malignant tumors, which usually have considerable cortical
destruction with extra-osseous extension. This resection includes
an extra-articular resection of the proximal fibula with 6 cm of
normal diaphysis, the anterior and lateral muscle compartments, the
anterior tibial artery and occasionally, the peroneal artery and
peroneal nerve.
Surgical management
A semisupine position (45º elevation of the operated
side) was used to permit easy access to the anterior and lateral
compartments and allow dissection of the popliteal space. A single
utilitarian approach provided safe and wide exposure of all four
compartments of the leg and popliteal fossa, thus allowing the
resection of proximal fibula tumors. The incision began
posteriorly, ~8 cm proximal to the midpoint of the transverse
popliteal skin crease, then curved gently forward and distally
toward the anterior tibial crest, finally passing anteriorly to the
fibular head and over Gerdy’s tubercule to a point just lateral of
the tibial crest. The incision extended 5 cm distally to the level
of the planned osteotomy. When a primary bone sarcoma was resected,
the previous biopsy tract, with a 2–3 cm margin, was included in
the incision. A large lateral flap and a smaller medial flap was
developed.
Five consecutive steps were performed in the type II
resection: i) Exploration of the common peroneal nerve, which
encompasses the base of the fibular head, to enter the peroneus
longus tunnel; ii) exploration of the popliteal space and blood
vessels. Large tumors of the proximal fibula may reach the midline
posteriorly and push on the popliteal vessels. The predominant
vessels were exposed by reflecting the lateral gastrocnemius muscle
through its length and, if required, released the proximal
tendinous origin from the lateral femoral condyle; iii) excision of
the anterior and lateral muscle compartments. The anterior and
lateral musculature and the overlying deep fascia were excised. The
distal level of transection was at the musculotendinous junction.
The LCL and the biceps femoris tendon were released 2.5 cm
proximally to their fibular insertion to prepare for subsequent
reconstruction; iv) extra-articular resection of the PTFJ. A
semicircular incision was made directly through the popliteus
muscle towards the posterior aspect of the lateral tibial condyle;
including the proximal fibula, part of the lateral condyle of the
tibia and the PTFJ. Following osteotomy, it was important to
inspect the lateral tibial condyle. If the knee joint capsule was
exposed and opened, it was repaired in order to prevent a potential
synovial fistula; iv) soft-tissue reconstruction. The LCL and
biceps femoris tendon were attached to the lateral tibial
metaphysic using 5.0-mm suture anchors (DePuy Mitek Inc., Raynham,
MA, USA) with a 20º knee flexion (0.3 cm below the PTFJ, a Bunnell
braided suture was recommended to reinforce the fixation with
nonabsorbable sutures to the overlying iliotibial band and fascia)
(Fig. 2). The exposed tibia and
soft-tissue defect was closed and covered. It was possible to
rotate the lateral gastrocnemius muscle to cover the defect when
the muscle was released close to its origin through the muscle
substance and from its tendinous insertion at the distal end. Care
was taken to preserve its proximal pedicle, the lateral sural
artery, throughout the dissection.
Postoperative management
Suction drainage and prophylactic antibiotics were
continued for 3–5 days. The leg was kept elevated during this time.
The extremity was immobilized at a 20º knee flexion for 2–3 weeks
to allow soft-tissue healing and posterior capsule reattachment.
Full weight-bearing was allowed when the limb had been immobilized
in a cast. An ankle-foot orthosis was required following a type II
resection unless tenodesis of the anterolateral compartment to the
tibial shaft had been performed. Patients with high-grade sarcomas
were treated with postoperative chemotherapy. Patients with Ewing’s
sarcoma were further treated with radiation therapy consisting of
external beam radiation of 6,000–7,000 cGy.
Data analysis
Data with regard to histological diagnoses, surgical
techniques of tumor resection and reconstruction, complications,
knee stability and Musculoskeletal Tumor Society (MSTS) functional
score were recorded. Patients were evaluated by plain radiography
and a physical examination every three months for the first two
postoperative years.
Lateral knee stability was assessed by measuring the
degree of lateral joint space opening using valgus-varus stress
radiographs with a 30º knee flexion and in neutral tibial rotation.
Instability was scored as grades 1–3: grade 1, an opening of 1–5
mm; grade 2, 6–10 mm; and grade 3, ≥11 mm (e.g. complete LCL
dysfunction). Grade was determined by comparing the results with
that of a normal contralateral knee (12).
Functional evaluation was conducted according to the
MSTS functional scoring system (13). With this system, each answer was
scored based on frequency of symptoms using a five-point scale
between zero (indicating a significant problem) and five
(indicating no problem or full, normal function). Responses to all
questions were combined for a composite score ranging between 0 and
30, with higher scores indicating improved knee function. The
results are expressed as the proportion of full normal function in
all six categories (pain, function, emotional acceptance, supports,
walking and gait) and are based on the values recorded during each
patient’s most recent follow-up.
Statistical analysis
Data were analyzed using the SPSS 16.0 software
package (SPSS Inc., Chicago, IL, USA). Comparison of functional
parameters and knee stability following surgery was performed using
the Wilcoxon Two-Sample test. P<0.05 was considered to indicate
a statistically significant difference.
Results
The mean follow-up period was 42.8±20.9 months
(range 24–117) for the reconstruction group and 40.8±26.0 months
(range 24–117) for the non-reconstruction group. The reconstruction
group consisted of 11 males and 7 females, with an average age of
31.5±13.5 years (range, 18–45 years). The non-reconstruction group
consisted of 6 males and 5 females, aged 32.5±14.5 years (range,
18–47 years).
No complications were identified in association with
the surgical excision, including skin necrosis, infection, hematoma
or thrombophlebitis and synovial fistula. All 7 patients (3 in the
reconstruction group and 4 in the non-reconstruction group) who
received type II resections had an expected iatrogenic permanent
loss of peroneal nerve function. Three patients (2 in the
reconstruction group and 1 in the non-reconstruction group) who
received type I resections had a transient peroneal nerve palsy
that resolved spontaneously within three to six months.
Fifteen patients (83.3%) in the reconstruction group
had stable knee (12 with type I resection, 3 with type II
resection), 1 (5.6%) had grade 1 instability and 2 (11.1%) had
grade 2 instability (Table II).
The patient with grade 1 instability was asymptomatic and did not
require knee support for ambulation. Four of the 11 patients
(36.4%) in the control group had a stable knee, 3 (27.3%) patients
had grade 1 instability, 1 (9.1%) had grade 2 instability and 2
(27.3%) had grade 3 instability (Table II). The patients with grade 2 and
3 instability in the control group required a knee brace and
occasionally, a cane for ambulation. Overall, patients in the
reconstruction group had a higher rate of knee stability than those
in the control group (P=0.0099). Furthermore, the results revealed
that for type I resections, the reconstruction group had a higher
rate of knee stability than the non-reconstruction group
(P=0.0165), while for type II resections, no statistical difference
was observed (P=0.0615).
 | Table IIKnee stability in the reconstruction
and non-reconstruction groups. |
Table II
Knee stability in the reconstruction
and non-reconstruction groups.
| Reconstruction group
(n=18) | Non-reconstruction
group (n=11) |
|---|
|
|
|
|---|
| Outcome | Type I | Type II | Type I | Type II |
|---|
| Stable knee | 12 | 3 | 4 | 0 |
| Lateral knee
instability |
| Grade 1 | 0 | 1 | 2 | 1 |
| Grade 2 | 0 | 2 | 0 | 1 |
| Grade 3 | 0 | 0 | 1 | 2 |
MSTS function scores were available for 16 patients
in the reconstruction group and 10 patients in the
non-reconstruction group. There was 1 fatality in the
reconstruction group and 2 in the non-reconstruction group due to
metastases (bone and lung). In the reconstruction group, the
composite scores ranged between 93 and 100%, with a median of 93%.
In the non-reconstruction group, the composite scores ranged
between 60 and 100%, with a median of 87%. In general, patients in
the reconstruction group had higher composite MSTS function scores
than those in the non-reconstruction group (P<0.05). For the
reconstruction and non-reconstruction groups, in all categories of
MSTS, patients who had received type I resection scored higher
(improved knee function) than those who had received type II
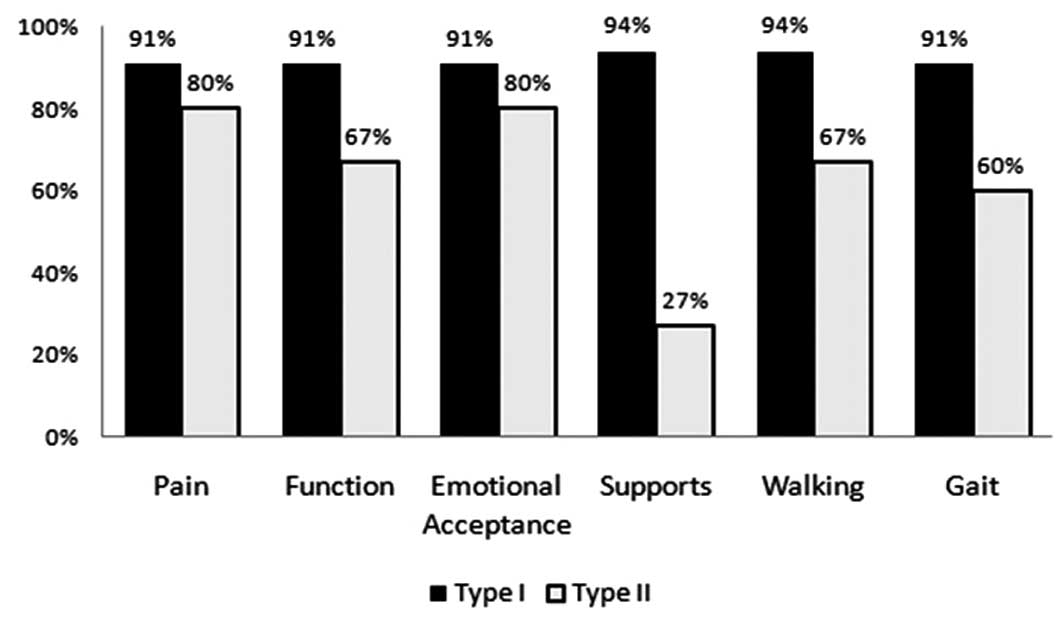
resection (Figs. 3 and 4). By analyzing MSTS function scores
restricted to those who received type I resection, results
suggested that in all categories of MSTS, those who received
reconstruction scored higher than those without reconstruction
(P<0.05) (Fig. 5). Similarly,
among those that received type II resection, patients who received
reconstruction scored higher than those without in all categories
of MSTS, with the exception of function and emotional acceptance;
the reconstruction group had higher composite scores than those in
the non-reconstruction group (P<0.05) (Fig. 6).
In the reconstruction and non-reconstruction groups,
patients with type I resection had a higher rate of knee stability
(P<0.01 and P<0.05, respectively) and an improved functional
outcome (P<0.001 and P<0.05, respectively) than those with
type II resection.
Discussion
Tumors of the proximal fibula are rare. Patients
with locally aggressive tumors require surgical management and
various extensile approaches of the fibula and popliteal vessels
have been described for limb-salvage procedures (14,15).
The proximal fibula, serving as the point of insertion of the LCL
and biceps femoris, is integral in the lateral stabilization of the
knee. Therefore excision of the proximal fibula may disrupt lateral
stability. It is essential to repair the LCL and biceps femoris
following proximal fibular excision (11). The present study communicates our
reconstruction technique and analyzed lateral knee stability and
functional outcome following resection the proximal fibula. This
technique included fixation of the LCL and biceps femoris tendon to
the tibial metaphysis, immobilization and protected
weight-bearing.
Siddiqui et al(16) demonstrated that, following
resection of the proximal fibula chondroblastic osteosarcoma, the
knee function remained stable, although there was no attempt to
reconstruct the lateral soft tissue structures.
Kanazawa et al(17) demonstrated promising results in
three stage IIB proximal fibula osteosarcomas by preserving the
common peroneal nerve through intentional marginal excision without
surgical reconstruction.
Einoder and Choong (8) reported that the knee remains
functionally stable following resection of proximal fibula tumors,
without reconstruction of the LCL for four years of follow-up.
However, a 1 cm joint space widening was detected in two cases
(which may result in osteoarthritis in long term follow-up).
Takahashi et al(9) observed 13 osteosarcomas of the
proximal fibula. The LCL and biceps femoris tendon was reattached
to the lateral wall of the tibia with a staple in two cases, with a
suture anchor in one case and with simple sutures to the soft
tissues in six cases. No patient presented with knee instability or
exhibited valgus instability on physical examination. It was
therefore indicated that surgical reconstruction of the LCL was not
required to achieve optimal function. This may be due to the
sparing of other stabilizing structures, including the cruciate
ligaments.
Draganich et al(3) reported six patients with proximal
fibular resection where repair of the LCL and biceps femoris was
performed. Gait and knee stability was evaluated with an
instrumented system and increased anterior and anteroposterior
translation of the knee during flexion, varus-valgus rotations at
20º flexion and several abnormalities in ground reaction forces
were identified. It was concluded that proximal fibular resection
without ligamentous reattachment resulted in gait abnormalities and
knee instability, and it is possible to minimize these disorders by
proper reattachment of the LCL and biceps tendon at their novel
insertion site.
Abdel et al(18,19)
reported that in 112 malignant and 121 benign proximal fibula
tumors, no long-term knee instability was observed in the patients
who underwent resection with the LCL and biceps femoris tendon
reconstruction by staple or suture anchor.
Faezypour et al(20) described a similar technique of
reconstructing the LCL and biceps femoris tendon; however, the 5
patients in this study had benign tumors and underwent type I
resections of the proximal fibula.
Saini et al(21) communicated observations following
Malawer type II resection in 8 patients with proximal fibular
osteosarcomas. Following tumor resection, nonabsorbable sutures
(no. 5 Ethibond; Ethicon Endo-Surgery (Europe) GmbH, Norderstedt,
Germany) were used to reattach the stumps of the LCL and biceps
femoris through drill holes in the lateral wall of the proximal
tibia. The LCL and biceps femoris were reinforced by suturing them
to the overlying iliotibial band. According to follow-up varus
stress radiographs, two patients had stable knees, 5 had grade 1
laxity and 1 had grade 2 laxity.
In the present study a group of patients who
underwent two types of resections for benign (type I resection) and
malignant (type II resection) diagnoses were analyzed and the
stability and functional outcomes between the reconstruction group
and the control group were compared. This is a relatively small
series (and therefore may limit the statistical power of the data);
however, it was possible to identify differences in lateral knee
joint stability and functional outcome with the two types of
resection.
Consideration of the resection type (I vs. II) may
be a significant factor contributing to knee stability following
surgery; therefore knee stability was compared by surgical type
between the reconstruction and non-reconstruction groups. Knee
stability was assessed by the surgeons who were aware of the type
of fibular resection performed on the patients at the time of
assessment. Generally, patients with type I resection had a higher
rate of knee stability than those with type II resection. For type
I resections, it was observed that the reconstruction group had a
higher rate of knee stability than the non-reconstruction group,
while for type II resections, no statistical difference was
revealed.
The patients who received type II resection of the
fibula had greater lateral knee instability and lower functional
outcome scores, requiring an orthotic device and sometimes
additional surgery, such as tenodesis of the toe extensors and the
anterior tibial muscle. The majority of patients who received type
I resection exhibited a stable knee or a mild grade 1 instability
that was asymptomatic and did not require knee support. We
hypothesize that the reason for the increased instability after a
type II resection may be the shorter LCL and biceps femoris tendon
stumps, which provide a short lever arm for knee function and less
viable adjacent soft tissue to support healing. Furthermore,
delayed healing is anticipated in patients receiving postoperative
chemotherapy. In addition to the impairment of the LCL and biceps
femoris tendon function, patients who have a type II resection lose
the peroneal nerve and a considerable quantity of muscle tissue
from the anterolateral compartment of the leg. These losses are
considered to be the reason for their inferior functional outcome
when compared with outcomes of patients who received a type I
resection. Of all functional parameters assessed, the most profound
difference between the two groups was the requirement for supports
due to the use of peroneal braces in patients who received a type
II resection.
When comparing knee stability between the
reconstruction and control groups following type II resection, no
statistical differences were identified, this was not the expected
outcome. This may be due to the small sample size of patients with
type II resections in the reconstruction (n=3) and
non-reconstruction (n=4) groups, limiting the statistical power of
the data. Further reasons for the lack of a statistical difference
in knee stability between the reconstruction and non-reconstruction
groups, following type II resection, may be associated with the
lateral knee joint stability structures, which have been resected
to a greater degree. The LCL and biceps femoris tendon stumps are
subsequently shorter, rendering reconstruction difficult and less
effective as there is less, viable adjacent soft tissue to support
its healing. In type II resection, a gastrocnemius flap is used for
soft tissue reconstruction. This procedure alone results in
additional mechanical problems. Kramers et al noted that
following a gastrocnemius flap procedure, the knee develops a
compensatory mechanism during the swing phase of the gait by
increasing peak knee flexion; however, knee motion remains normal
in the stance phase (22).
LCL may provide the main resistance to varus
rotation at the knee, whereas the biceps femoris may be a
significant dynamic restraint to anterior displacement of the tibia
(23). In conclusion, the
reconstruction of the LCL and biceps femoris tendon to the lateral
tibial metaphysis, with suture anchors, was observed to be a safe
and reliable technique to reconstruct knee stability following
resection of the proximal fibula. It provided stability and optimal
function in the majority of patients. This technique is simple to
perform and associated with minimal levels of morbidity. However, a
multicenter study and a greater number of long-term follow-up dates
is required to demonstrate whether this method of reconstruction
delays the occurrence of knee osteoarthritis.
References
|
1
|
Dorfman HD and Czerniak B: General
considerations. Bone Tumors. Dorfman HD and Czerniak B: CV Mosby;
St Louis, MO: pp. 1–33. 1998
|
|
2
|
Unni KK: Dahlin’s Bone Tumors: General
Aspects and Data on 11,087 Cases. 5th edition. Lippincott-Raven;
Philadelphia, PA: 1996
|
|
3
|
Draganich LF, Nicholas RW, Shuster JK,
Sathy MR, Chang AF and Simon MA: The effects of resection of the
proximal part of the fibula on stability of the knee and on gait. J
Bone Joint Surg Am. 73:575–583. 1991.PubMed/NCBI
|
|
4
|
Erler K, Demiralp B, Ozdemir MT and
Basbozkurt M: Treatment of proximal fibular tumors with en bloc
resection. Knee. 11:489–496. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grood ES, Noyes FR, Butler DL and Suntay
WJ: Ligamentous and capsular restraints preventing straight medial
and lateral laxity in intact human cadaver knees. J Bone Joint Surg
Am. 63:1257–1269. 1981.PubMed/NCBI
|
|
6
|
Draganich LF and Vahey JW: An in vitro
study of anterior cruciate ligament strain induced by quadriceps
and hamstrings forces. J Orthop Res. 8:57–63. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McDonald DJ, Sim FH, McLeod RA and Dahlin
DC: Giant-cell tumor of bone. J Bone Joint Surg Am. 68:235–242.
1986.PubMed/NCBI
|
|
8
|
Einoder PA and Choong PF: Tumors of the
head of the fibula: good function after resection without ligament
reconstruction in 6 patients. Acta Orthop Scand. 73:663–666.
2002.PubMed/NCBI
|
|
9
|
Takahashi S, Ogose A, Tajino T, Osanai T
and Okada K: Osteosarcoma of the proximal fibula. An analysis of 13
cases in the northern Japan. Ups J Med Sci. 112:366–372. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takeda A, Tsuchiya H, Mori Y, Tanaka S,
Kikuchi S and Tomita K: Anatomical aspects of biopsy of the
proximal fibula. Int Orthop. 24:335–337. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Malawer MM: Surgical management of
aggressive and malignant tumors of the proximal fibula. Clin Orthop
Relat Res. 186:172–181. 1984.PubMed/NCBI
|
|
12
|
Bickels J, Kollender Y, Pritsch T, Meller
I and Malawer MM: Knee stability after resection of the proximal
fibula. Clin Orthop Relat Res. 454:198–201. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Enneking WF, Dunham W, Gebhardt MC,
Malawar MM and Pritchard DJ: A system for the functional evaluation
of reconstructive procedures after surgical treatment of tumors of
the musculoskeletal system. Clin Orthop Relat Res. 286:241–246.
1993.PubMed/NCBI
|
|
14
|
Danese CA and Singer A: Lateral approach
to the popliteal artery trifurcation. Surgery. 63:588–598.
1968.PubMed/NCBI
|
|
15
|
Dardik H, Dardik I and Veith FJ: Exposure
of the tibial-peroneal arteries by a single lateral approach.
Surgery. 75:377–382. 1974.PubMed/NCBI
|
|
16
|
Siddiqui YS, Pathania VP, Mendiratta M and
Rahman N: Chondroblastic osteosarcoma of proximal fibula. Oncol
Gastroenterol Hepatol Reports. 1:41–44. 2012. View Article : Google Scholar
|
|
17
|
Kanazawa Y, Tsuchiya H, Nonomura A,
Takazawa K, Yamamoto N and Tomita K: Intentional marginal excision
of osteosarcoma of the proximal fibula to preserve limb function. J
Orthop Sci. 8:757–761. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Abdel MP, Papagelopoulos PJ, Morrey ME, et
al: Malignant proximal fibular tumors: surgical management of 112
cases. J Bone Joint Surg Am. 94:e1652012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abdel MP, Papagelopoulos PJ, Morrey ME,
Wenger DE, Rose PS and Sim FH: Surgical management of 121 benign
proximal fibula tumors. Clin Orthop Relat Res. 468:3056–3062. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Faezypour H, Davis AM, Griffin AM and Bell
RS: Giant cell tumor of the proximal fibula: surgical management. J
Surg Oncol. 61:34–37. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saini R, Bali K, Gill SS, Mootha AK and
Dhillon MS: En bloc resection of osteosarcoma of the proximal
fibula. An analysis of 8 cases. Acta Orthop Belg. 76:806–810.
2010.PubMed/NCBI
|
|
22
|
Kramers-de Quervain IA, Läuffer JM, Käch
K, Trentz O and Stüssi E: Functional donor-site morbidity during
level and uphill gait after a gastrocnemius muscle-flap procedure.
J Bone Joint Surg. 83-A:239–246. 2001.PubMed/NCBI
|
|
23
|
Kirgis A and Albrecht S: Palsy of the deep
peroneal nerve after proximal tibial osteotomy. An anatomical
study. J Bone Joint Surg Am. 74:1180–1185. 1992.PubMed/NCBI
|