Introduction
Leber’s hereditary optic neuropathy (LHON) is a
maternally inherited disease characterized by acute or subacute
bilateral visual loss in young adulthood, particularly in males,
with a median age of onset of 24 years (1,2). A
study identified that >95% of LHON cases were caused by three
point mutations of mitochondrial DNA (mtDNA): G11778A, T14484C and
G3460A (3). However, the
incomplete penetrance implicates that the mtDNA mutations are
necessary but do not determine LHON, and additional genetic or
environmental factors are required to trigger the pathological
processes (4).
Histopathological descriptions of molecularly
characterized patients with LHON have demonstrated a marked loss of
retinal ganglion cells and their axons (5,6). The
small-caliber fibers of the papillomacular bundle (PMB) are
selectively lost at a very early stage of the pathological process,
which eventually extends to the rest of the nerve, resulting in
optic atrophy (7). According to
disease duration, LHON may be divided into three stages: The
preclinical stage, the acute/subacute stage (defined as ‘early’
within 6 months from onset; E-LHON) and the atrophic phase (>6
months; A-LHON), with 6-months being the mean time for the
development of optic atrophy (8).
A follow-up study showed evident optic atrophy and a stable visual
acuity remaining at the lowest level after 6 months (9).
Optical coherence tomography (OCT) is a novel
noninvasive, noncontact diagnostic technology, which is capable of
performing high-resolution imaging of the transverse section of the
retina in vivo and in real time (10). OCT has been used extensively to
measure the retinal nerve fiber layer (RNFL) thickness and the
macula lutea in patients with optic nerve and retinal diseases.
Since the RNFL thickness begins to change prior to
disease onset, analyzing only the changes in RNFL thickness
following disease onset may not be sufficient. Thus far, to the
best of our knowledge, no previous studies have identified the
changes of RNFL thickness that are associated with a cycling period
in patients with LHON. In the present study, the changes in RNFL
thickness in each quadrant were examined in patients with LHON at
different disease durations, and the correlation between RNFL
thickness and the best corrected visual acuity (BCVA) was
investigated.
Patients and methods
Ethical considerations
This study was approved by the ethics committee of
the Chinese PLA General Hospital (Beijing, China). The ethics
committee approved the screening, inspection and data collection of
these patients, and all patients provided written informed consent.
All experiments followed the provisions of the Declaration of
Helsinki.
Patients
All patients with LHON diagnosed by mtDNA analysis
in the Chinese PLA General Hospital (Haidian, China) between
September 1, 2011 and March 31, 2013 were recruited. These patients
were evaluated prospectively by ophthalmic tests, comprising BCVA,
non-contact intraocular pressure measurements, slit-lamp
microscopy, ophthalmoscopy and OCT. Patients were excluded
according to the following criteria: Patients with retinal diseases
and/or optic nerve diseases other than LHON; patients who were
unable to accept OCT examination; patients with nystagmus whose OCT
images were not stable; and patients with an OCT signal intensity
of <6.
The recruited patients with LHON were divided into 5
diagnostic groups according to the duration of eye symptoms: Group
1, ≤3 months; group 2, 4–6 months; group 3, 7–9 months; group 4,
10–12 months; and group 5, >12 months.
Age- and gender-matched control individuals were
recruited following the routine visual acuity testing of volunteers
at the hospital. The control individuals underwent the same tests
as those used to evaluate the patients with LHON. Based on OCT
results, the eye with the better OCT signal was selected in each
individual.
OCT analysis
OCT scanning was performed by Cirrus high
definition-OCT (software version 3.0, model 4000; Carl Zeiss
Meditec, Inc. Dublin, CA, USA). Real-time image scans (27,000
A-scans/sec) were performed, an axial resolution of 5 microns was
adopted and data were restructured as a 3-dimensional cube. RNFL
thickness measurements were acquired using the optic disk cube
200×200 protocol and were analyzed the using optic nerve head (ONH)
and RNFL oculus utro (OU) analysis protocols. BCVA examinations
were performed using the logMAR visual testing chart (11).
All OCT scanning was performed in a darkroom by the
same technician. Patients with a pupil diameter of <2 mm
underwent mydriasis. In these patients, internal fixation was used
whenever possible. If the patient was not able to see the internal
fixation, they were asked to observe the external fixation using
the fellow eye. If the method described above was infeasible for a
patient, they were asked to move their eyes laterally during the
scan acquisition until the image of the optic disc appeared on the
screen of the operator. Each eye was rescanned until a good quality
was obtained and an image was recorded for each eye. Statistical
analyses were performed for the 360°-average RNFL thickness and the
RNFL thickness in the temporal, superior, nasal and inferior
quadrants.
Statistical analysis
Statistical analysis was performed with SPSS
software, version 19.0 (SPSS Inc., Chicago, IL, USA). Quantitative
data were analyzed by the method of variance analysis with least
significant difference multiple comparisons post hoc test. Linear
correlation analysis was used for comparisons between the RNFL
thickness and the BCVA. P<0.05 was considered to indicate a
statistically significant difference.
Results
Demographic data of patients
A total of 68 eyes from patients with LHON (males,
n=61; females, n=7) and 15 eyes from healthy individuals (males,
n=10; females, n=5) were included. Table I presents demographic data of the
study cohorts. Patients with a LHON duration of >12 months had a
relatively older age and a longer mean duration of the disease; in
this group, the longest duration of LHON was 3 years, but the
LogMAR evaluation showed no statistically significant difference
between disease groups. Fig. 1
demonstrates the OCT scanning visual-field report of three typical
patients; the degree of the central visual field defect was
aggravated gradually to diffuse defects in these three
patients.
 | Table IDemographic information of the
patients in each group. |
Table I
Demographic information of the
patients in each group.
| Demographics | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Control |
|---|
| Gender |
| Male | 14 | 13 | 10 | 9 | 15 | 10 |
| Female | 0 | 1 | 0 | 1 | 5 | 5 |
| Age, years
(range) | 15.4 (4–29) | 19 (12–34) | 21.5 (7–45) | 17.7 (7–28) | 28.9 (15–45) | 24.9 (7–43) |
| Onset age, years
(range) | 15.1 (4–29) | 18.8 (12–33) | 20.7 (6–44) | 17.1 (6–28) | 17.6 (12–36) | - |
| ADV, months
(range) | 1.3 (0.3–3) | 4.3 (3.3–5) | 7.2 (6–9) | 10.5 (9–12) | 137.5 (14–360) | - |
| LogMAR BCVA
score | 1.5 (0.4–2.9) | 1.6 (1.0–2.9) | 1.6 (0.3–2.4) | 1.6 (0.1–2.5) | 1.8 (0.1–4.1) | - |
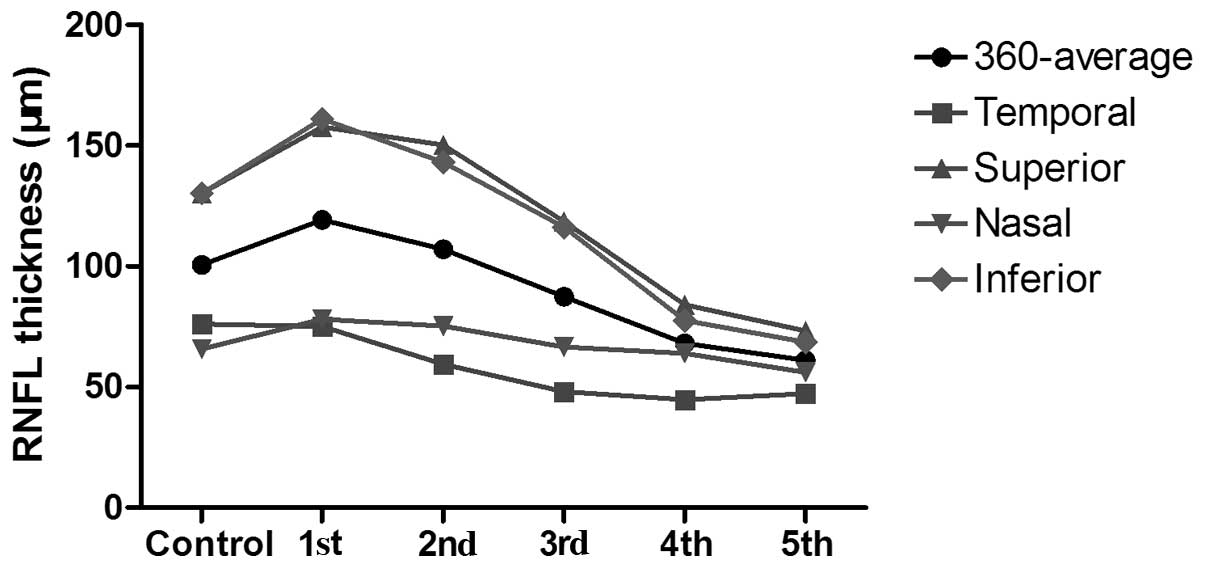
RNFL thickness variation
To compare RNFL thickness by OCT in patients with
LHON and the control group, the changes in RNFL thickness were
investigated at 3, 6, 9 and 12 months following onset. The mean
RNFL thickness in each group is shown in Table II. The OCT scans show the RNFL to
be temporarily relatively thicker in patients with LHON within 3
months from the time of disease onset. After 6 months, the
360°-average RNFL thickness and the RNFL in all quadrants
(temporal, superior, nasal and inferior) became thinner and
progressively thinned over 12 months. The changes in RNFL thickness
in each quadrant and the 360° averages for the different time
course groups are displayed in Fig.
2.
 | Table IIMean values of the 360°-average RNFL
thickness and the RNFL thickness in the temporal, superior, nasal
and inferior quadrants in each group. |
Table II
Mean values of the 360°-average RNFL
thickness and the RNFL thickness in the temporal, superior, nasal
and inferior quadrants in each group.
| Group | 360°-average
(μm) | T (μm) | S (μm) | N (μm) | I (μm) |
|---|
| 1 | 119.3±31.6 | 75.1±30.1 | 157.8±48.8 | 78.1±19.5 | 161.1±46.1 |
| 2 | 107.1±19.3 | 59.4±16.5 | 150.3±36.8 | 75.3±12.9 | 143.1±28.2 |
| 3 | 87.4±12.7 | 48.1±9.5 | 118.5±16.1 | 66.6±12.5 | 116.3±25.8 |
| 4 | 68.1±11.0 | 44.7±8.3 | 84.0±18.4 | 63.9±9.1 | 77.6±18.4 |
| 5 | 61.1±10.8 | 47.3±2.6 | 73.2±21.3 | 56.1±7.7 | 68.6±17.1 |
| Control | 100.5±8.7 | 76.1±16.0 | 130.2±16.2 | 65.7±10.2 | 130.3±14.0 |
Changes in the superior and inferior
quadrant and 360°-average RNFL thickness
Compared with the control group value, the
360°-average RNFL thickness was significantly higher in group 1
(P=0.026), and lower in groups 3, 4 and 5 (P=0.005, <0.001 and
<0.001, respectively). The 360°-average RNFL thickness in groups
3, 4 and 5 was significantly increased compared with those in group
1 (P=0.016, 0.001 and <0.001, respectively) and group 2
(P=0.006, <0.001 and <0.001, respectively). In groups 4 and
5, the 360°-average RNFL thickness was significantly increased
compared with that of group 3 (P=0.002 and <0.001,
respectively), while the RNFL thickness was not observed to be
significantly different between groups 4 and 5.
The changes in RNFL thickness in the superior and
inferior quadrants were comparable with those in the 360°-average
RNFL thickness.
Changes in RNFL thickness in the nasal
quadrant
The RNFL thickness in the nasal quadrant was
significantly increased in groups 1, 2 and 5 compared with that of
the control group (P=0.046, 0.023 and 0.005, respectively). The
RNFL thickness of the nasal quadrant was significantly reduced in
groups 4 and 5 compared with that in group 1 (P=0.048 and 0.002,
respectively), and in groups 3, 4 and 5 compared with that in group
2 (P=0.048, 0.019 and <0.001, respectively). The RNFL thickness
of the nasal quadrant was significantly reduced in group 5 compared
with those in group 3 (P=0.01) and group 4 (P=0.013).
Changes in RNFL thickness in the temporal
quadrant
The RNFL thickness of the temporal quadrant was
significantly increased in groups 2, 3, 4 and 5 compared with that
in the control group (P=0.005, <0.001, <0.001 and <0.001,
respectively). The temporal RNFL thickness was significantly
decreased in groups 3, 4 and 5 compared with that in group 1
(P=0.019, 0.002 and 0.002, respectively), and significantly
decreased in groups 4 and 5 compared with that in group 2 (P=0.016
and 0.043, respectively). No other statistically significant
differences were identified between the groups.
Correlation between RNFL thickness and
BCVA
In the present study, logMAR values were used as a
measure of the BCVA in each group. Following analysis, the RNFL
thickness in the four quadrants and the 360° average showed no
linear correlation with BCVA (P>0.05; data not shown).
Discussion
With the development and continuous upgrading of
technology, OCT has become one of the most effective technologies
with which to study optic nerve and retinal diseases (12,13),
particularly regarding the anatomical structure of the retina and
RNFL thickness. Recently, OCT has been commonly applied in optic
neuropathy research, such as the study of glaucoma, optic neuritis
and multiple sclerosis (14,15).
However, studies of LHON are limited in the literature.
A previous study of LHON by OCT showed that
unaffected carriers demonstrated thicker RNFL in the temporal and
inferior quadrants than the control (16). The RNFL thickness in patients with
E-LHON and A-LHON also differs. In a cross-sectional study, eyes
with E-LHON showed a thicker RNFL in the temporal quadrant compared
with that of the healthy control group, and no significant changes
were detected in other quadrants, whereas eyes with A-LHON
demonstrated a thinner RNFL in all measurements (17). Furthermore, a cohort study of four
patients with molecularly defined LHON by Barboni et
al(18), demonstrated that the
temporal and inferior quadrants showed a statistically significant
increase of RNFL thickness between the presymptomatic stage and
disease onset. With the exception of the temporal quadrant, the
RNFL thickness showed a statistically significant increase between
the presymptomatic stage and the 3-month follow-up. A significant
reduction of RNFL thickness was detected in all but the nasal
quadrant between the presymptomatic stage and the 9 month follow-up
(18).
A previous study showed that the RNFL thickness
increased significantly in the temporal quadrant and marginally
increased in the inferior quadrant of non-invasive carriers
(16). Combined with the findings
of the present study, this suggests that RNFL in the temporal and
inferior quadrants thickens prior to the occurrence of the disease,
but swelling in the temporal quadrant recovers gradually within 3
months following disease onset. In the pathological process,
small-caliber fibers of the PMB are selectively lost at a very
early stage and this loss is then extended to the other nerve
fibers, resulting in diffused optic atrophy (7,19).
As the RNFL in the temporal quadrant is mainly composed of the PMB,
its thickness was altered earlier than that of the other quadrants.
Between 4–6 months, RNFL in the temporal quadrant was significantly
reduced, suggesting that the RNFL in the temporal quadrant started
to atrophy and the swelling of RNFL in other quadrants began to
subside. Between 7 and 9 months, the RNFL in the superior, nasal
and inferior quadrants had started to shrink with the reduction in
the superior and inferior quadrants being apparent while the nasal
quadrant only showed a tendency to thin. The results of the present
study are consistent with the findings of Barboni et
al(18). The mean values of
RNFL thickness in the nasal quadrant decreased at 10–12 months,
which indicated that the RNFL in the nasal quadrant was thinning
continuously. After 12 months, all measurements of RNFL showed
significant thinning, and significant atrophy of RNFL in the nasal
quadrant was observed.
Furthermore, no significant differences were
identified in the BCVA between groups in the present study, and no
linear correlation between the BCVA and RNFL thickness was
observed. These results are consistent with the clinical
observations of patients with LHON, since their visual acuity
remained stable at the lowest level 6 months following disease
onset (9,20). Certain patients even showed visual
recovery to a certain degree while their RNFLs continued to shrink.
In five patients with a disease duration of >20 years, the
360°-average RNFL thickness was relatively low, but not the lowest
among all the study participants, indicating that the RNFL
thickness varied in patients with LHON according to the time
sequence recorded. A larger sample size may further elucidate this
phenomenon.
In conclusion, the present study demonstrates the
unique features of changes in RNFL thickness from the onset of LHON
to 18 months and provided noteworthy information.
References
|
1
|
Nikoskelainen EK, Huoponen K, Juvonen V,
Lamminen T, Nummelin K and Savontaus ML: Ophthalmologic findings in
Leber hereditary optic neuropathy, with special reference to mtDNA
mutations. Ophthalmology. 103:504–514. 1996. View Article : Google Scholar
|
|
2
|
Leo-Kottler B and Wissinger B: Leber’s
hereditary optic neuropathy. Ophthalmologe. 108:1179–1192. 2011.(In
German).
|
|
3
|
Mao YJ, Qu J and Guan MX: The influence of
mitochondrial haplogroup on Leber’s hereditary optic neuropathy.
Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 25:45–49. 2008.(In
Chinese).
|
|
4
|
Carelli V, Giordano C and d’Amati G:
Pathogenic expression of homoplasmic mtDNA mutations needs a
complex nuclear-mitochondrial interaction. Trends Gene. 19:257–262.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sadun AA, Kashima Y, Wurdeman AE, Dao J,
Heller K and Sherman J: Morphological findings in the visual system
in a case of Leber’s hereditary optic neuropathy. Clini Neurosci.
2:165–172. 1994.
|
|
6
|
Kerrison JB, Howell N, Miller NR, Hirst L
and Green WR: Leber hereditary optic neuropathy. Electron
microscopy and molecular genetic analysis of a case. Ophthalmology.
102:1509–1516. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sadun AA, Win PH, Ross-Cisneros F, Walker
SO and Carelli V: Leber’s hereditary optic neuropathy
differentially affects smaller axons in the optic nerve. Trans Am
Ophthalmol Soc. 98:223–232. 2000.
|
|
8
|
Nikoskelainen E, Hoyt WF, Nummelin K and
Schatz H: Fundus findings in Leber’s hereditary optic
neuroretinopathy. III Fluorescein angiographic studies. Arch
Ophthalmol. 102:981–989. 1984.
|
|
9
|
Riordan-Eva P, Sanders MD, Govan GG,
Sweeney MG, Da Costa J and Harding AE: The clinical features of
Leber’s hereditary optic neuropathy defined by the presence of a
pathogenic mitochondrial DNA mutation. Brain. 118:319–337.
1995.
|
|
10
|
Huang D, Swanson EA, Lin CP, et al:
Optical coherence tomography. Science. 254:1178–1181. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Laidlaw DA, Tailor V, Shah N, Atamian S
and Harcourt C: Validation of a computerised logMAR visual acuity
measurement system (COMPlog): comparison with ETDRS and the
electronic ETDRS testing algorithm in adults and amblyopic
children. Br J Ophthalmol. 92:241–244. 2008. View Article : Google Scholar
|
|
12
|
Aydin A, Wollstein G, Price LL, Fujimoto
JG and Schuman JS: Optical coherence tomography assessment of
retinal nerve fiber layer thickness changes after glaucoma surgery.
Ophthalmology. 110:1506–1511. 2003. View Article : Google Scholar
|
|
13
|
Garcia-Martin E, Pinilla I, Sancho E, et
al: Optical coherence tomography in retinitis pigmentosa:
reproducibility and capacity to detect macular and retinal nerve
fiber layer thickness alterations. Retina. 32:1581–1591. 2012.
View Article : Google Scholar
|
|
14
|
Garcia-Martin E, Pinilla I, Sancho E, et
al: Optical coherence tomography in retinitis pigmentosa:
reproducibility and capacity to detect macular and retinal nerve
fiber layer thickness alterations. Retina. 32:1581–1591. 2012.
View Article : Google Scholar
|
|
15
|
He XF, Liu YT, Peng C, Zhang F, Zhuang S
and Zhang JS: Optical coherence tomography assessed retinal nerve
fiber layer thickness in patients with Alzheimer’s disease: a
meta-analysis. Int J Ophthalmol. 5:401–405. 2012.
|
|
16
|
Savini G, Barboni P, Valentino ML, et al:
Retinal nerve fiber layer evaluation by optical coherence
tomography in unaffected carriers with Leber’s hereditary optic
neuropathy mutations. Ophthalmology. 112:127–131. 2005.PubMed/NCBI
|
|
17
|
Barboni P, Savini G, Valentino ML, et al:
Retinal nerve fiber layer evaluation by optical coherence
tomography in Leber’s hereditary optic neuropathy. Ophthalmology.
112:120–126. 2005.
|
|
18
|
Barboni P, Carbonelli M, Savini G, et al:
Natural history of Leber’s hereditary optic neuropathy:
longitudinal analysis of the retinal nerve fiber layer by optical
coherence tomography. Ophthalmology. 117:623–627. 2010.
|
|
19
|
Barcella V, Rocca MA, Bianchi-Marzoli S,
et al: Evidence for retrochiasmatic tissue loss in Leber’s
hereditary optic neuropathy. Hum Brain Mapp. 31:1900–1906.
2010.PubMed/NCBI
|
|
20
|
Smith KH, Johns DR, Heher KL and Miller
NR: Heteroplasmy in Leber’s hereditary optic neuropathy. Arch
Ophthalmol. 111:1486–1490. 1993.
|