Introduction
Polyacrylamide hydrogel (PAHG) has been widely used
for injection augmentation mammaplasty in Russia, China and Iran
for more than two decades (1).
Although numerous studies have indicated that PAHG injection for
soft-tissue augmentation leads to a good result (1,2), a
number of other studies have reported high complication rates
following the use of PAHG. Reported adverse effects associated with
PAHG injection for breast augmentation include indurations, lumps,
hematoma, inflammation, infection, persistent mastodynia, poor
cosmetic results, glandular atrophy, gel migration, loss of ability
to breastfeed and delayed diagnosis of breast cancer (1,3–8).
Since April 2006, when the China State Food and Drug
Administration announced that PAHG was prohibited from production
and clinical application in plastic surgery, significant social
concern was raised concerning the use of PAHG injections as soft
tissue fillers. It was estimated that ~200,000 patients have
received PAHG breast augmentation in the last decade (1). Between 2005 and 2012, 407 patients
came to the Plastic Surgery Hospital (Beijing, China) for PAHG
removal due to indurations, lumps, hematoma, inflammation,
infection, persistent mastodynia, poor cosmetic results, gel
migration or loss of ability to breastfeed. Removal of PAHG often
leads to immediate postoperative breast deformity and a significant
challenge facing plastic surgeons involves the restoration of a
pair of aesthetically pleasing breasts, in terms of fullness and
shape. Implant insertion is an effective option for selected
patients. However, the majority of patients underwent the PAHG
injections in small clinics and often did not receive information
concerning the PAHG, including the volume. Therefore, the selection
of suitable implants has been difficult.
It is vital to estimate the precise volume and depth
of the PAHG injected for breast augmentation. It is not only a
significant factor in the preoperative evaluation of breast
augmentation, but may also directly affect the postoperative shape
of the breast. Therefore, a reliable method for calculating the
volume of the injected PAHG is required. Magnetic resonance imaging
(MRI) scans are commonly used to analyze the position of the
injected PAHG. Therefore, the development of a rapid and precise
method of estimating the volume of PAHG on the basis of MRI scans
would be of benefit. The purpose of the present study was to define
a volume measurement method. In addition, the reliability and
precision of the volume measurement method were monitored.
Materials and methods
Between 2005 and 2012, 407 patients underwent PAHG
removal breast surgery in the Department of Aesthetic and Plastic
Breast Surgery of the Plastic Surgery Hospital. Each patient
underwent breast MRI pre-operatively. Ten patients that had never
had breast surgery prior to the study were randomly selected and
enrolled in the study. Clinical characteristics of the patients are
shown in Table I. Patient age
ranged between 25 and 46 years (mean, 34.1 years). The time between
the initial PAHG injection to the removal of the PAHG ranged
between 2 and 10 years (mean, 6.6 years). Complications included
palpable indurations, masses, pain and psychological problems. The
study was approved by the institutional review board of Plastic
Surgery Hospital of Peking Union Medical College. Prior to breast
MRI, informed consents were obtained either from the patients or
the patients’ family.
 | Table IClinical characteristics of the
patients. |
Table I
Clinical characteristics of the
patients.
| Patient | Age, years | Duration of PAHG,
years | Complications |
|---|
| 1 | 32 | 9 | Pain |
| 2 | 45 | 5 | Pain and
hardness |
| 3 | 46 | 5 | Pain and
indurations |
| 4 | 38 | 10 | Psychological
problems |
| 5 | 31 | 9 | Psychological
problems |
| 6 | 29 | 7 | Indurations |
| 7 | 26 | 7 | Pain, indurations and
psychological problems |
| 8 | 39 | 8 | Indurations |
| 9 | 30 | 2 | Psychological
problems |
| 10 | 25 | 4 | Masses and
psychological problems |
All patients were scanned prior to the operative
procedures by MRI. A 1.5-T MRI scanner (Siemens Magnetom Vision;
Siemens AG, Munich, Germany) with dedicated breast coils was used
for the imaging. The standard protocol included axial T2-weighted
images with and without fat depression and sagittal T1- and
T2-weighted images with fat depression. The DICOM images of the MRI
scans were imported into Mimics software (Materialise Company,
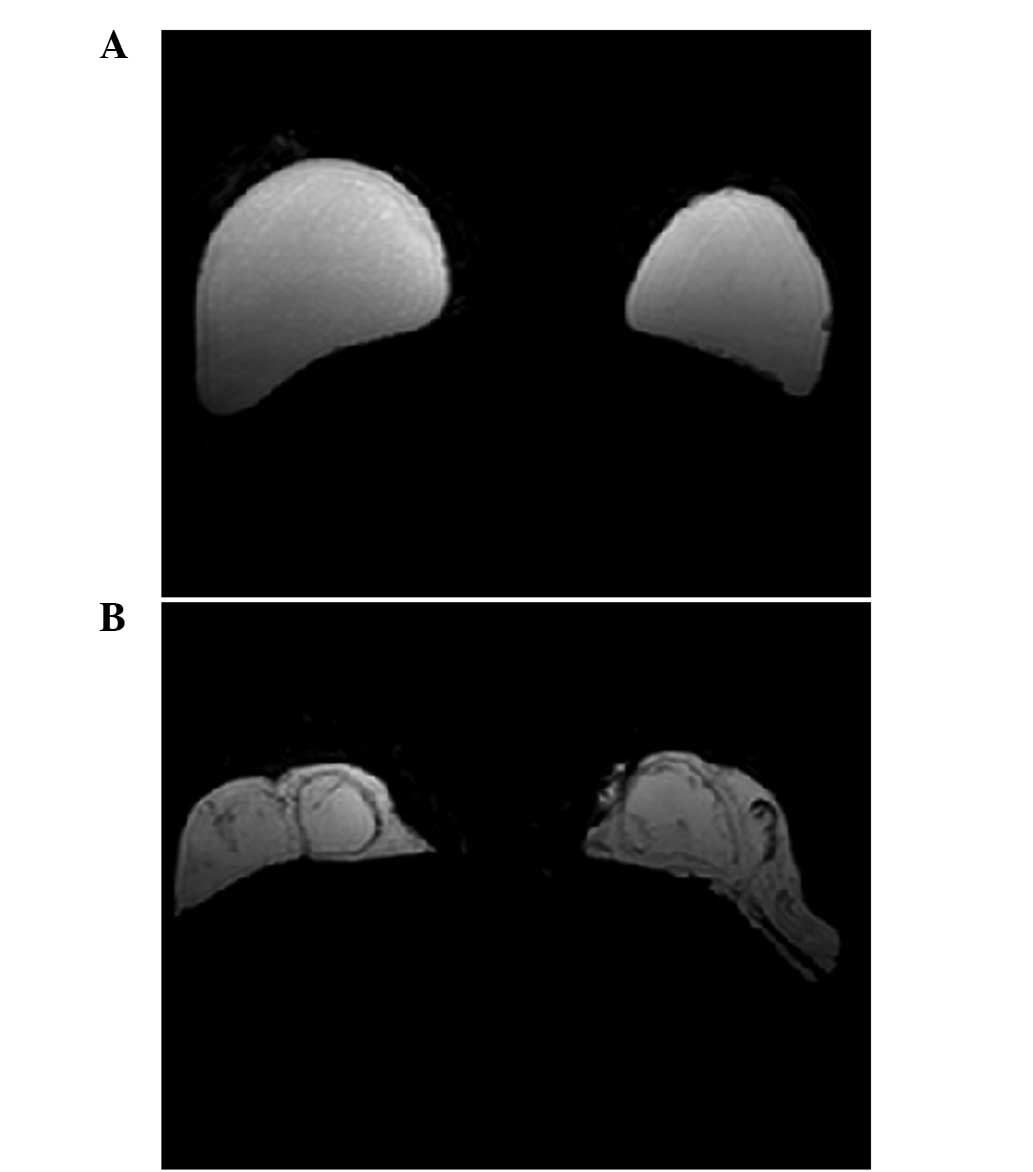
Leuven, Belgium). The axial T2-weighted images with fat suppression
(Fig. 1) were used for the
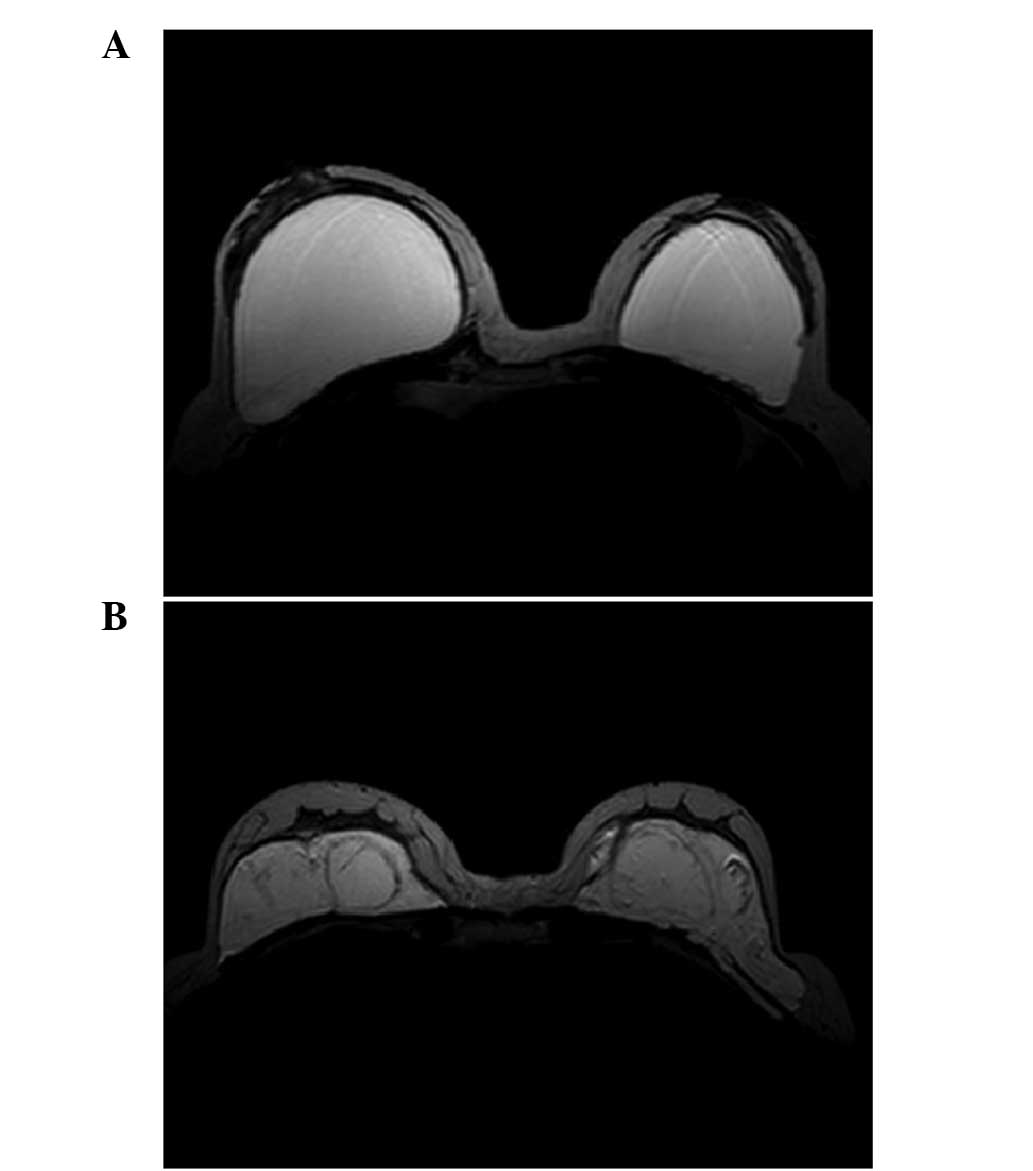
reconstruction. On the basis of imaging without fat suppression
(Fig. 2), a suitable threshold was
selected to label the PAHG. It was necessary to ensure that the
colorization threshold was adjusted to label as much of the
injected PAHG as possible, while coloring little or no areas around
the PAHG. As PAHG has a bright signal, similar to that of water, it
may be mistaken for vessels. Based on the T2-weighted imaging
without fat suppression, the mislabeled vessels were excluded.
Following the imaging, a 3-D reconstruction was performed and the
volume of PAHG was calculated using Mimics software.
Three plastic surgeons independently carried out the
volumetric measurement. Each patient was measured ten times by
three independent plastic surgeons. Calculated PAHG volume data are
presented in Table II. The
volumes of PAHG injected for breast augmentation were analyzed to
calculate the intra- and inter-observer correlation coefficients.
Analysis of variance with repeated measurement was performed to
analyze inter-observer variance using a global significance level
of P<0.05. SPSS version 16.0 for Windows was used for
statistical analysis (SPSS, Inc., Chicago IL, USA).
 | Table IICalculated volumes of PAHG in ml, mean
± SD. Each patient was measured ten times by three independent
plastic surgeons. |
Table II
Calculated volumes of PAHG in ml, mean
± SD. Each patient was measured ten times by three independent
plastic surgeons.
| Parameters | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | Patient 10 |
|---|
| Surgeon 1 | 417.80±3.80 | 551.24±3.51 | 320.57±2.56 | 265.99±3.15 | 376.45±3.83 | 450.07±3.77 | 499.68±4.23 | 496.69±3.37 | 615.84±5.18 | 881.74±4.52 |
| Surgeon 2 | 417.65±2.62 | 552.53±1.41 | 316.23±1.24 | 270.15±2.37 | 374.48±3.67 | 452.84±5.35 | 497.97±2.21 | 495.58±4.20 | 602.49±3.61 | 891.81±3.61 |
| Surgeon 3 | 414.13±3.44 | 544.64±3.13 | 319.04±2.87 | 262.74±2.42 | 373.95±3.77 | 452.92±5.20 | 505.33±2.76 | 508.23±6.75 | 603.57±7.74 | 881.08±4.67 |
| Mean ± SD | 416.53±2.08 | 549.47±4.23 | 318.61±2.20 | 266.29±3.71 | 374.96±1.32 | 451.94±1.62 | 500.99±3.85 | 500.17±7.01 | 607.30±7.42 | 884.88±6.01 |
PAHG was injected in different layers and
distributed differently in different patients. In patient 8, PAHG
was injected into the subglandular space and distributed regularly
(Fig. 1A and 2A). In patient 1, PAHG infiltrated into
the surrounding tissue and was distributed irregularly (Fig. 1B and 2B).
Results
Calculated PAHG volume data are presented in
Table II. The mean volumes of
PAHG injected for breast augmentation ranged between 266.29 and
884.88 ml. No significant differences in the calculated volume of
injected PAHG were identified among the three observers (P=0.173).
The intra-observer correlation coefficient was 0.964, which
indicated high measurement precision and observer independency.
Discussion
The purpose of the present study was to determine a
method for precisely measuring the volumes of injected PAHG. The
results demonstrate that the volume of the injected material may be
precisely calculated, which is significant for the preoperative
evaluation of patients prior to the removal of PAHG. Information
concerning the distribution of the injected PAHG and the extent of
the tissue infiltration was obtained. It was therefore possible to
estimate the difficulty and the risks involved in the removal of
the PAHG, as well as ensuring the feasibility of immediate breast
augmentation and the postoperative shape of the breast. On the
basis of the estimated volume, appropriately sized implants may be
selected prior to breast augmentation, following the removal of the
PAHG. However, there are specific restrictive indications for
immediate breast shape repair. Luo et al considered the
indications were as follows: Absence of breast neoplasm and
infection; removal of >90% of the injected gel; no residual
hydrogel in pectoral muscles and/or the subpectoral space; enough
healthy mammary tissue and pectoral muscle present to cover the
breast prostheses; inframammary folds are intact or are able to be
reconstructed simultaneously; and no systemic or psychological
problems (9). The present study
highlighted additional rules that should be followed. Firstly, the
cavity containing the injected PAHG should not be larger than the
pocket of the breast implant. Secondly, there should be no residual
hydrogel during the closure of the lacunar.
The measurement method may also be used to calculate
the volume of the residual hydrogel. The effectiveness of various
approaches for the removal of PAHG from the breast may be evaluated
on the basis of the estimated volume of residual hydrogel.
There have been few studies analyzing volume
measurement of PAHG. However, several studies have discussed the
volume estimation of silicone gel-filled breast implants from MRI
images (8–12). Previously, Rudolph and Forcier
reported a volume measurement method. The authors used MRI plus
computer-assisted detection to perform volume calculations
(13), which made the calculations
more rapid and accurate. However, PAHG breast implants differ from
those that are usually confined to capsules. The filler material
has been shown to migrate easily, due to muscular activity or the
effect of gravity, particularly when the capsules are broken by
incorrect massage or incidental force (14). Therefore, it is likely that PAHG
implants result in a number of complications, including pain,
infection, masses, breast disfigurement and distant migration. The
PAHG may also infiltrate into the surrounding tissues and cause
degeneration. All traits of PAHG lead to its irregular
distribution, which makes the volume measurement more complex. Sun
et al have reported a method using a 3-D MRI reconstruction
technique to determine the volume and distribution of the PAHG
(15). However, the study mainly
focused on investigating an effective diagnostic method and did not
analyze the precision of the measurement method.
In the present study, it was not possible to compare
the calculated volumes of injected PAHG with the actual volumes,
since information concerning the preoperatively injected PAHG
volumes was not available. In addition, it was not possible to
compare these volumes with the volume of the removed materials.
Firstly, since PAHG is a hydrophilic filler material, some of the
material may be easily aspirated following saline dilution. During
the surgical procedure, the cavity was repeatedly irrigated with a
large quantity of normal saline intraoperatively. Therefore, the
amount of the removed PAHG may not be fully consistent with the
estimated volume of PAHG. Secondly, the PAHG, as well as the
degenerated tissue, were removed intraoperatively leading to the
volume of all excised tissue being inconsistent with the estimated
volume of PAHG. Thirdly, some of the PAHG may have been injected
into another area, including the subcutaneous or intercostal
muscles. In consideration of the intraoperative safety and the
postoperative shape of the breast, it may not be possible to remove
the PAHG completely. This also caused the volume of the materials
removed to differ from the calculated volume of PAHG.
In conclusion, MRI imaging offers a precise method
for the volumetric measurement of injected PAHG. This is
significant for pre- and post-operative evaluation and the
selection of implants for immediate reconstruction.
References
|
1
|
Wang ZX, Luo DL, Dai X, Yu P, Tao L and Li
SR: Polyacrylamide hydrogel injection for augmentation mammaplasty:
loss of ability for breastfeeding. Ann Plast Surg. 69:123–128.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Christensen LH, Breiting BV, Aasted A,
Jørgensen A and Kebuladze I: Long-term effects of polyacrylamide
hydrogel on human breast tissue. Plast Reconstr Surg.
111:1883–1890. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Acartürk S, Gencel E and Tuncer I: An
uncommon complication of secondary augmentation mammoplasty:
bilaterally massive engorgement of breasts after pregnancy
attributable to postinfection and blockage of mammary ducts.
Aesthetic Plast Surg. 29:274–279; discussion 280, 2005.
|
|
4
|
Cheng NX, Wang YL, Wang JH, Zhang XM and
Zhong H: Complications of breast augmentation with injected
hydrophilic polyacrylamide gel. Aesthetic Plast Surg. 26:375–382.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cheng NX, Liu LG, Hui L, Chen YL and Xu
SL: Breast cancer following augmentation mammaplasty with
polyacrylamide hydrogel (PAAG) injection. Aesthetic Plast Surg.
33:563–569. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Leung KM, Yeoh GP and Chan KW: Breast
pathology in complications associated with polyacrylamide hydrogel
(PAAG) mammoplasty. Hong Kong Med J. 13:137–140. 2007.PubMed/NCBI
|
|
7
|
Khan UD: Breast autoinflation with sterile
pus as a marker of implant rupture: single-stage treatment and
outcome for five consecutive cases. Aesthetic Plast Surg. 33:58–65.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nachbar JM and Orrison WW Jr: Validation
of quantification of breast implant capsule surface area and volume
using magnetic resonance imaging. Ann Plast Surg. 27:321–326.
1991.PubMed/NCBI
|
|
9
|
Luo SK, Cheng GP, Sun ZS and Cheng NX: Our
strategy in complication management of augmentation mammaplasty
with polyacrylamide hydrogel injection in 235 patients. J Plast
Reconstr Aesthet Surg. 64:731–737. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Akbas H, Sahin B, Eroglu L, et al:
Estimation of breast prosthesis volume by the Cavalieri principle
using magnetic resonance images. Aesthetic Plast Surg. 28:275–280.
2004.PubMed/NCBI
|
|
11
|
Mineyev M, Kramer D, Kaufman L, Carlson J
and Frankel S: Measurement of breast implant volume with magnetic
resonance imaging. Ann Plast Surg. 34:348–351. 1995.PubMed/NCBI
|
|
12
|
Tuncali D and Ozgür F: Spontaneous
autoinflation of saline-filled mammary implants: postoperative
volume determination by magnetic resonance imaging. Aesthetic Plast
Surg. 23:437–442. 1999.
|
|
13
|
Rudolph R and Forcier N: Calculation of
silicone breast implant volumes using breast magnetic resonance
imaging. Aesthetic Surg J. 29:310–313. 2009.PubMed/NCBI
|
|
14
|
Cheng NX, Xu SL, Deng H, Ding XB, Zhang
XM, Wu DH, Zhong H and Sun ZH: Migration of implants: a problem
with injectable polyacrylamide gel in aesthetic plastic surgery.
Aesthetic Plast Surg. 30:215–225. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun JM, Yuan Q, Guo K, Guo NQ, Peng C,
Zhang Y and Wang JC: 3-Dimensional reconstruction of MRI in
patients with polyacrylamide hydrogel injection for augmentation
mammoplasty. Zhonghua Zheng Xing Wai Ke Za Zhi. 24:371–373.
2008.(In Chinese).
|