Introduction
Functional bowel disorder (FBD) is the generic term
for disorders of bowel motor and secretory function in the absence
of organic changes. These disorders are diagnosed according to
symptoms following the exclusion of lesions caused by inflammation,
infection, tumor and other structural disorders (1,2). The
incidence of FBD in the elderly is ~53%, although in patients
>75 years, it is even higher (3). Inflammatory bowel polyps are benign
proliferative lesions characterized by increased stromal cells and
infiltration of inflammatory cells (4,5).
Chymase, a chymotrypsin-like protease, is a
non-angiotensin-converting enzyme (ACE) angiotensin II (Ang
II)-generating enzyme with low expression in healthy intestinal
mucosa (6,7). The expression of chymase has been
shown to be upregulated in response to inflammatory stimulation,
indicating its possible role in the pathogenesis of intestinal
inflammatory polyps (8,9). In the present study, chymase
expression was evaluated in intestinal inflammatory polyps and its
correlation with the pathogenesis of intestinal inflammatory polyps
in elderly patients with FBD was analyzed.
Materials and methods
Patients
Between August 2005 and August 2008, 45 consecutive
outpatients of the Nanjing Jin Ling Hospital (Nanjing, China) were
enrolled in this study. The study population comprised 28 males and
17 females (average age, 76.53±8.37 years) with inflammatory polyps
and FBD. The study was conducted in accordance with the Declaration
of Helsinki and approval was obtained from the Ethics Committee of
Nanjing Jin Ling Hospital. Written informed consent was obtained
from all participants. All cases were diagnosed according to the
Rome III process (10) and divided
into four subgroups. The characteristics of each subgroup are shown
in Table I. A total of 44 healthy
individuals (32 males, 12 females; average age, 76.68±6.41 years)
were selected as controls.
 | Table ICharacteristics of patients with
FBD. |
Table I
Characteristics of patients with
FBD.
| Category | Cases (n) | Age (years) | History (years) |
|---|
| Irritable bowel
syndrome (C1) | 8 (M 5, F 3) | 76.53±8.37
(65–87) | 9.05±6.99 (2–26) |
| Functional (C2) | 10 (M 6, F4) | 77.23±7.84
(65–87) | 9.37±8.23 (5–36) |
| Functional
constipation (C3) | 14 (M 9, F 5) | 77.37±6.18
(65–88) | 10.44±6.25
(4–36) |
| Functional diarrhea
(C4) | 13 (M 8, F 5) | 77.17±6.18
(65–87) | 9.99±8.17 (2–36) |
All participants with demographic and clinical data
signed the informed consent form. Ultrasound, computed tomography
(CT) scan and colonoscopy were performed to exclude
gastrointestinal organic diseases and other systemic diseases. None
of the patients had received immunologic treatment in the three
months prior to enrollment. Serial sections of paraffin-embedded
intestinal mucosa tissues confirmed by pathologists were collected
by biopsy during colonoscopy.
Immunohistochemistry (IHC)
IHC staining was performed using a
Streptavidin-Peroxidase (SP) kit (SP-9000, anti-mouse/anti-rabbit
IgG; Zhongshan Laboratories, Zhongshan, China) (11). In brief, slides were dewaxed in
xylene, rehydrated in alcohol, and incubated in 0.3% (v/v) hydrogen
peroxide in methanol to block endogenous peroxidase activity.
Antigens were retrieved by microwaving the sample on high-power for
15 min with two 5-min intervals using a 0.01-M sodium citrate
buffer. Subsequent to incubating with normal goat serum for 1 h,
the sections were incubated with an anti-human chymase polyclonal
antibody (dilution 1:50; Zhongshan Belling Biotechnology Co., Ltd.,
Zhongshan, China) overnight at 4°C. The sections were then
incubated with biotin-labeled goat anti-rabbit serum (1:2,000;
Zhongshan Dongqiang Laboratories Co. Ltd., Zhongshan, China) for 30
min. Subsequent to washing with phosphate-buffered saline (PBS),
the sections were treated with diaminobenzidine (DAB). The sections
were then counterstained with hematoxylin, rinsed, dehydrated, and
mounted. Sections incubated with PBS instead of primary antibody
were used as negative controls. All sections were examined and
scored independently by two experienced pathologists who had no
knowledge of clinical or pathological information regarding the
samples.
Quantitative polymerase chain reaction
(PCR)
Total RNA was isolated from tissues with TRIzol
reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) according
to the manufacturer’s instructions (11). The reverse transcription reaction
was performed using the First-Strand cDNA Synthesis kit (MBI
Fermentas, Vilnius, Lithuania) according to the manufacturer’s
instructions. Chymase primer sequences were as follows:
5′-GGAAATGTGAGCCAGATAGTGCAGTC-3′ (forward);
5′-AATCCGGAGCTGGAGAACTCTTGTC-3′ (reverse). The TaqMan®
stem-loop quantitative PCR method was used to assess chymase
expression with kits from Applied Biosystems (Foster City, CA,
USA). All quantitative PCR experiments were performed on a Chromo4™
Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). PCR
conditions were as follows: 93°C for 2 min, 93°C for 45 sec, 63°C
for 45 sec, 35 cycles. CT values were applied to determine the mRNA
level of target genes in each sample.
Statistical analysis
Numerical count data are presented as the mean ±
standard deviation. P<0.05 was considered to indicate a
statistically significant difference. All statistical tests,
including the Student’s t-test, χ2 test and Fisher’s
exact test, were two-sided and performed with SAS statistical
software (version 9.1; SAS Institute, Cary, NC, USA).
Results
IHC
Chymase expression was evaluated by IHC in 45
patients with inflammatory polyps and FBD compared with healthy
controls. According to the present study, chymase was expressed
predominantly in the cytoplasm of mucosa cells (Fig. 1). In addition, chymase-positive
mast cells were distributed diffusely in the intestinal mucosa. An
increased number of chymase-positive mast cells were observed in
inflammatory polyps compared with healthy intestinal mucosa
(P<0.05).
Chymase mRNA level
To determine chymase mRNA expression, quantitative
PCR was performed. As shown in Table
II, 45 cases of inflammatory polyps had increased chymase mRNA
levels compared with healthy controls. However, there were no
significant differences among FBD subgroups.
 | Table IIChymase mRNA level in each group. |
Table II
Chymase mRNA level in each group.
| Category | Cases (n) | Chymase mRNA
level |
|---|
| Control | 44 | 0.60±0.11 |
| FBD | 45 | 0.81±0.10a |
| C1 subgroup | 8 | 0.82±0.11 |
| C2 subgroup | 10 | 0.84±0.10 |
| C3 subgroup | 14 | 0.80±0.09 |
| C4 subgroup | 13 | 0.80±0.11 |
Chymase polymorphism analyses
The chymase genotype in patients with intestinal
inflammatory polyps was further determined by PCR-restriction
fragment length polymorphism (RFLP). It was shown that the
frequency of the GG genotype in the intestinal mucosa of patients
with FBD was significantly higher than that in healthy controls
(66.67 versus 40.91%, P<0.05). A similar tendency was observed
in the G allele type (81.11 versus 63.63%, P<0.05). The
frequency of the AA genotype in patients with FBD was significantly
lower than that in healthy controls (4.44 versus 13.6%, P<0.05).
There were no significant differences in the frequency of the AG
genotype and the A allele type between patients and controls (28.89
versus 45.45%, 18.89 versus 36.36%, both P>0.05).
The frequencies of different chymase genotypes in
subtypes of FBD were further analyzed. It was observed that the
frequency of the G allele type in the intestinal mucosa of the C4
subgroup was significantly higher than that in controls. However,
in other subgroups, there was no difference between patients and
controls (Table III).
 | Table IIIFrequencies of chymase genotypes. |
Table III
Frequencies of chymase genotypes.
| | Genotype (%) | Allele (%) |
|---|
| |
|
|
|---|
| Category | Cases (n) | AA | AG | GG | A | G |
|---|
| Control | 44 | 6 (13.63) | 20 (45.45) | 18 (40.91) | 32 (36.36) | 56 (63.63) |
| FBD | 45 | 2 (4.44)a | 13 (28.89) | 30 (66.67)a | 17 (18.89) | 73 (81.11)a |
| C1 subgroup | 8 | 1 (12.50) | 1 (12.50) | 6 (75.00) | 3 (18.75) | 13 (81.25) |
| C2 subgroup | 10 | 1 (10.00) | 3 (30.00) | 6 (60.00) | 5 (25.00) | 15 (75.00) |
| C3 subgroup | 14 | 0 (0.00) | 5 (35.71) | 9 (64.29) | 5 (17.86) | 23 (82.14) |
| C4 subgroup | 13 | 0 (0.00) | 4 (30.77) | 9 (69.23) | 4 (15.38) | 22 (84.62)a |
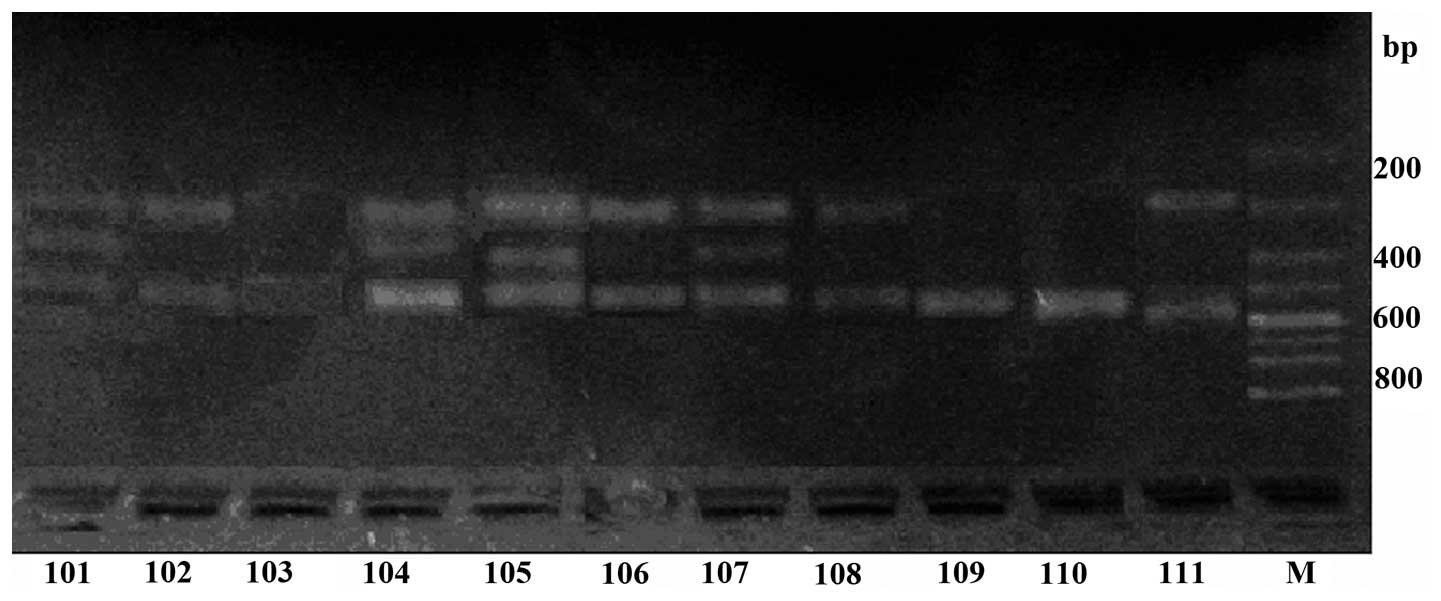
The results of the electrophoresis are shown in
Fig. 2. Samples in lanes 101–103
were from healthy controls, while samples in lanes 104–11 were from
patients with FBD. The genotype of lanes 101, 104, 105 and 107 was
AG heterozygote, while lanes 109 and 110 were AA homozygote and
lanes 102, 103, 106, 108 and 111 were GG homozygote. The density of
the lanes from patients with FBD was higher than the control lanes.
The chymase G allele was observed as two bands at 467 and 186 bp,
the AA homozygous genotype as one band at 654 bp, and the AG
heterozygous genotype as three bands at 654, 467 and 186 bp
(Fig. 2).
Discussion
FBD is common in the elderly and often seriously
affects quality of life. The molecular mechanism of neuroendocrine
system abnormalities in FBD has become a particular focus of study
(12). The effects of brain-gut
peptides on the intestinal tract partly account for the pathology
of FBD.
Chymase is a non-ACE that functions in the
conversion of Ang I to Ang II in the non-circulating ACE pathway.
Chymase is frequently present in secretion granules of mast cells,
which are widely distributed in the intestinal mucosa, blood
vessels, heart and other tissues (13). In our previous study, elevated
chymase levels were observed in patients with FBD (14). Intestinal polyps are usually
detected in FBD. In the present study, IHC staining was performed
to determine the expression of chymase in mast cells of
inflammatory bowel polyps and healthy mucosa. It was observed that,
in inflammatory polyps, there was a significantly greater number of
chymase-positive mast cells than in controls. These results
suggested a close correlation between chymase levels and
proliferation of inflammatory polyps.
In recent years, the correlation between mast and
interstitial cells has raised particular concern. Chymase-producing
mast cells are the precursors of interstitial cells. Therefore,
with the proliferation of intestinal mucosa, the number of mast
cells increases. In response to inflammatory stimuli, mast cells
degranulate and chymase accumulates in intestinal tissues. The
increased chymase converts Ang I into Ang II, and Ang II induces
functional disorder of smooth muscle in the intestinal tract, which
leads to the exacerbation of FBD (15,16).
The present study showed that chymase was
overexpressed in inflammatory polyps at the mRNA and protein level.
In an earlier study, we demonstrated that elevated chymase levels
associated with hypertension induced target organ damage, including
myocardial hypertrophy, modest elevated creatinine levels and
microalbuminuria (3). Accumulation
of chymase may also induce microvascular lesions in the intestinal
tract, which may stimulate neuroendocrine cells to degranulate and
cause functional disorder.
Currently available literature indicates that RAS
dysfunction in abdominal sympathetic ganglia and the central
nervous system is important in the pathogenesis of FBD (17). RAS overexpression in
non-circulating tissues and its hyperactivity may stimulate
angiogenesis, thus increasing the proliferation of polyps, and this
may induce gene mutation and the development of malignancy
(18).
Chymase has been demonstrated to be a potential
target in the blockade of organ damage. Chymase inhibitors have
shown efficacy in the intervention of aortic aneurysm, diabetic
retinopathy, cardiac dysfunction and fibrosis. In gastrointestinal
diseases, administration of dextran sulfate sodium (DSS) to mice
yielded a significant increase in chymase activity (19–22).
Thus, data from the present and previous studies suggest that
chymase is involved in intestinal inflammatory diseases and that it
may be a potential therapeutic target for patients with FBD.
References
|
1
|
Keating E, Lemos C, Monteiro R, Azevedo I
and Martel F: The effect of a series of organic cations upon the
plasmalemmal serotonin transporter, SERT. Life Sci. 76:103–119.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mykletun A, Heradstveit O, Eriksen K, et
al: Health anxiety and disability pension award: The HUSK Study.
Psychosom Med. 71:353–360. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ji HZ, Wu XW, Xu XB, et al: The
correlation between functional bowel disorder in the elderly and
hypertensive target organ damage. Chin J Prev Contr Chron Dis.
15:365–366. 2007.(In Chinese).
|
|
4
|
Corleto VD, Pagnini C, Cattaruzza MS, et
al: Is proliferative colonic disease presentation changing? World J
Gastroenterol. 18:6614–6619. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu X, Cokkinides V, Chen VW, et al:
Associations of subsite-specific colorectal cancer incidence rates
and stage of disease at diagnosis with county-level poverty, by
race and sex. Cancer. 107:1121–1127. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Andoh A, Deguchi Y, Inatomi O, et al:
Immunohistochemical study of chymase-positive mast cells in
inflammatory bowel disease. Oncol Rep. 16:103–107. 2006.PubMed/NCBI
|
|
7
|
Daemen MJ, Lombardi DM, Bosman FT and
Schwartz SM: Angiotensin II induces smooth muscle cell
proliferation in the normal and injured rat arterial wall. Circ
Res. 68:450–456. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maltby S, Khazaie K and McNagny KM: Mast
cells in tumor growth: angiogenesis, tissue remodelling and
immune-modulation. Biochim Biophys Acta. 1796:19–26.
2009.PubMed/NCBI
|
|
9
|
He SH: Key role of mast cells and their
major secretory products in inflammatory bowel disease. World J
Gastroenterol. 10:309–318. 2004.PubMed/NCBI
|
|
10
|
Drossman DA: The functional
gastrointestinal disorders and the Rome II process. Gut. 45(Suppl
2): II1–II5. 1999.PubMed/NCBI
|
|
11
|
Du W, Ji H, Cao S, et al: EpCAM: a
potential antimetastatic target for gastric cancer. Dig Dis Sci.
55:2165–2171. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Drossman DA: The functional
gastrointestinal disorders and the Rome III process.
Gastroenterology. 130:1377–1390. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takai S, Jin D, Sakaguchi M and Miyazaki
M: Chymase-dependent angiotensin II formation in human vascular
tissue. Circulation. 100:654–658. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu XW, Sun Q, Ji HZ, et al: Chymase
genenic polymorphism of elderly patients with functional bowel
disorders. Chin J Gerontol. 30:158–160. 2010.
|
|
15
|
Matsuzuka T, Miller K, Pickel L, Doi C,
Ayuzawa R and Tamura M: The synergistic induction of
cyclooxygenase-2 in lung fibroblasts by angiotensin II and
pro-inflammatory cytokines. Mol Cell Biochem. 320:163–171. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Riaz AA, Wang Y, Schramm R, et al: Role of
angiotensin II in ischemia/reperfusion-induced
leukocyte-endothelium interactions in the colon. FASEB J.
18:881–883. 2004.PubMed/NCBI
|
|
17
|
Mohan M, Jaiswal BS and Kasture S: Effect
of Solanum torvum on blood pressure and metabolic alterations in
fructose hypertensive rats. Ethnopharmacol. 126:86–89. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu XB and Ji HZ: Anti-tumor activity of
angiotensin-converting-enzyme inhibitors. J Med Postgraduates.
21:325–329. 2008.
|
|
19
|
Urata H: Pathological involvement of
chymase-dependent angiotensin II formation in the development of
cardiovascular disease. J Renin Angiotensin Aldosterone Syst. 1(2
Suppl): S35–S37. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jin D, Takai S, Yamada M, et al: Impact of
chymase inhibitor on cardiac function and survival after myocardial
infarction. Cardiovasc Res. 60:413–420. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kakimoto K, Takai S, Murano M, et al:
Significance of chymase-dependent matrix metalloproteinase-9
activation on indomethacin-induced small intestinal damages in
rats. J Pharmacol Exp Ther. 332:684–689. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takai S, Jin D and Miyazaki M: New
approaches to blockade of the renin-angiotensin-aldosterone system:
chymase as an important target to prevent organ damage. J Pharmacol
Sci. 113:301–309. 2010. View Article : Google Scholar : PubMed/NCBI
|