Introduction
The primary treatment for hemorrhagic shock is to
control the source of bleeding as quickly as possible and to
replace fluid (1). In controlled
hemorrhagic shock (CHS), where the source of bleeding has been
occluded, fluid replacement is aimed towards the normalization of
hemodynamic parameters. In uncontrolled hemorrhagic shock (UCHS),
in which the bleeding has temporarily stopped as a result of
hypotension, vasoconstriction and clot formation, the aim of fluid
treatment is to restore a radial pulse, restore the sensorium or
maintain a blood pressure of 80 mmHg with aliquots of 250 ml
Ringer’s lactate (RL) solution (hypotensive resuscitation)
(2).
When the expected evacuation time is <1 h
(usually urban trauma), it is necessary to immediately evacuate the
patient to a surgical facility, once the airway and breathing have
been secured (3); the introduction
of an intravenous line wastes time. When the expected evacuation
time is >1 h, an intravenous line is introduced and fluid
treatment is initiated prior to evacuation. The resuscitation must
occur prior to, or concurrently with, any diagnostic studies
(4).
In patients with hemorrhagic shock, hypertonic
saline has the theoretical benefit of increasing intravascular
volume with only small volumes of fluid (5). The combination of dextran and
hypertonic saline may be beneficial in situations where the
infusion of large volumes of fluid has the potential to be harmful,
such as in elderly individuals with impaired cardiac activity
(6). However, additional trials
are required before this combination is accepted as a standard of
care.
There are recognized risks involved with the
transfusion of large quantities of concentrated red blood cells
(CRBCs) (7). As a result,
alternative modalities are being investigated. One such modality is
hemoglobin-based oxygen carriers (HBOCs). The clinical application
of the HBOCs has been limited by the toxic effect profile. However,
investigations are ongoing into the use of these products (8–10).
Hemorrhagic shock is a common acute and critical
illness, and the complication and mortality rates are high
(11). The treatment of
hemorrhagic shock necessitates the removal of the cause as soon as
possible. In addition, timely and effective fluid resuscitation is
important (12), in order to
improve the oxygen supply to the tissues, and restore the oxygen
supply-demand balance and normal cell function. It has been shown
that when crystal and colloid droplets are titrated to the same
level of filling pressure, they are able to restore tissue
perfusion to the same extent (13). However, it has not been elucidated
whether the effects of different types of fluid resuscitation on
the potential morphological and functional injuries to liver cells
during hemorrhagic shock are the same. Apoptosis is a significant
form of cell death following ischemia-reperfusion injury. To a
certain extent, mitochondrial damage promotes apoptosis. Succinate
dehydrogenase (SDH) is an important functional enzyme in the
mitochondrial respiratory chain, and measuring the activity of SDH
is a method that indirectly reflects the mitochondrial oxidative
phosphorylation activity. The reduction of mitochondrial membrane
potential (ΔΨm) is considered to be an irreversible event in early
apoptosis (14), which occurs
before the morphological and biochemical changes in the apoptotic
cells. Therefore, inhibiting the reduction in membrane potential
may prevent apoptosis.
In the present study a model of hemorrhagic shock
was induced in rats, in order to assess the effects of different
types of fluid resuscitation on mitochondrial ultrastructure, ΔΨm,
SDH activity and liver cell function in rat liver cells. In
addition, the development and progression of hemorrhagic shock and
the pathophysiological changes in hepatic mitochondria were
studied, in order to elucidate the mechanisms underlying the
protective effects of different types of fluid resuscitation on
liver cells. This may provide an important theoretical basis for
clinical treatment.
Materials and methods
Animals and grouping
Forty healthy, adult, male Wistar rats, which were
supplied by the Shihezi University Laboratory Animal Center
(Shihezi, China), were randomly divided into five groups: i) Sham
surgery (Sham group, n=8); ii) shock (Shock group, n=8); iii) RL
resuscitation (RL group, n=8); iv) hydroxyethyl starch (HES)
resuscitation (HES group, n=8); and v) autologous blood
resuscitation (BL group, n=8). A comparison of the weights of the
rats in the five groups did not reveal any statistically
significant differences. This study was performed in strict
accordance with the recommendations in the Guide for the Care and
Use of Laboratory Animals of the National Institutes of Health (8th
edition, 2011). The animal use protocol was reviewed and approved
by the Institutional Animal Care and Use Committee (IACUC) of
Shihezi University.
Induction of hemorrhagic shock
All animal experiments were performed in accordance
with the National Research Council Ethical Guidelines for the use
of animals in and with standard operating procedures (SOP). The
anesthetized rats were fixed, the left common carotid artery was
isolated, carotid occlusion, proximal, distal occlusion, proximal
and distal occlusion in the use of 24G intravenous catheter at the
arterial cannulation fixed, the entire pipeline system precharge of
heparin saline, carotid artery catheter connected pressure
transducer, monitors, continuous hemodynamic monitoring and blood.
Similarly the right external jugular vein was isolated for the
infusion. Intermittent bleeding until blood pressure stabilized,
the rat’s mean arterial blood pressure fell to 40 mmHg, after 1 h
shock, group via the right external jugular vein for fluid
resuscitation.
Recovery program
In the Sham group, only the insertion of the
arterial catheter was performed, without the bleeding. The Shock
group received no fluid resuscitation. Following successful
modeling, in the RL group, Ringer’s lactate was infused; in the HES
group, the rats were infused with HES 130/0.6 in a sodium chloride
injection (Fresenius Kabi Deutschland Gmbh, Beijing, China); in the
BL group, the rats were infused with autologous blood, following
anticoagulant treatment, and were then infused with Ringer’s
lactate. The recovery objective was to maintain the mean arterial
pressure (MAP) of the rats at 80 mmHg. Two hours subsequent to the
end of the recovery experiment, the rats were sacrificed.
Specimen collection and observation
methods
Morphological changes
Samples of fresh liver tissue, measuring ~1
mm3, were fixed with 2.5% glutaraldehyde, prior to being
dehydrated, embedded, sliced, double-stained and cut into ultrathin
sections. Following this, the sections were examined under an
electron microscope and the morphological changes in the
mitochondria were observed.
SDH activity
The liver tissue was homogenized in 0.25 mol/l
sucrose solution, pH 7.5. Following this, the homogenate was
centrifuged at 1,142 × g for 15 min, prior to the supernatant
undergoing further centrifugation at 11,282 × g for 10 min. The
mitochondria from the precipitate were subsequently suspended in
isolation medium and frozen at −20°C. Twenty four hours subsequent
to defrosting, the SDH activity was assessed, in accordance with
the kit’s instructions (Kaiji Biological Technology development
Co., Ltd., Nanjing, China).
Membrane potential
The liver cells (50 μl/100 mg) were suspended in 500
μl JC-1 working solution (Haimen BiYunTian Biotechnology Research
Institute, Nantong, China), and a confocal laser microscope (ZEISS
LSM510; Germany) was used to observe the changes in membrane
potential. In the JC-1 solution there were 50 μl/100 mg cells
suspended. The mitochondrial extraction kit was purchased from
Haimen BiYunTian Biotechnology Research Institute (number: C3606).
The mitochondrial membrane potential detection kit (JC-1) was
purchased from Haimen BiYunTian Biotechnology Research Institute
(number: C2006). The laser confocal microscope (ZEISS LSM510) was
purchased from Carl Zeiss (Thornwood, NY, USA).
Pathological changes
The liver tissue was treated using conventional
methods to produce paraffin sections, which were then stained with
hematoxylin and eosin (H&E). Following this, the pathological
changes were observed. The pathological and histological changes
were observed under light and the liver tissue pathology integral
was calculated.
Apoptosis
A terminal deoxynucleotidyl transferase-mediated
dUTP nick end labeling (TUNEL) assay (Haimen BiYunTian
Biotechnology Research Institute; number C1008) was used to assess
the apoptosis of the liver cells. The apoptosis index (AI) was
recorded under a light microscope, and apoptotic cells were
observed under a fluorescence microscope (CHK optical microscope;
Olympus, Tokyo, Japan). The AI was calculated by randomly selecting
10 high-magnification views (x400) under the microscope and, for
each high-magnification field of vision, calculating the number of
cells positive for apoptosis, per 100 cells. The AI value was
expressed as a percentage of positive cells.
Statistical analysis
SPSS version 16.0 statistical software (SPSS, Inc.,
Chicago, IL, USA) was used for the statistical analysis. All
measurement data are expressed as the mean ± standard deviation
(SD). The overall comparison was performed using analysis of
variance (ANOVA), while two samples were compared using the
Student-Newman-Keuls (SNK-q) test. The incidence of adverse
reactions was compared using the Fisher’s exact probabilities test.
The semi-quantitative determination of multiple independent samples
(class variables) was performed using the rank sum test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Electron microscopy
Sham group liver tissue
Liver cell blood sinus, the bile duct surface was
rich in microvilli and the tight junctions connecting the surface
structures were clear. In addition, the membrane structure was
normal and the dense cytoplasm was observed to be rich in
mitochondria and well organized. The developed endoplasmic
reticulum was shown to be rich in glycogen, with few lysosomes and
occasional lipid droplets. The nuclei were round and rich in finely
granular chromatin.
Shock group liver tissue
Liver cell blood sinus, the bile duct surface was
rich in microvilli and the tight junctions connecting the surface
structures were clear. The membrane structure was observed to be
normal; however, although the dense cytoplasm was rich in
mitochondria, there was an increased volume and a decreased number
of mitochondria, and degeneration was apparent. The developed
endoplasmic reticulum was rich in glycogen, with few lysosomes;
however, there was a slightly increased presence of lipid droplets
compared with the Sham group. Rounded nuclei were observed, with
fine granular chromatin (Fig.
1A).
RL group liver tissue
Liver cell blood sinus, the bile duct surface was
rich in microvilli and the tight junctions connecting the surface
structures were evident. The membrane structure was normal and the
dense cytoplasm was rich in mitochondria. Furthermore, the volume
increase was partially attenuated and the degeneration of the
mitochondrial cristae was observed to have disappeared. The
developed endoplasmic reticulum was glycogen rich, with few
lysosomes and it was observed that there was an increased presence
of lipid droplets compared with the Sham group. The nuclei were
round and rich in finely granular chromatin. The vacuolar area
without cell structures showed an uneven distribution of the
mitochondrial matrix condensation and there was an uneven
distribution of organelles. In addition, a reduction in the number
of ribosomes on the rough endoplasmic reticulum, and nucleolar
margination was observed, with a greater number of nucleoli
(Fig. 1B).
Colloid group (HES) liver tissue
Liver cell blood sinus, the bile duct surface was
rich in microvilli and the tight junctions connecting the surface
structures were clearly visible. The membrane structure was normal,
the cytoplasm of mitochondrial centralized, a form of dissolved
swelling, and no mitochondrial cristae were present. In the
developed endoplasmic reticulum there was an abundance of glycogen,
few lysosomes and a significant increase in the number of lipid
droplets. The nuclei were round, finely granular and chromatin rich
(Fig. 1C).
BL group liver tissue
Examination under a microscope revealed a good liver
cell morphology, sinusoid surface, and a bile duct surface rich in
microvilli. The tight junctions connecting the surface structures
were evident. In addition, the membrane structure was normal and
the cytoplasm was significantly increased in volume. Furthermore,
the deformation of the mitochondria and the degeneration of the
mitochondrial cristae were reduced. There was a reduction in the
endoplasmic reticulum, glycogen is rich, few lysosomes and a
markedly increased presence of lipid droplets. The nuclei were
round and rich in finely granular chromatin.
Hepatic mitochondrial SDH
Significant differences in the specific activity of
mitochondrial SDH were observed between certain groups of rats
(P<0.05). The specific activity of SDH in the RL and HES groups
was significantly different compared with the Sham group
(P<0.05). In the BL group, the activity was reduced compared
with the Sham group; however, the difference was not statistically
significant (P>0.05). The difference between the BL and Shock
groups was significant (P<0.05); furthermore, compared with the
BL group, the specific activity of SDH in the mitochondria was
significantly different in the RL and HES groups (P<0.05).
However, there was no significant difference between the RL and HES
groups (P>0.05; Table I).
 | Table IEffect of different types of fluid
resuscitation on hepatic mitochondrial succinate dehydrogenase
(SDH). |
Table I
Effect of different types of fluid
resuscitation on hepatic mitochondrial succinate dehydrogenase
(SDH).
| Group | No. of cases | Enzyme-specific
activity (U/mg protein) |
|---|
| Sham | 8 | 167.88±82.49 |
| Shock | 8 | 11.29±18.40a |
| RL | 8 | 29.90±13.29a,b |
| HES | 8 | 33.27±12.13a,b |
| BL | 8 | 84.70±47.70c |
| F-value | | 2.754 |
| P-value | | 0.045 |
Hepatic ΔΨm
With regard to the ΔΨm of the liver cells, there
were statistically significant differences in the RL and HES groups
compared with the Sham group (P<0.05). The ΔΨm was reduced in
the BL group compared with the Sham group; however, the difference
was not statistically significant (P>0.05). There were
significant differences in the ΔΨm of the BL and HES groups
compared with the Shock group (P<0.05). With regard to the ΔΨm
of the three different fluid resuscitation groups, the ΔΨm of the
BL and HES groups was significantly different from that of the RL
group (P<0.05). No significant difference was observed between
the ΔΨm of the BL and HES groups (P>0.05; Table II and Fig. 2)
 | Table IIEffect of fluid resuscitation on
hepatic mitochondrial membrane potential. |
Table II
Effect of fluid resuscitation on
hepatic mitochondrial membrane potential.
| Group | No. of cases | Membrane potential
(red/green) |
|---|
| Sham | 27 | 1.3271±0.6243 |
| Shock | 16 |
0.1519±0.1230a |
| RL | 31 |
0.2816±0.1941a |
| HES | 25 |
0.7786±0.3784a-c |
| BL | 15 |
0.8646±0.3222b |
| F-value | | 6.631 |
| P-value | | 0.000 |
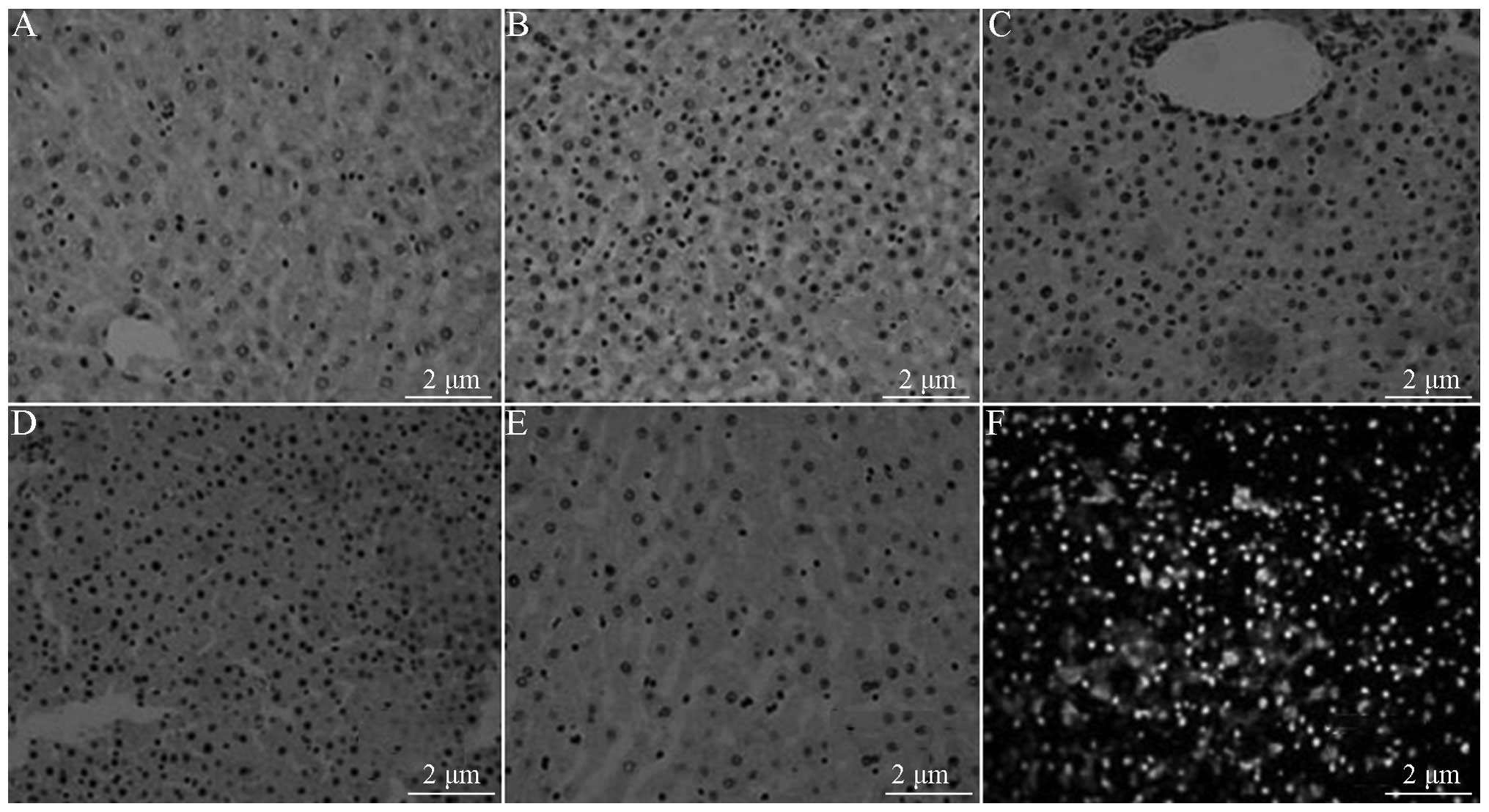
H&E staining
Sham group liver tissue
The microscopic examination revealed complete
hepatic lobules, with polygonal hepatic cells arranged radially
around a central vein, and visible sinusoids neatly arranged into
hepatic cords. The basic structure of the hepatic lobule portal
area was clearly visible, with no inflammatory cell infiltration
(Fig. 3A).
Shock group liver tissue
The structural integrity of the hepatic lobules was
observed. Low-magnification light microscopy revealed staining of
the liver cells, with extensive hydropic degeneration, some
congestion of the lobular central vein and sinusoids, vascular
dilatation and congestion of the portal area. In addition, the
centrilobular portion of the liver denatured, in particular the
subcapsule was significant (Fig.
3B).
RL group liver tissue
Light microscopy revealed lobular structural
integrity, water from the central part of lobule degeneration, the
lesion was significantly under the capsule. Vascular dilatation and
congestion were observed, in addition to hydropic degeneration of
the liver cells and some liver cell necrosis. Furthermore, there
was sinusoidal expansion and the infiltration of inflammatory
cells, mainly neutrophils (PMNs), under the capsule (Fig. 3C).
Colloid group (HES) liver tissue
Light microscopy revealed lobular structural
integrity and mild congestion of the central vein and sinusoids. In
addition, the focal liver cells were lightly stained. Furthermore,
partial liver cell that is nearly subcapsule hydropic degeneration,
focal hepatic sinus expansion (Fig.
3D).
BL group liver tissue
Light microscopy showed the structural integrity of
the hepatic lobules. In the central area of the lobule there was
focal hydropic degeneration of the liver cells, in addition to a
small amount of subcapsular hydropic degeneration of the liver
cells (Fig. 3E).
Cell AI
The experimental results revealed that there was a
small number of weakly colored positive cells in the Sham group and
a large number of deeply colored apoptotic cells in the Shock
group. In the recovery groups, it was observed that there was also
an increased number of apoptotic cells, mainly around the central
vein in the hepatic lobules. In addition, inflammatory necrosis was
present in the portal area. The single-factor ANOVA was F=15.755,
P=0.000 (P<0.05), which may be considered as the difference
between the five groups in general. The pairwise comparison results
indicated that the minimum AI was in the Sham group (4.29±3.73),
while the highest AI was in the Shock group (42.75±16.42); compared
with the three recovery groups, the AIs in the Shock group were
statistically significant (P<0.05). With regard to the
comparisons among the three different fluid resuscitation group,
the AI for the RL (26.63±11.54) and the HES (26.50±8.47) groups was
significantly higher than that of BL group (15.25±5.97)
(P<0.05). No significant difference was identified between the
RL and HES groups (P>0.05; Table
III).
 | Table IIITUNEL assay results for the liver
cells in each group. |
Table III
TUNEL assay results for the liver
cells in each group.
| Group | n | AI |
|---|
| Sham | 8 | 4.29±3.73a |
| Shock | 8 | 42.75±16.42b |
| RL | 8 | 26.63±11.54b,c |
| HES | 8 | 26.50±8.47b,c |
| BL | 8 | 15.25±5.97b |
| F-value | | 15.755 |
| P-value | | 0.000 |
TUNEL assay
TUNEL-positive apoptotic cells showed small
condensed nuclei and a circumscribed nuclear membrane, as the
nucleus was stained brown.
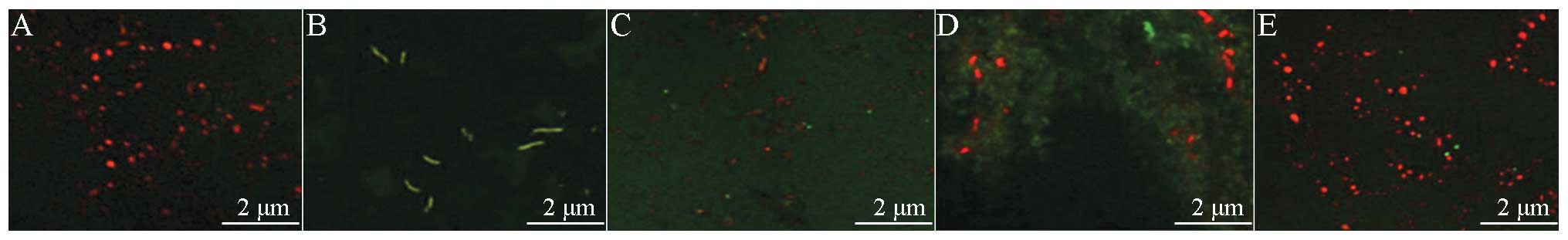
Sham group liver tissue
Under the microscope, a small number of
TUNEL-positive apoptotic cells (4.95±5.06)% were observed to be
scattered in the liver tissue (Fig.
4A).
Shock group liver tissue
When the liver cells were observed under the
microscope, extensive hydropic degeneration and a large number of
TUNEL-positive apoptotic cells scattered in the liver tissue were
observed. Inflammation and necrosis were apparent in a banded and
concentrated zone in the portal area. The AI was 73.13±10.51%,
which was increased significantly compared with that in the Sham
group (Fig. 4B).
RL group liver tissue
Under the microscope, an increased number of
TUNEL-positive apoptotic cells were observed to be scattered around
the central vein and portal area. The AI was 41.88±18.17%, which
was significantly less than that of the Shock group (Fig. 4C).
Colloid group (HES) liver tissue
Under the microscope, TUNEL-positive apoptotic cells
scattered around the blood vessels of the portal area were
frequently observed. The AI was 40.25±9.92%, which was
significantly reduced compared with that in the Shock group and
significantly greater compared with that in the BL group (Fig. 4D).
BL group liver tissue
Under the microscope, it was observed that a small
number of TUNEL-positive apoptotic cells (4.95±5.06%) were
scattered in the liver tissue. Compared with the Shock, RL and HES
groups, the number of TUNEL-positive apoptotic cells was
significantly reduced (Fig.
4E).
Fluorescence microscopy of apoptotic
cells in liver tissue
Under a fluorescence microscope, normal cells were
stained with light green fluorescence and apoptotic cells were
stained yellow-green fluorescence. The apoptotic cells showed
nuclear enrichment, nuclear fragmentation and apoptotic bodies, in
addition to other withered morphological characteristics of
apoptosis (Fig. 4F).
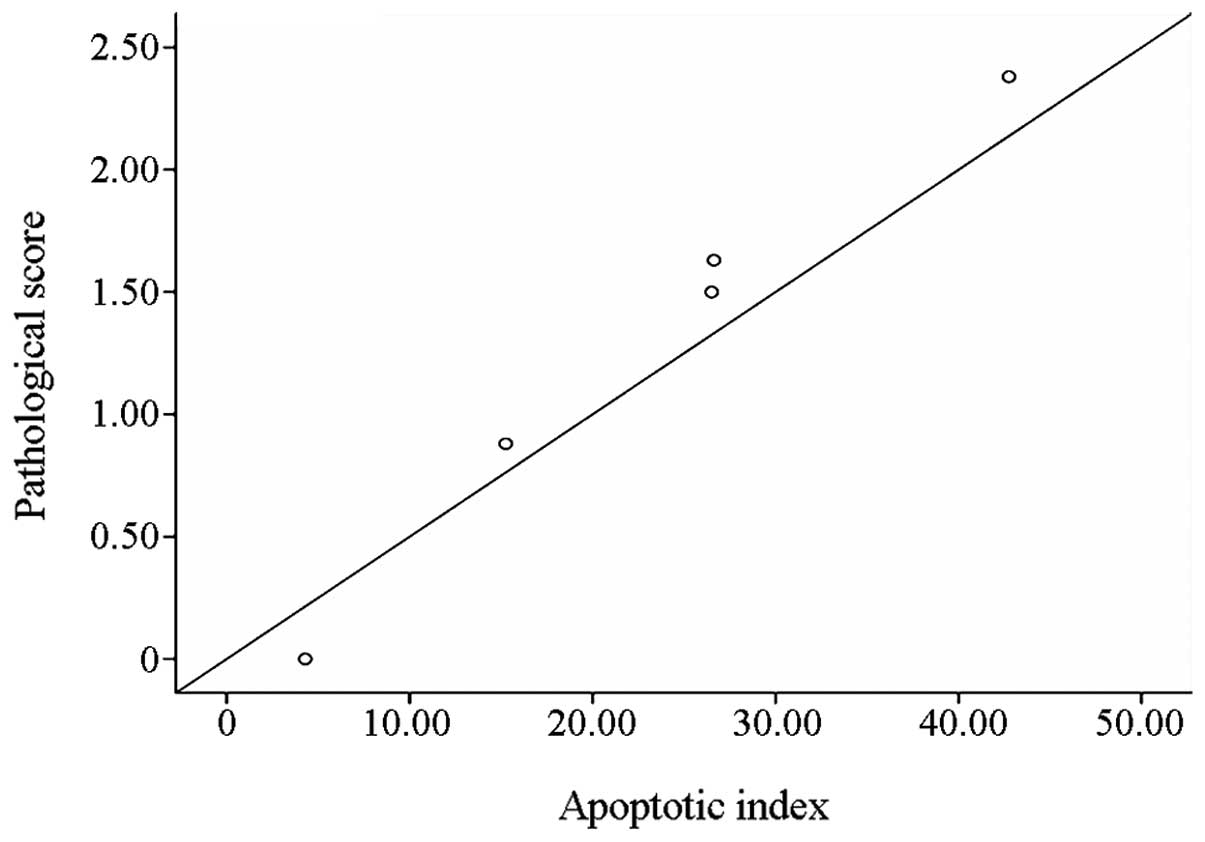
Correlation analysis
A linear correlation plot was drawn to show the
correlation between the liver cell AI and the pathological changes.
This is shown in Fig. 5.
Discussion
In hepatic ischemia-reperfusion, hypoxia-ischemia
leads to the production of a large number of oxygen free radicals.
The increased presence of oxygen radicals damages the mitochondrial
membrane, inhibits membrane protein function and damages
nucleotides and other macromolecules, thus undermining the
structure and function of the mitochondria (15). The electron microscopy results
showed that in the three fluid resuscitation groups, RL, HES and
BL, the hepatic mitochondrial injury and the degree of dissolution
were reduced, and the amount of damage to the mitochondria was
gradually reduced. Resuscitation with HES and blood has been shown
to inhibit the body’s inflammatory response, reduce the liver cell
and mitochondrial damage following the recovery of rats from
hemorrhagic shock and reduce the degree of injury from hepatic
ischemia-reperfusion; the recovery following blood resuscitation
has been shown to be better than that with HES (16,17).
With regard to the changes in mitochondrial morphology, the
recovery following resuscitation with autologous blood appears to
be better than the recovery following RL and HES resuscitation.
SDH is an enzyme in the mitochondrial respiratory
chain. Measuring the activity of SDH indirectly reflects the
vitality of the mitochondria (18). The present study showed that there
were significant differences between the specific activity of the
liver mitochondria SDH in the rats of certain groups. The
activities in the Shock, RL and HES groups were significantly lower
than that in the Sham group. The SDH activity in the BL group was
reduced compared with that in the Sham group; however, the
difference was not statistically significant. The mitochondrial
activity was higher in the BL group than those in the other two
resuscitation groups, indicating that the damage to mitochondrial
function and the liver ischemia-reperfusion injury was lower in the
BL group than that in the other two resuscitation groups.
ΔΨm is formed from the asymmetric distribution of
electrons on the two sides of the mitochondrial membrane. The low
permeability of the mitochondrial inner membrane and the
electrochemical proton gradient is the basis for maintaining the
membrane potential (19). Studies
have demonstrated that in the early stages of apoptosis, prior to
the onset of pathological changes in the nucleus, the ΔΨm is
reduced, suggesting that a decreased ΔΨm is an early stage of
apoptosis (17,20,21).
The inhibition of the decline in ΔΨm may prevent the occurrence of
apoptosis, indicating that cell ΔΨm changes are changes specific to
apoptosis (22).
In the present study, it was shown that the hepatic
ΔΨm was significantly lower in the Shock, RL and HES groups than in
the Sham group. However, although the ΔΨm was reduced in the BL
group compared with that in the Sham group, the difference was not
statistically significant. The ΔΨm in the BL group was higher than
that in the RL and HES groups. The reduction in the ΔΨm of the BL
group was smaller than that in the other two groups. It has been
suggested that the reduction in ΔΨm is irreversible in early
apoptotic events (23,24). With regard to the inhibition of the
reduction in transmembrane potential, compared with RL and HES,
blood was the ideal recovery liquid and was better able to prevent
apoptosis, the reduction in mitochondrial function and the level of
liver ischemia-reperfusion injury.
Following traumatic hemorrhagic shock, PMNs and
monocytes-macrophages are activated, leading to a broad
inflammatory cascade in the body, which is considered to be an
important factor leading to injury. The present study showed that
with regard to the pathological changes in the liver, the BL group
exhibited the minimum pathological liver injury score out of the
three recovery groups. The differences between the score in the BL
group and the scores in the RL and HES groups, respectively, were
statistically significant (P<0.05). This may be due to the fact
that RL and HES are only able to increase the circulating blood
volume in the body and improve the tissue perfusion in shock: RL
and HES do not exhibit an oxygen-carrying capacity, unlike blood
resuscitation. As a result of this, RL and HES are not able to
significantly attenuate the tissue hypoxia that occurs in shock or
reduce the pathological changes to the liver induced by
hypoxia.
The results indicated that the AI in the RL group
was higher than that in the BL group and significantly lower than
that in the Sham group (P<0.05). This result was consistent with
the results in the study by Murao et al(25). With regard to the effect of RL
solution and HES resuscitation on apoptosis, studies have shown
that, compared with plasma, whole blood or saline resuscitation,
the application of RL solution or HES resuscitation for hemorrhagic
shock in rats may significantly increase apoptosis in the liver and
small intestine or the lung (25,26).
These results were consistent with the results of the present
study, in which the AIs of the HES and RL groups were significantly
higher than that of the BL group. This may be associated with the
fact that the recovery of PMN oxidative burst activity was
significantly enhanced (27).
However, in the Scultetus recovery with fresh whole blood,
increased activation of PMNs was not observed (28). This result was consistent with the
fact that the AI for blood resuscitation was the lowest. In the
recovery process, the strength of the neutrophil activation is
dependent on infusion quantity and speed during the resuscitation
(29). PMN as an important
effector cells in inflammatory reaction, either in quantity or the
content of cytotoxic substance in intra-cytoplasmic in a core
position. Although it may kill pathogens by swallow, respiratory
burst and degranulation. However, the uncontrolled release of
inflammatory mediators may cause systemic inflammatory response
syndrome, causing tissue and organ damage and eventually leading to
multiple organ dysfunction syndrome. Therefore, PMNs are considered
a ‘double-edged sword’ (30),
PMN-induced apoptosis occurs mainly through the release of tumor
necrosis factor (TNF)-α, interferon (IFN)-7 and interleukin
(31), which promotes the
increased expression of receptors involved in apoptosis, leading to
the apoptosis of hepatocytes (32).
The present study showed that hepatocyte apoptosis
and the pathological changes in the liver tissue were positively
correlated (r=0.977). This indicated that, in the same group a high
liver AI was associated with serious pathological liver injury,
although there was some variation in the spatial distribution of
the apoptosis and pathological damage. Apoptotic cells more
commonly appeared in the lobule around the central veins. With the
occurrence of peripheral ischemia in the Shock group, a large
number of apoptotic cells were also observed in the hepatic lobule
and portal area. A possible explanation for this phenomenon may be
that the liver cells around the central vein undergo oxygen
exchange, so the apoptosis in this area was connected with hypoxia
that caused by so poor blood infusion in pathological
conditions.
In this study, how the three types of fluid
resuscitation led to apoptosis and why the AIs were different was
not elucidated. We propose that it may have been associated with
the composition of the resuscitation fluids and the different body
stress responses caused by the recovery. Therefore, whereas hepatic
ischemia-reperfusion directly leads to apoptosis, the recovery of
the cells following RL and HES resuscitation activates a large
number of mechanisms and factors associated with apoptosis, unlike
blood recovery, which has little effect on these related factors.
The specific factors that may induce apoptosis are as follows: i)
Recovery of the body following the loss of a large amount of blood
causes the body to produce large numbers of oxygen free radicals,
which may result in cell apoptosis; ii) blood loss and recovery
cause damage to mitochondrial morphology and function, which may
stimulate apoptosis; iii) in the blood loss and recovery stress
condition, the cells activate self-protective response mechanisms,
and endoplasmic reticular stress activates the apoptosis system;
iv) following blood loss, hypoxia leads to a decreased production
of ATP, and the occurrence of calcium overload also induces
apoptosis; and v) recovery of the body following blood loss may
lead to the production of a large number of cytokines, and these
cytokines may be involved in apoptosis. These factors do not occur
singly, but in numerous interactions.
In conclusion, fluid resuscitation following
hemorrhagic shock is a complex process. To achieve a recovery
effect, it is necessary to perform a comprehensive analysis and a
correct diagnosis, in order to avoid complications. The results of
this study, with regard to the ultrastructure of the tissue, may be
used to evaluate ischemia-reperfusion injury. In the future these
results are likely to provide an important reference for fluid
resuscitation in hemorrhagic shock.
Acknowledgements
The authors would like to express their gratitude to
all laboratory staff for their help during this study.
Abbreviations:
|
RL
|
Ringer’s lactate
|
|
HES
|
hydroxyethyl starch
|
|
BL
|
blood resuscitation
|
|
MAP
|
mean arterial pressure
|
|
TUNEL
|
TdT-mediated dUTP nick end
labeling
|
|
SDH
|
succinate dehydrogenase
|
|
SOP
|
standard operating procedures
|
|
AI
|
apoptosis index
|
|
ΔΨm
|
mitochondrial transmembrane
potential
|
|
PMNs
|
neutrophils
|
|
TNF
|
tumor necrosis factor
|
|
IFN
|
interferon
|
|
ATP
|
adenosine triphosphate
|
References
|
1
|
Helling H, Schenk HJ, Pindur G, Weinrich
M, Wagner B and Stephan B: Fibrinolytic and procoagulant activity
in septic and haemorrhagic shock. Clin Hemorheol Microcirc.
45:295–300. 2010.PubMed/NCBI
|
|
2
|
Junger WG, Rhind SG, Rizoli SB, et al:
Resuscitation of traumatic hemorrhagic shock patients with
hypertonic saline-without dextran-inhibits neutrophil and
endothelial cell activation. Shock. 38:341–350. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Spinella PC and Holcomb JB: Resuscitation
and transfusion principles for traumatic hemorrhagic shock. Blood
Rev. 23:231–240. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Narasaki R, Xu Z, Liang Z, et al: The
vitronectin-binding domain of plasminogen activator inhibitor-1
plays an important functional role in lipopolysaccharide-induced
lethality in mice. J Thromb Haemost. 10:2618–2621. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brotfain E, Leibowitz A, Dar DE, et al:
Severe traumatic brain injury and controlled hemorrhage in rats:
quest for the optimal mean arterial blood pressure after whole
fresh donor blood resuscitation. Shock. 38:630–634. 2012.
View Article : Google Scholar
|
|
6
|
Jin G, DeMoya MA, Duggan M, et al:
Traumatic brain injury and hemorrhagic shock: evaluation of
different resuscitation strategies in a large animal model of
combined insults. Shock. 38:49–56. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brockman EC, Bayır H, Blasiole B, et al:
Polynitroxylated-pegylated hemoglobin attenuates fluid requirements
and brain edema in combined traumatic brain injury plus hemorrhagic
shock in mice. J Cereb Blood Flow Metab. 33:1457–1464. 2013.
View Article : Google Scholar
|
|
8
|
White NJ, Wang X, Bradbury N, et al: Fluid
resuscitation of uncontrolled hemorrhage using a hemoglobin-based
oxygen carrier: effect of traumatic brain injury. Shock.
39:210–219. 2013.PubMed/NCBI
|
|
9
|
Blasiole B, Bayr H, Vagni VA, et al:
Effect of hyperoxia on resuscitation of experimental combined
traumatic brain injury and hemorrhagic shock in mice.
Anesthesiology. 118:649–663. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jin G, Duggan M, Imam A, et al:
Pharmacologic resuscitation for hemorrhagic shock combined with
traumatic brain injury. J Trauma Acute Care Surg. 73:1461–1470.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Subeq YM, Hsu BG, Lin NT, et al:
Hypothermia caused by slow and limited-volume fluid resuscitation
decreases organ damage by hemorrhagic shock. Cytokine. 60:68–75.
2012.PubMed/NCBI
|
|
12
|
Calzia E, Huber-Lang M, Ignatius A,
Radermacher P and Thiemermann AC: Modeling traumatic-hemorrhagic
shock - nothing is simple and easy. Shock. 38:685–686. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bennetts P, Shen QH, Thimmesch A, Clancy R
and Pierce J: Effects of ubiquinol on reactive oxygen species and
cellular injury in rats following hemorrhagic shock and fluid
resuscitation. Critical Care Medicine (Congress abstracts).
40:5382012.
|
|
14
|
Legrand M, Mik EG, Balestra GM, et al:
Fluid resuscitation does not improve renal oxygenation during
hemorrhagic shock in rats. Anesthesiology. 112:119–127. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Taguchi K, Ogaki S, Watanabe H, et al:
Fluid resuscitation with hemoglobin vesicles prevents
Escherichia coli growth via complement activation in a
hemorrhagic shock rat model. J Pharmacol Exp Ther. 337:201–208.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Morrison CA, Carrick MM, Norman MA, et al:
Hypotensive resuscitation strategy reduces transfusion requirements
and severe postoperative coagulopathy in trauma patients with
hemorrhagic shock: preliminary results of a randomized controlled
trial. J Trauma. 70:652–663. 2011. View Article : Google Scholar
|
|
17
|
Hussmann B, Lefering R, Taeger G, Waydhas
C and Ruchholtz S: Influence of prehospital fluid resuscitation on
patients with multiple injuries in hemorrhagic shock in patients
from the DGU trauma registry. J Emerg Trauma Shock. 4:465–471.
2011.PubMed/NCBI
|
|
18
|
Flaherty DC, Hoxha B, Sun J, et al:
Pyruvate-fortified fluid resuscitation improves hemodynamic
stability while suppressing systemic inflammation and myocardial
oxidative stress after hemorrhagic shock. Mil Med. 175:166–172.
2010. View Article : Google Scholar
|
|
19
|
Yamamoto M, Horinouchi H, Kobayashi K, et
al: Fluid resuscitation of hemorrhagic shock with hemoglobin
vesicles in beagle dogs: pilot study. Artif Cells Blood Substit
Immobil Biotechnol. 40:179–195. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Messmer C, Yalcin O, Palmer AF and
Cabrales P: Small-volume resuscitation from hemorrhagic shock with
polymerized human serum albumin. Am J Emerg Med. 30:1336–1346.
2012.PubMed/NCBI
|
|
21
|
Maier S, Holz-Hölzl C, Pajk W, et al:
Microcirculatory parameters after isotonic and hypertonic colloidal
fluid resuscitation in acute hemorrhagic shock. J Trauma.
66:337–345. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Balbino M, Capone Neto A, Prist R,
Ferreira AT and Poli-de-Figueiredo LF: Fluid resuscitation with
isotonic or hypertonic saline solution avoids intraneural calcium
influx after traumatic brain injury associated with hemorrhagic
shock. J Trauma. 68:859–864. 2010. View Article : Google Scholar
|
|
23
|
Santry H and Alam H: Fluid resuscitation:
past, present, and the future. Shock. 33:229–241. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
White NJ, Martin EJ, Brophy DF and Ward
KR: Coagulopathy and traumatic shock: characterizing hemostatic
function during the critical period prior to fluid resuscitation.
Resuscitation. 81:111–116. 2010. View Article : Google Scholar
|
|
25
|
Murao Y, Isayama K, Saito F, et al: Effect
of hypertonic saline resuscitation on CD4+CD25+ regulatory T cells
and gammadelta T cells after hemorrhagic shock and resuscitation in
relation to apoptosis and iNOS. J Trauma. 67:975–982. 2009.
|
|
26
|
Nishi K, Takasu A, Shinozaki H, Yamamoto Y
and Sakamoto T: Hemodilution as a result of aggressive fluid
resuscitation aggravates coagulopathy in a rat model of
uncontrolled hemorrhagic shock. J Trauma Acute Care Surg.
74:808–812. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pierce JD, Knight AR, Slusser JG, Gajewski
BJ and Clancy RL: Effects of fluid resuscitation and dopamine on
diaphragm performance, hydrogen peroxide, and apoptosis following
hemorrhagic shock in a rat model. Mil Med. 176:336–342. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Scultetus A, Arnaud F, Kaplan L, et al:
Hemoglobin-based oxygen carrier (HBOC-201) and escalating doses of
recombinant factor VIIa (rFVIIa) as a novel pre-hospital
resuscitation fluid in a swine model of severe uncontrolled
hemorrhage. Artif Cells Blood Substit Immobil Biotechnol. 39:59–68.
2011. View Article : Google Scholar
|
|
29
|
Drabek T, Kochanek PM, Stezoski J, et al:
Intravenous hydrogen sulfide does not induce hypothermia or improve
survival from hemorrhagic shock in pigs. Shock. 35:67–73. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Duke MD, Guidry C, Guice J, et al:
Restrictive fluid resuscitation in combination with damage control
resuscitation: time for adaptation. J Trauma Acute Care Surg.
73:674–678. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guerci P, Tran N, Menu P, Losser MR,
Meistelman C and Longrois D: Impact of fluid resuscitation with
hypertonic-hydroxyethyl starch versus lactated ringer on
hemorheology and microcirculation in hemorrhagic shock. Clin
Hemorheol Microcirc. Dec 27–2012.(Epub ahead of print).
|
|
32
|
Seishi Y, Horinouchi H, Sakai H and
Kobayashi K: Effect of the cellular-type artificial oxygen carrier
hemoglobin vesicle as a resuscitative fluid for prehospital
treatment: experiments in a rat uncontrolled hemorrhagic shock
model. Shock. 38:153–158. 2012. View Article : Google Scholar
|