Introduction
Cerebral ischaemia is caused by a number of factors,
including advanced age, hypertension, previous stroke or transient
ischaemic attack and cardiac arrest (1). It predominantly results in neuronal
apoptosis or neuronal death in the brain regions that are most
intrinsically vulnerable, including the CA1 region of the
hippocampus and the striatum (2,3). The
extent of brain damage is determined by the severity of the primary
injury and the intensity of the secondary injury cascades that
contribute to delayed cellular destruction (4).
Following the onset of ischaemia, oxygen and glucose
supply is interrupted and this causes cell mortality cascades,
resulting in the breakdown of the blood-brain barrier and cerebral
oedema (5). Brain oedema is one of
the most common causes of disability and mortality in patients
suffering from ischaemia (6).
Reperfusion that occurs following focal cerebral ischaemia
exacerbates brain swelling (7).
Ischaemic oedema is possibly initiated by Na+ influx
associated with energy failure. Higher osmolarity conditions induce
water influx into the cells, resulting in ionic oedema (8). This early oedema phase or cytotoxic
oedema may last for several hours prior to the leakage of large
volumes of water in the brain, resulting in vasogenic oedema
(9). Adverse brain oedema further
reduces the blood flow supplying the neurons, causing irreversible
apoptosis (10). Studies have
shown that the expression of aquaporin-4 (AQP4) is upregulated
following cerebral ischaemic injuries (11,12).
AQP4 functions in the formation of cerebral oedema and neuronal
mortality. Caspase-3 is one of the most critical downstream
apoptotic proteases in the caspase cascade ‘waterfall’ and affects
neuronal apoptosis (13). Numerous
extracellular signals activate caspase-8 and caspase-9 in cerebral
ischaemia-reperfusion (I/R) injury. These signals induce caspase-3
to hydrolyse cell-specific proteins and poly (ADP-ribose)
polymerase to induce apoptosis (14).
The overexpression of AQP4 and caspase-3 aggravates
neural damage following cerebral ischaemic injury. The expression
of AQP4 and caspase-3 in brain tissue may be altered by
administering an effective drug to relieve cerebral ischaemia. For
instance, lactuside B (LB) is a single compound extracted and
isolated from the root of Pterocypsela elata, which grows in
Tongbai County (Henan, China). In our previous study, LB was
observed to reduce brain infarct volume and increase the bcl-2/bax
mRNA ratio of the cerebral cortex which has an anti-apoptotic
effect on nerve cells in rats (15). The hippocampus and the striatum are
sensitive areas affected during cerebral ischaemic injury. In the
present study, the levels of AQP4 and caspase-3 were evaluated. To
study the mechanism by which LB protects against cerebral
ischaemia, the effects of LB on AQP4 and caspase-3 mRNA expression
in the hippocampus and striatum of rats were investigated.
Materials and methods
Animals
Adult male Sprague-Dawley rats (280–320 g; clean
grade II) were purchased from the Henan Experimental Animal Centre
(Zhengzhou, China; certification no., SYXK Henan 2005–0012). All
rats were maintained at a controlled temperature (23±1°C) with a
12-h dark/light cycle and free access to water and food. The rats
were cared for in accordance with the guidelines for the treatment
of experimental animals published by the Ministry of Science and
Technology of the People’s Republic of China in 2006. This study
was carried out in strict accordance with the recommendations in
the Guide for the Care and Use of Laboratory Animals of the
National Institutes of Health (8th version, 2010). The animal use
instructions were reviewed and approved by the Institutional Animal
Care and Use Committee of Xinxiang Medical University (Xinxiang,
China).
LB
The LB used in this study was provided by the
Department of Medicinal Chemistry (Xinxiang Medical University). LB
was isolated and purified from 8 kg P. elata grown in
Tongbai County. LB is a white amorphous water-soluble and
chemically stable powder with a purity of >99%.
Cerebral ischaemia establishment
Focal cerebral ischaemia was induced in the rats by
intraluminally occluding the middle cerebral artery (MCA) as
described by Longa et al (16). The blocking line, a fishing line of
size 1.5 with a diameter of 0.2 mm (DaDong Yang, Zhejiang, China),
was inserted into the entry point of the MCA from the external
carotid arteries (ECAs) via the bifurcation of the common carotid
artery and the internal carotid artery (ICA). The line was
continuously inserted until the 2.0-cm mark was reached. In the
sham surgery group, the lines were inserted into the ICA until the
0.5-cm mark was reached and the remaining surgical procedures were
the same as those in the other groups. Following blockage of the
arterial flow for 2 h, the lines were withdrawn from the ECAs to
allow brain reperfusion. The rats were then returned to their cages
and closely monitored. Once the rats had regained consciousness
from anaesthesia, they were evaluated for their neurological
behavior at various times according to the method described by
Longa et al (16). The rats
with scores from one to four were considered successful models.
Neural functional defects were evaluated prior to the rats in each
group being sacrificed. A high score indicated the highest severity
of neural functional defect.
Grouping and administration of drugs
A total of 112 rats were randomly divided into five
groups: sham surgery; cerebral I/R (I/R); I/R + LB 12.5 mg/kg (I/R
+ LL); I/R + LB 25 mg/kg (I/R + LM); and I/R + LB 50 mg/kg (I/R +
LH). The sham surgery group comprised 16 rats. The remaining four
groups comprised 24 rats each. All rats in the sham surgery group
survived. In the cerebral ischaemic models, the rat survival rate
was 70–80%. Once reperfusion was established, all rats were
intraperitoneally injected with 5 ml/kg/day of the corresponding
drug. The rats in the sham surgery and model (I/R) groups were also
treated with normal saline. The animals in the I/R + LL, I/R + LM
and I/R + LH groups were treated with 12.5, 25 and 50 mg/kg LB,
respectively. From each group, eight rats were sacrificed 24 h
following treatment and used to determine the brain water content.
The remaining animals from each group were sacrificed at 24 and 72
h. AQP4 and caspase-3 mRNA expression levels in the hippocampus and
the striatum were detected by reverse transcription polymerase
chain reaction (RT-PCR).
Brain water content
Rats were sacrificed at 24 h following focal
cerebral ischaemia and their brains were immediately removed. The
brain water content was determined as described by Young et
al (17). A neutral filter
paper was used to absorb and remove bloodstains from the brain. The
wet weight of each right hemisphere was measured using an FA2004
chemical balance (Shanghai Liangping Instrument Co., Ltd.,
Shanghai, China) within 90 sec of isolation. Next, the brain was
dried in an oven at 110°C for 15 h and the dry weight was obtained.
The water content of the brain was calculated using the following
equation: Brain water content = (wet weight − dry weight)/wet
weight × 100%.
RT-PCR
RT-PCR was performed to determine the AQP4 and
caspase-3 mRNA expression levels in the hippocampus and the
striatum. Following sacrifice of the rats, the hippocampus and
striatum were obtained and ground in liquid nitrogen. Total RNA was
extracted using TRIzol reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA) and transcribed to cDNA using a PrimeScript™
RT-PCR kit [Takara Biotechnology (Dalian) Co., Ltd., Dalian,
China], in which 1 μl cDNA was used as a template for amplification
and 50 μl PCR solution was used. The PCR conditions for AQP4 were
set as follows: 95°C for 5 min; 30 cycles of 95°C for 30 sec, 59°C
for 30 sec, 72°C for 1 min; and 72°C for 10 min. For caspase-3, the
annealing temperature was 54°C and the other conditions were the
same as those for AQP4. The PCR products were separated on a 2%
agarose gel [Gene tech (Shanghai) Co., Ltd., Shanghai, China] and
images of the bands were captured by photography using a Tocan 240
gel imaging system (Tocan Biotechnology Co., Shanghai, China). The
greyscale images of each band were detected using Quantity One
image analysis software (Bio-Rad, Hercules, CA, USA). The
quantities of each PCR product were normalised by dividing the
average grey level of the signal by the average grey level of the
corresponding β-actin amplicon. These quantities were determined as
a semi-quantitative value of the target fragments. The PCR-specific
primers of AQP4 (330 bp) and caspase-3 (282 bp), as well as the
internal reference primers of β-actin (208 bp), were designed. For
AQP4, the upstream and downstream primers were
5′-GGGTTGGACCAATCATAGGCGCT-3′ and 5′-GCAGGAAATCTGAGGCCAGTTCTAGG-3′,
respectively. For caspase-3, the upstream and downstream primers
were 5′-ACGGTACGCGAAGAAAAGTGAC-3′ and 5′-TCCTGACTTCGTATTTCAGGGC-3′,
respectively. For β-actin, the upstream and downstream primers were
5′-CCTTCCTGGGCATGGAGTCCTG-3′ and 5′-GGAGCAATGATCTTGATCTTC-3′,
respectively.
Statistical analysis
Data are presented as mean ± SD. SPSS 17.0 (SPSS,
Inc., Chicago, IL, USA) and Excel (Microsoft, Redmond, WA, USA)
software were used for statistical analysis. Data were analysed by
ANOVA and the Student-Newman-Keuls post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Neurological deficit score
Rats in the sham surgery group showed normal
activities without any neurological deficit symptoms once
consciousness had been regained. Neurological deficits were
evaluated and scored at 24 and 72 h following the treatments.
Table I shows that the
neurological deficit scores were significantly higher in the I/R
injury group (24 and 72 h following drug administration, 3.45±0.80
and 3.26±0.62, respectively) than in the sham surgery group
(P<0.01). The groups intraperitoneally injected with LB at doses
of 12.5, 25 and 50 mg/kg showed decreased neurological deficit
scores (24 h following drug administration, 2.60±0.52, 2.23±0.43
and 2.18±0.42, respectively; 72 h following drug administration,
2.45±0.26, 1.88±0.33 and 1.59±0.29, respectively) compared with
those in the I/R injury group (P<0.05). The neurological deficit
scores of the groups treated with 25 and 50 mg/kg LB at 72 h were
significantly lower than those of the groups treated at 24 h
(1.88±0.33 and 1.59±0.29; P<0.05). LB decreased the neurological
deficit score of the rats following brain I/R injury. This result
was evident in the groups treated with 25 and 50 mg/kg LB at 72
h.
 | Table IEffects of LB on neurological deficits
scores following I/R injury in rats (mean ± SD). |
Table I
Effects of LB on neurological deficits
scores following I/R injury in rats (mean ± SD).
| Groups | n | Dose, mg/kg | 24-h LB
treatment | 72-h LB
treatment |
|---|
| Sham | 16 | - | 0.00±0.00 | 0.00±0.00 |
| I/R | 17 | - | 3.45±0.80a | 3.26±0.62a |
| I/R + LL | 17 | 12.5 | 2.60±0.52a,b | 2.45±0.26a,b |
| I/R + LM | 18 | 25.0 | 2.23±0.43a,c | 1.88±0.33a,c,d |
| I/R + LH | 20 | 50.0 | 2.18±0.42a,c | 1.59±0.29a,c,d |
Brain water content
Brain water content was determined following drug
administration (Fig. 1). Following
2 h MCA occlusion and 24 h reperfusion, the brain water content of
the ipsilateral hemisphere in the I/R group significantly increased
compared with that in the sham group (82±2 vs. 78±1%; P<0.05).
The brain water content significantly decreased in the I/R + LB
groups compared with that in the I/R group (LL, 79±1; LM, 76±1; and
LH 78±1% vs. 82±2%; P<0.05). LB significantly decreased the
brain water content of the rats following brain I/R injury. A
dose-response correlation was observed between I/R + LL and I/R +
LM groups (76±1 vs. 79±1%; P<0.01).
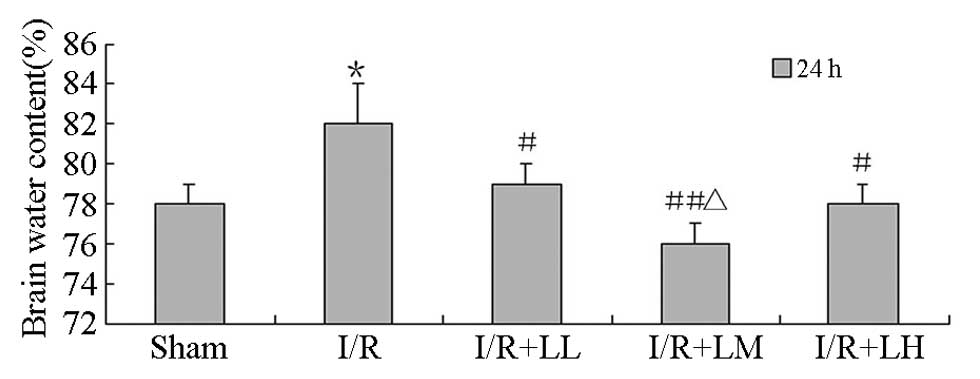 | Figure 1Effect of LB (12.5, 25 and 25 mg/kg)
on brain water content following brain I/R injury in rats, all by
intraperitoneal injections. Each column represents the mean (%) ±
SD. Results were analyzed by ANOVA followed by Student-Newman-Keuls
as the post hoc test (each group, n=8). *P<0.05, vs.
sham; #P<0.05 and ##P<0.01, vs. I/R;
ΔP<0.05, vs. I/R + LL and I/R + LH. LB, lactuside B;
I/R, ischaemia-reperfusion; LL, 12.5 mg/kg LB; LM, 25 mg/kg LB; LH,
50 mg/kg LB. |
RT-PCR
Table II and
Fig. 2 show that LB decreased AQP4
and caspase-3 mRNA expression in the hippocampus following brain
I/R. AQP4 and caspase-3 mRNA expression was significantly higher in
the I/R group than in the sham surgery group at 24 and 72 h
following drug administration (AQP4, 1.49±0.15 and 1.44±0.13;
caspase-3, 0.48±0.05 and 0.50±0.06). AQP4 and caspase-3 mRNA
expression was significantly downregulated in the I/R + LB groups
(LL, LM and LH groups) at 24 h following drug administration (AQP4,
1.23±0.10, 0.95±0.08 and 0.83±0.07; caspase-3, 0.36±0.06, 0.32±0.05
and 0.29±0.03, respectively) compared with the I/R group. The
results observed at 72 h following treatment were similar to those
at 24 h (AQP4, 1.24±0.10, 0.86±0.09 and 0.72±0.08; caspase-3,
0.34±0.06, 0.26±0.05 and 0.25±0.04, respectively). The most
significant reduction in AQP4 and caspase-3 mRNA expression was
noted in the I/R + LM and I/R + LH groups at 72 h following drug
administration. This expression showed a dose-dependent
correlation.
 | Figure 2Effect of LB (12.5, 25 and 25 mg/kg)
on AQP4 and caspase-3 mRNA expression in the hippocampus. RT-PCR
was used to measure (A) AQP4 and (B) caspase-3 mRNA expression
levels in the hippocampus. Lane 1, sham; 2, I/R; 3, I/R + LL; 4,
I/R + LM; 5, I/R + LH; and Marker. LB, lactuside B; AQP4,
aquaporin-4; I/R, ischaemia-reperfusion; LL, 12.5 mg/kg LB; LM, 25
mg/kg LB; LH, 50 mg/kg LB. |
 | Table IIEffects of LB on AQP4 and caspase-3
mRNA expression in the hippocampus following I/R injury in rats
(mean ± SD). |
Table II
Effects of LB on AQP4 and caspase-3
mRNA expression in the hippocampus following I/R injury in rats
(mean ± SD).
| | | 24-h LB
treatment | 72-h LB
treatment |
|---|
| | |
|
|
|---|
| Groups | n | Dose, mg/kg | AQP4 | Caspase-3 | AQP4 | Caspase-3 |
|---|
| Sham | 8 | - | 0.64±0.06 | 0.23±0.02 | 0.64±0.07 | 0.24±0.04 |
| I/R | 9 | - | 1.49±0.15a | 0.48±0.05a | 1.44±0.13a | 0.50±0.06a |
| I/R+LL | 9 | 12.5 | 1.23±0.10a,b | 0.36±0.06a,b | 1.24±0.10a,b | 0.34±0.06a,b |
| I/R+LM | 10 | 25.0 | 0.95±0.08a,b | 0.32±0.05a,b | 0.86±0.09a,c,e | 0.26±0.05a,b,d |
| I/R+LH | 12 | 50.0 | 0.83±0.07a,b | 0.29±0.03a,b | 0.72±0.08a,c,e | 0.25±0.04a,b,d |
Fig. 3 shows that
LB decreased AQP4 and caspase-3 mRNA expression in the striatum
following brain I/R. AQP4 and caspase-3 mRNA expression of the
striatum was significantly higher in the I/R group than in the sham
surgery group at 24 and 72 h following drug administration (AQP4,
1.39±0.10 and 1.42±0.13; caspase-3, 0.48±0.09 and 0.47±0.07,
respectively). LB significantly reduced AQP4 and caspase-3 mRNA
expression in the striatum compared with that in the I/R group at
24 h: (AQP4, 1.09±0.08, 1.00±0.06 and 0.90±0.04; caspase-3,
0.38±0.04, 0.28±0.05 and 0.22±0.06, respectively) and 72 h (AQP4,
1.01±0.09, 0.82±0.08 and 0.70±0.05; caspase-3, 0.31±0.06, 0.22±0.04
and 0.20±0.03, respectively) following drug administration. The
effects became stronger as the LB doses were increased. The most
significant reductions in AQP4 and caspase-3 mRNA expression were
noted in the I/R + LM and I/R + LH groups at 72 h following drug
administration.
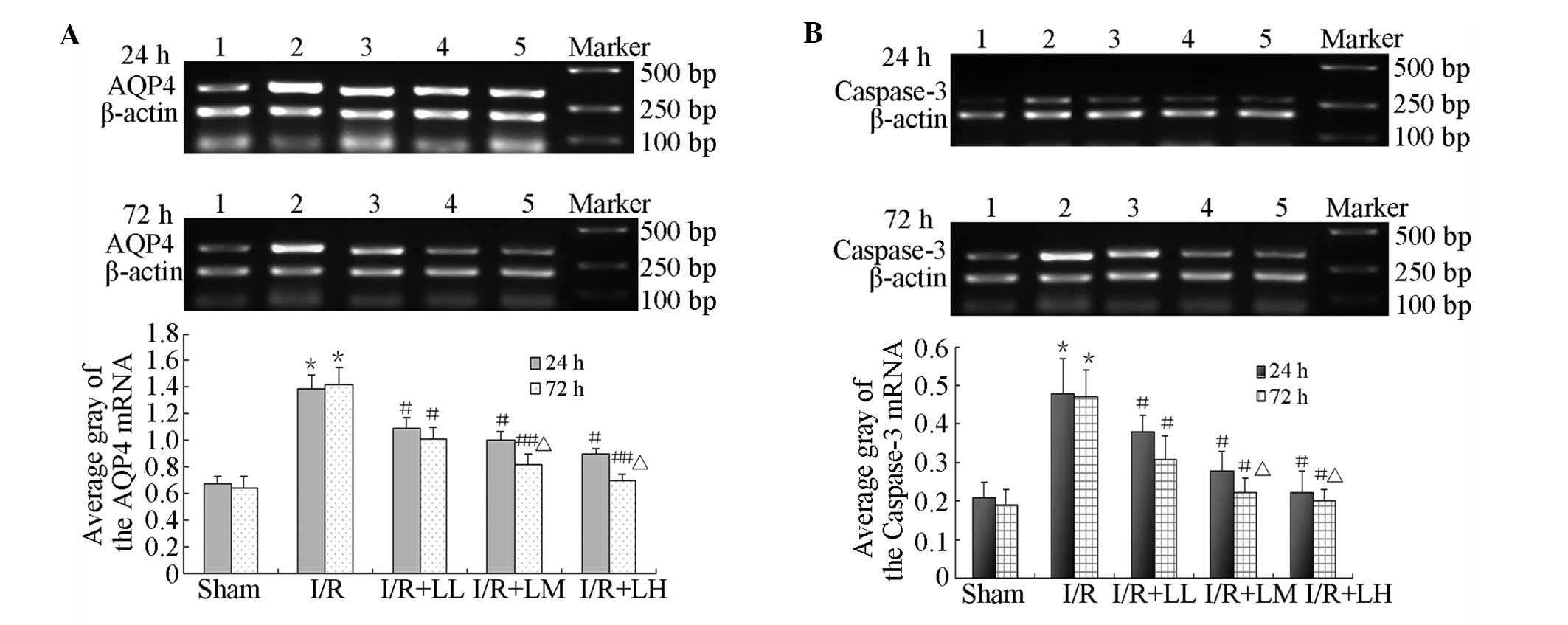 | Figure 3Effect of LB (12.5, 25 and 25 mg/kg)
on AQP4 and caspase-3 mRNA expression in the striatum. RT-PCR was
used to measure (A) AQP4 and (B) caspase-3 mRNA expression levels
in the striatum. *P<0.01, vs. sham group;
#P<0.05 and ##P<0.01, vs. I/R (model)
group; ΔP<0.05, vs. 24 h treatment. Lane 1, sham; 2,
I/R; 3, I/R + LL; 4, I/R + LM; 5, I/R + LH; and Marker. LB,
lactuside B; AQP4, aquaporin-4; I/R, ischaemia-reperfusion; LL,
12.5 mg/kg LB; LM, 25 mg/kg LB; LH, 50 mg/kg LB. |
Discussion
In the present study, AQP4 mRNA was expressed in the
hippocampus and the striatum, but the expression levels were low.
AQP4 is a protein that functions as a water channel in the central
nervous system and is highly expressed in the end-feet of
astrocytes. AQP4 allows water to diffuse across the membrane. A
normal expression of AQP4 may help maintain water balance in the
brain. In this study, AQP4 mRNA expression in the hippocampus and
the striatum was significantly higher in the I/R group than in the
sham surgery group. This increased expression was considered as
mRNA overexpression in these regions. However, specific differences
were observed between the peak expression levels of AQP4 mRNA in
the hippocampus and striatum. In particular, the peak expression
levels in the hippocampus and the striatum were observed at 24 and
72 h following I/R injury, respectively. These results may be
induced by the sensitivity of the hippocampus to injury and oedema
following cerebral ischaemia. The results indicated that the peak
expression of AQP4 mRNA was reached at almost the same time as
brain oedema occurred; the peak expression was reached at 1–3 days
and then reduced gradually.
As a significant channel of fast-flowing water in
acute primary cerebral oedema, AQP4 functions in the exchange of
water between normal physiological processes and cerebral
ischaemia. Following 2 h MCA occlusion and 24 h reperfusion,
serious oedema was observed in the right side of the brain.
The two consistent results show that AQP4
overexpression may elicit a negative effect on cerebral oedema
caused by cerebral I/R. Experimental study has also found that LB
reduces AQP4 mRNA expression in the hippocampus and striatum in a
dose-dependent manner. For instance, Igarashi et al
(18) observed that an AQP4
inhibitor significantly reduced ischaemic cerebral oedema. Another
study noted that AQP4 knockout mice exhibit increased intracranial
pressure, in which brain I/R injury is aggravated, showing an
enlarged infarct size, a more marked loss of CA1 neurons and
astrocyte hypertrophy (19). A
constant level of AQP4 expression is capable of attenuating
cellular oedema following cerebral ischaemia to a certain degree
(20). These studies have
hypothesised that the actions of LB against cerebral ischaemia are
associated with lower AQP4 mRNA expression levels in the
hippocampus and the striatum.
As cerebral ischaemia and cerebral oedema develop,
neurons tend to undergo irreversible cell necrosis in the ischaemic
core region and may remain viable for several hours or days in the
peri-infarct region (penumbra) (14,21).
The neurons of the penumbra are prone to apoptosis. Following
cerebral ischaemia, the apoptotic cells, which are mainly located
in a specific infarcted area, including the preoptic region, corpus
striatum, inner margin cortex of infarcted boundary, corpus
striatum, hippocampus and olfactory tubercle, reach the highest
number in 24–48 h (22). Previous
studies have found that caspase-3 expression is increased as cell
apoptosis in the brain is increased during local and focal
ischaemia (23,24). The present study identified that
caspase-3 mRNA expression in the hippocampus and the striatum was
significantly higher in the I/R group than in the sham surgery
group. However, expression levels were essentially the same at 24
and 72 h following I/R injury. These results are consistent with
those observed in a previous study (25). Therefore, the results indicate that
LB reduces caspase-3 mRNA expression with a dose-effect correlation
in the hippocampus and the striatum. These results also revealed
that the actions of LB against cerebral ischaemia are associated
with low levels of caspase-3 mRNA expression in the hippocampus and
the striatum.
Brain damage caused by cerebral infarction is
confined to the cerebral cortex and results in neuronal mortality
of the hippocampus and the striatum (26,27).
The results of the present study showed that LB reduced the
neurological deficit scores and the brain water content. These
effects may be associated with the decreased AQP4 and caspase-3
mRNA expression levels in the hippocampus and the striatum
following the administration of LB. These results provide novel
information concerning the mechanism of action of LB in brain
ischaemia. Therefore, LB may potentially be used as a new
neuroprotective agent.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (no. 81172953) and Foundation
of Henan Educational Committee, China (no. 2009A310009).
References
|
1
|
Donnan GA, Fisher M, Macleod M and Davis
SM: Stroke. Lancet. 371:1612–1623. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Giffard RG and Swanson RA:
Ischemia-induced programmed cell death in astrocytes. Glia.
50:299–306. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ohk TG, Yoo KY, Park SM, et al: Neuronal
damage using fluoro-jade B histofluorescence and gliosis in the
striatum after various durations of transient cerebral ischemia in
gerbils. Neurochem Res. 37:826–834. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li M, Zhang X, Cui L, et al: The
neuroprotection of oxymatrine in cerebral ischemia/reperfusion is
related to nuclear factor erythroid 2-related factor 2
(nrf2)-mediated antioxidant response: role of nrf2 and
hemeoxygenase-1 expression. Biol Pharm Bull. 34:595–601. 2011.
View Article : Google Scholar
|
|
5
|
Taniguchi M, Yamashita T, Kumura E, et al:
Induction of aquaporin-4 water channel mRNA after focal cerebral
ischemia in rat. Brain Res Mol Brain Res. 78:131–137. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tang Z, Sun X, Huo G, et al: Protective
effects of erythropoietin on astrocytic swelling after
oxygen-glucose deprivation and reoxygenation: mediation through
AQP4 expression and MAPK pathway. Neuropharmacology. 67:8–15. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gartshore G, Patterson J and Macrae IM:
Influence of ischemia and reperfusion on the course of brain tissue
swelling and blood-brain barrier permeability in a rodent model of
transient focal cerebral ischemia. Exp Neurol. 147:353–360. 1997.
View Article : Google Scholar
|
|
8
|
Simard JM, Kent TA, Chen M, Tarasov KV and
Gerzanich V: Brain oedema in focal ischaemia: molecular
pathophysiology and theoretical implications. Lancet Neurol.
6:258–268. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mori K, Miyazaki M, Iwase H and Maeda M:
Temporal profile of changes in brain tissue extracellular space and
extracellular ion (Na+, K+) concentrations
after cerebral ischemia and the effects of mild cerebral
hypothermia. J Neurotrauma. 19:1261–1270. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Spatz M: Past and recent BBB studies with
particular emphasis on changes in ischemic brain edema: dedicated
to the memory of Dr. Igor Klatzo Acta Neurochir Suppl. 106:21–27.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Badaut J, Lasbennes F, Magistretti PJ and
Regli L: Aquaporins in brain: distribution, physiology, and
pathophysiology. J Cereb Blood Flow Metab. 22:367–378. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Potterat O: Goji (Lycium barbarum
and L. chinense): Phytochemistry, pharmacology and safety in
the perspective of traditional uses and recent popularity. Planta
Med. 76:7–19. 2010.
|
|
13
|
Prabhakar G, Vona-Davis L, Murray D,
Lakhani P and Murray G: Phosphocreatine restores high-energy
phosphates in ischemic myocardium: Implication for off-pump cardiac
revascularization. J Am Coll Surg. 197:786–791. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Broughton BR, Reutens DC and Sobey CG:
Apoptotic mechanisms after cerebral ischemia. Stroke. 40:e331–e339.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li SY, Sun J, Niu BX, Yan FL and Zhan HQ:
Effect of LB on the expression of bcl-2 and bax mRNA and their
protein in rats’ cerebral cortex after cerebral
ischemia-reperfusion injury. Yao Xue Xue Bao. 46:1314–1320.
2011.(In Chinese).
|
|
16
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Young W, Rappaport ZH, Chalif DJ and Flamm
ES: Regional brain sodium. potassium and water changes in the rat
middle cerebral artery occlusion of ischemia. Stroke. 18:751–759.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Igarashi H, Huber VJ, Tsujita M and Nakada
T: Pretreatment with a novel aquaporin 4 inhibitor, TGN-020,
significantly reduces ischemic cerebral edema. Neurol Sci.
32:113–116. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zeng XN, Xie LL, Liang R, Sun XL, Fan Y
and Hu G: AQP4 knockout aggravates ischemia/reperfusion injury in
mice. CNS Neurosci Ther. 18:388–394. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shin JA, Choi JH, Choi YH and Park EM:
Conserved aquaporin 4 levels associated with reduction of brain
edema are mediated by estrogen in the ischemic brain after
experimental stroke. Biochim Biophys Acta. 1812:1154–1163. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song DY, Oh KM, Yu HN, et al: Role of
activating transcription factor 3 in ischemic penumbra region
following transient middle cerebral artery occlusion and
reperfusion injury. Neurosci Res. 70:428–434. 2011. View Article : Google Scholar
|
|
22
|
Moroni F: Poly (ADP-ribose) polymerase 1
(PARP-1) and postischemic brain damage. Curr Opin Pharmacol.
8:96–103. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ness JM, Harvey CR, Washington JD, Roth
KA, Carroll SL and Zhang J: Differential activation of c-fos and
caspase-3 in hippocampal neuron subpopulations following neonatal
hypoxia-ischemia. J Neurosci Res. 86:1115–1124. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yip KK, Lo SC, Leung MC, So KF, Tang CY
and Poon DM: The effect of low-energy laser irradiation on
apoptotic factors following experimentally induced transient
cerebral ischemia. Neuroscience. 190:301–306. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu XH, Zhang SM, Yan WM, et al:
Development of cerebral infarction, apoptotic cell death and
expression of X-chromosome-linked inhibitor of apoptosis protein
following focal cerebral ischemia in rats. Life Sci. 78:704–712.
2006. View Article : Google Scholar
|
|
26
|
Cincioglu M, Kismali G, Ugur SA, et al:
Indinavir inhibits the expression of cytoplasmic aromatase and
nuclear SREBP in the hippocampus of reperfusion injury-induced
ischemic rats. J Steroid Biochem Mol Biol. 130:81–89. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Belayev L, Busto R, Zhao W and Ginsberg
MD: HU-211, a novel noncompetitive N-methyl-D-aspartate antagonist,
improves neurological deficit and reduces infarct volume after
reversible focal cerebral ischemia in the rat. Stroke.
26:2313–2319. 1995.
|

















