Introduction
Non-alcoholic fatty liver disease (NAFLD), commonly
known as fatty liver, is a disease characterized by an abnormal
accumulation of fats [triglycerides (TGs)] inside liver cells
(1). Thus far, the prevalence of
NAFLD has consistently increased with lifestyle changes (2). Steatohepatitis is histologically
characterized by a significant accumulation of hepatic lipids and
lobular necro-inflammation in NAFLD, which may be progressive and
eventually induce liver fibrosis and cirrhosis (3,4).
Although much progress has been made in recent
years, the pathogenesis of steatohepatitis has not been fully
elucidated. It is known that defects in fat metabolism are
responsible for the pathogenesis of NAFLD, which may be due to an
imbalance in lipid storage and consumption in hepatocytes.
Peroxisome proliferator-activated receptor-γ (PPARγ) signaling is
important in hepatic fat accumulation, transport and utilization
(5). PPARγ, also known as the
thiazolidines ligand, plays a critical role in regulating fatty
acid storage and metabolism. A previous study identified that PPARγ
exhibited an anti-steatohepatitic effect by reducing the production
of hepatic pro-inflammatory cytokines, such as tumor necrosis
factor-α (TNF-α) and interleukin-6 (IL-6) (6). Furthermore, the activation of PPARγ
may inhibit inflammatory responses by preventing the activation of
nuclear transcription factors, such as nuclear factor-κB (NF-κB)
(7). For these reasons, PPARγ may
be a potential therapeutic target in the treatment of
steatohepatitis. Additional clinical studies have demonstrated that
PPARγ agonists, such as thiazolidinediones (TZDs), not only
increase insulin sensitivity in adipose, liver and skeletal muscle
tissue, but also affect hepatic fat accumulation by enhancing fatty
acid oxidation (8,9). Despite their validated efficacy and
widespread use, TZDs possess a number of side-effects, including
significant weight gain and peripheral edema (10,11).
In serious cases, TZDs may result in severe hepatotoxicity
(12). In this regard, it is
necessary to develop novel agents that target PPARγ, but with
reduced adverse effects.
Medicinal plants, also called herbal medicines, have
been traditionally used for treating liver disease worldwide
(13). There are numerous herbal
products that are believed to have therapeutic benefit on NAFLD,
such as silymarin (milk thistle), glycyrrhizin (licorice root
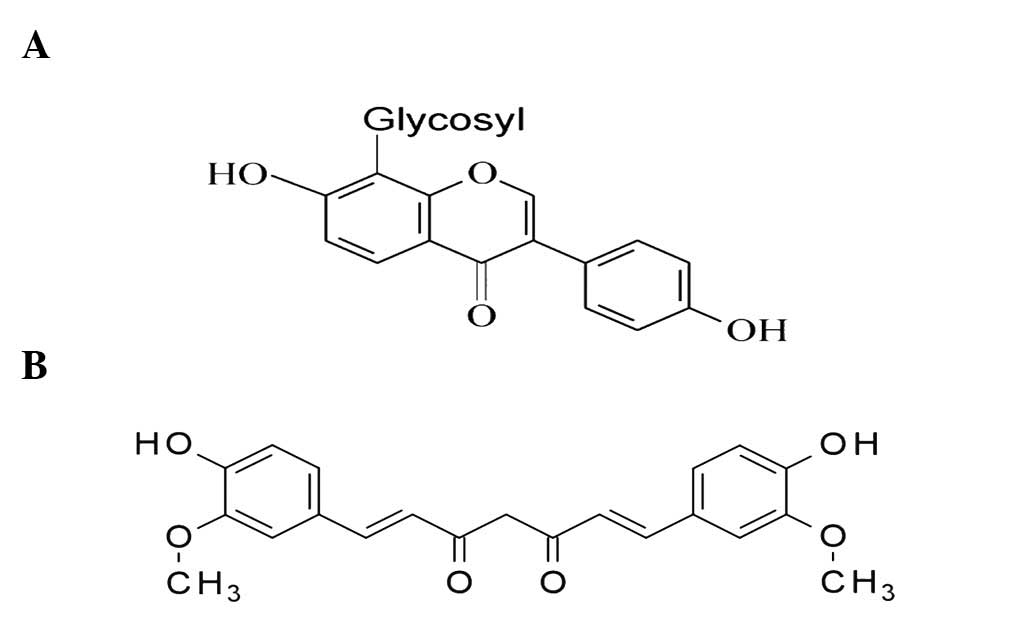
extract) and curcumin (turmeric extract) (14). Among them, curcumin (Fig. 1B) is the best known and has been
used for centuries in Asia as a dietary spice, a food coloring and
a treatment for inflammation, wounds, and gastrointestinal and
liver disorders (15). A previous
study demonstrated that curcumin may alleviate steatohepatitis and
inhibit liver fibrogenesis (16).
The possible mechanisms of curcumin may involve the stimulation of
PPARγ activity and the inhibition of hepatic stellate cell (HSC)
activation (17).
Previous studies have investigated the
anti-oxidative and anti-inflammatory activities effects of puerarin
(Fig. 1A) (18). Puerarin has been shown to exhibit
therapeutic effects on NAFLD by acting as an antioxidant, lowering
cholesterol levels and improving leptin signal transduction
(19,20). In the present study, the
intervening actions of puerarin and curcumin were compared on mice
models of steatohepatitis induced by a methionine- and
choline-deficient (MCD) diet. With regard to previous studies
concerning the roles of curcumin and puerarin in PPARγ signaling,
it may be hypothesized that curcumin and puerarin have potential as
therapeutic agents for the treatment of steatohepatitis.
Materials and methods
Chemicals and reagents
Feeds for the methionine-choline-sufficient (MCS)
diet and methionine-choline-deficient (MCD) diet were provided by
the Trophic Animal Feed High-tech Co., Ltd. (Nantong, China).
Curcumin and puerarin were purchased from Xi’an Guanyu Bio-tech
Co., Ltd. (Shaanxi, China). Colorimetric kits for the testing of
triglyceride (TG), total cholesterol (TC), high density lipoprotein
(HDL) and low density lipoprotein (LDL) were purchased from BioSino
Bio-technology and Science, Inc. (Beijing, China). Enzyme-linked
immunosorbent assay (ELISA) kits for TNF-α and IL-6 and were
purchased from Shanghai Yanji Biotechnology Co., Ltd. (Shanghai,
China). Anti-NF-κB (p65, ab31481), anti-PPARγ (ab27649), and
anti-GAPDH antibodies (ab9483) were purchased from Abcam
(Cambridge, UK). Additional reagents were obtained from
Sigma-Aldrich (St. Louis, MO, USA).
Animal handling procedure
Thirty-two C57BL/6 mice (weight, 20–25 g) were
purchased from Vital River Laboratories (Beijing, China;
certificate no. SCXK-2006–0009) and divided into four groups: The
normal control group, the model control group, the curcumin
treatment group and the puerarin treatment group. Mice were housed
in a temperature-, humidity- and light-controlled environment
(temperature, 22±2°C, a 12-h light/dark cycle, 50–60% humidity)
with access to rodent feed and water ad libitum. Normal
control mice were fed the MCS diet and the remaining mice were fed
the MCD diet for 2 weeks.
Drug administration
Curcumin and puerarin were administered orally in a
volume of 0.1 ml/10 g body weight once a day for 2 weeks at the
same time as MCD feeding. Drugs were dissolved in dimethyl
sulfoxide (DMSO) and then diluted in distilled water to a
concentration of 90 mg/ml (18,21).
Vehicle solution (DMSO mixed with distilled water) was administered
to the normal control mice. The experimental procedures were
reviewed and approved by the Animal Care and Use Committee in the
Beijing University of Chinese Medicine (Beijing, China) prior to
the animal experiments being performed.
Detection of TG, TC, HDL and LDL levels
in the blood serum
Blood was harvested after the mice were
anesthetized. At the end of treatment, animals were anesthetized
using 4% chloral hydrate after a 12-h over night fast. The blood
samples were obtained from the inferior vena cava. Following
centrifugation at 644 × g for 10 min at 4°C, the serum was
collected to measure the levels of TG, TC, HDL and LDL. All these
measurements were determined using enzymatic colorimetric kits and
were performed according to the manufacturer’s instructions.
Histopathological analysis
One fresh section of liver tissue from each mouse
was kept in liquid nitrogen for 4–10 sec for the running frozen
section technique. Sections were stained with Oil red O
(Sigma-Aldrich). An additional section of liver tissue was fixed by
immersion in 10%-buffered formalin for paraffin embedding, and
hematoxylin and eosin staining.
ELISA for the detection of TNF-α, and
IL-6 levels in the serum
The concentrations of TNF-α and IL-6 in the serum of
the mice were analyzed using commercially available ELISA kits
according to the manufacturer’s instructions. Briefly, all the
samples were diluted at 1:10. The absorbance was read at 450 nm
using a microplate reader (Multiskan MK3; Thermo Scientific,
Rockford, IL, USA). Samples and standards were run three times.
Western blotting to detect PPARγ and
NF-κB expression
Proteins in the liver tissue homogenates were
extracted using ice-cold tissue lysis buffer. Protein
concentrations were determined using a BCA protein assay kit
(Promega, Madison, WI, USA). Samples were separated by 10% SDS-PAGE
and transferred onto polyvinylidene difluoride membranes. The
membranes were immunoblotted with primary antibodies that
recognized PPARγ (1:2,000), NF-κB (1:400) and GAPDH (1:5,000).
Peroxidase-conjugated secondary antibodies [goat polyclonal
secondary antibody to mouse (ab6006); Abcam] and an enhanced
chemiluminescence detection system [ECL Western Blotting Substrate
Kit - 500 Tests (ab65628); Abcam] were used according to routine
methods. The intensities of the protein bands were analyzed using
Gel-Pro Analyzer, version 3.2 software (Bio-Rad Gel Doc 2000
digital gel imaging system; Bio-Rad, Hercules, CA, USA). GAPDH
protein was used as the internal control to normalize for protein
loading.
Statistical analysis
Data are expressed as the mean ± standard error.
Differences between the mean values of normally distributed data
were assessed using a one-way analysis of variance and the
Student-Newman-Keuls test. Analyses were performed using Excel and
Paint software for Windows. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of puerarin and curcumin on lipid
metabolism
All animals tolerated the experimental procedures
well and no deaths occurred during the 2 week study. The levels of
serum TG, TC, HDL and LDL were analyzed in each group. The results
showed that there were no significant differences in TG and TC
levels between the normal control group and the MCD group, which
indicated that the MCD diet does not induce hyperlipidemia in mice
(P>0.05). Notably, puerarin treatment significantly reduced the
levels of TG and TC in the serum to lower than those of the normal
mice (P<0.05, versus the MCS group; Fig. 2A and B). Compared with the values
in the MCS group (normal control), the HDL level decreased but the
LDL level increased significantly in the MCD group (P<0.01 and
P<0.05, respectively). Compared with the value in the MCD model
group, the serum LDL level was reduced only in the mice treated
with puerarin (P<0.01; Fig. 2C and
D).
Effects of puerarin and curcumin on the
levels of TNF-α and IL-6 in mice serum
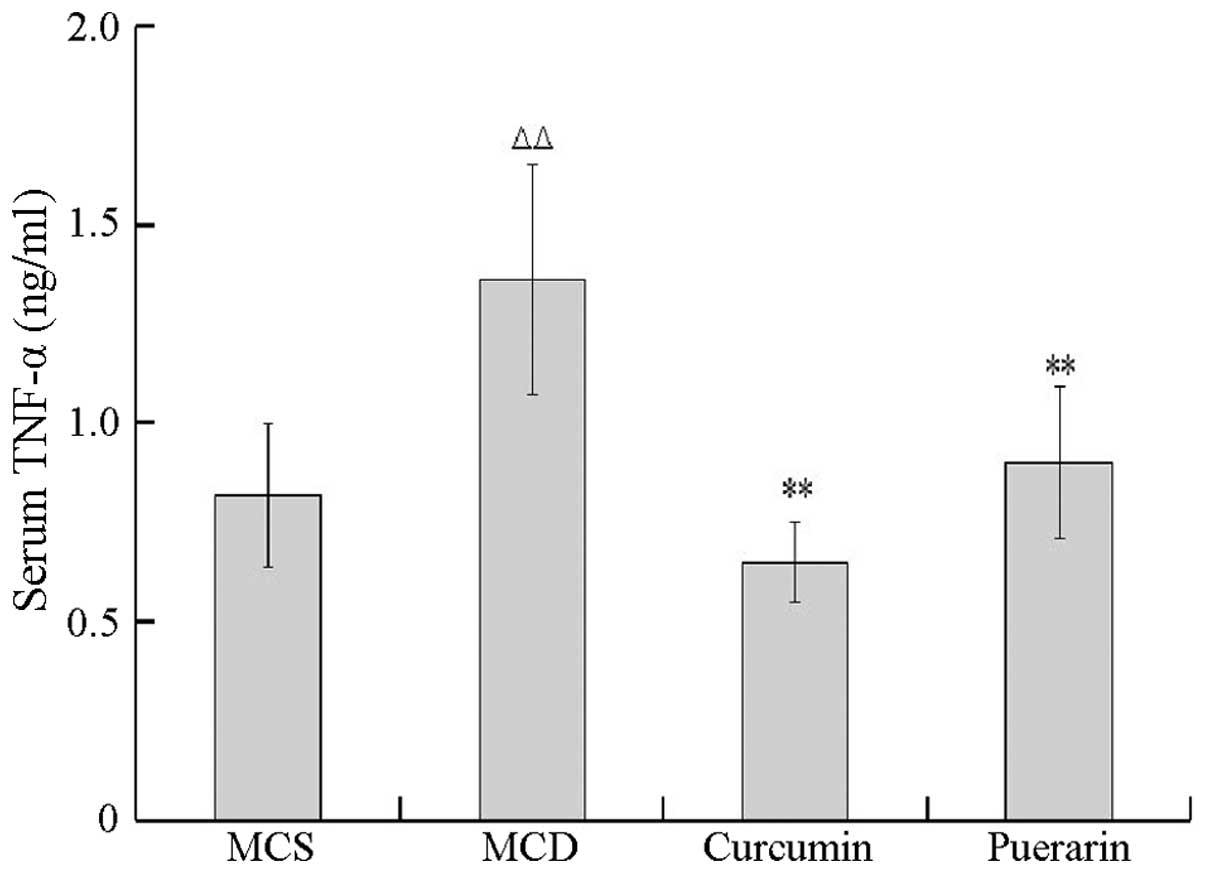
The inhibitory activities of puerarin and curcumin
on TNF-α and IL-6 levels were tested using an ELISA method. As
shown in Fig. 3, the level of
TNF-α in the serum was significantly increased in the MCD group
compared with that of the MCS group (P<0.01). In addition, the
levels of TNF-α decreased in mice treated with curcumin and
puerarin, respectively, compared with that of the MCD group
(P<0.01), suggesting that curcumin and puerarin markedly
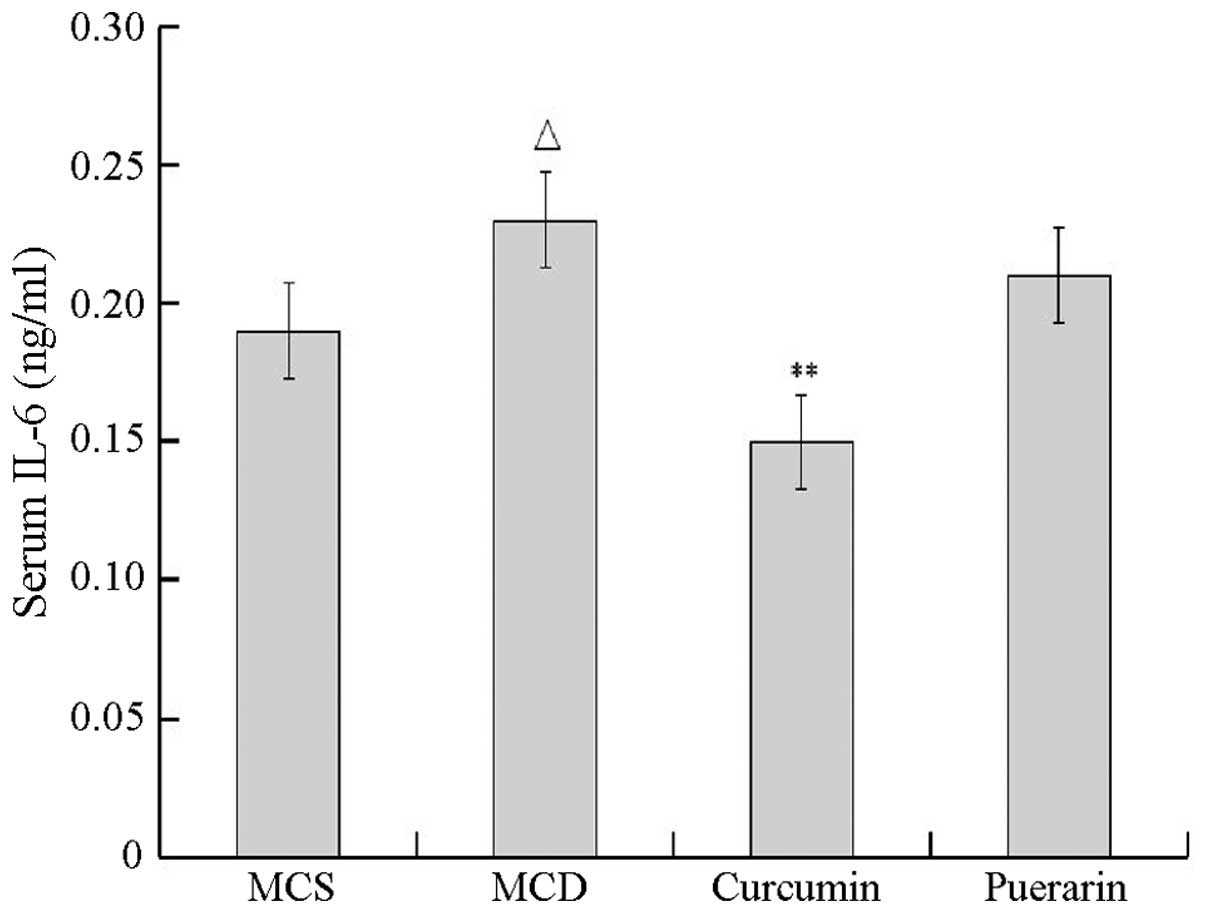
inhibited TNF-α secretion. As shown in Fig. 4, the level of IL-6 also
significantly increased in the MCD group compared with that of the
normal control group (P<0.05). Curcumin but not puerarin
inhibited IL-6 secretion compared with that of the MCD group
(P<0.01).
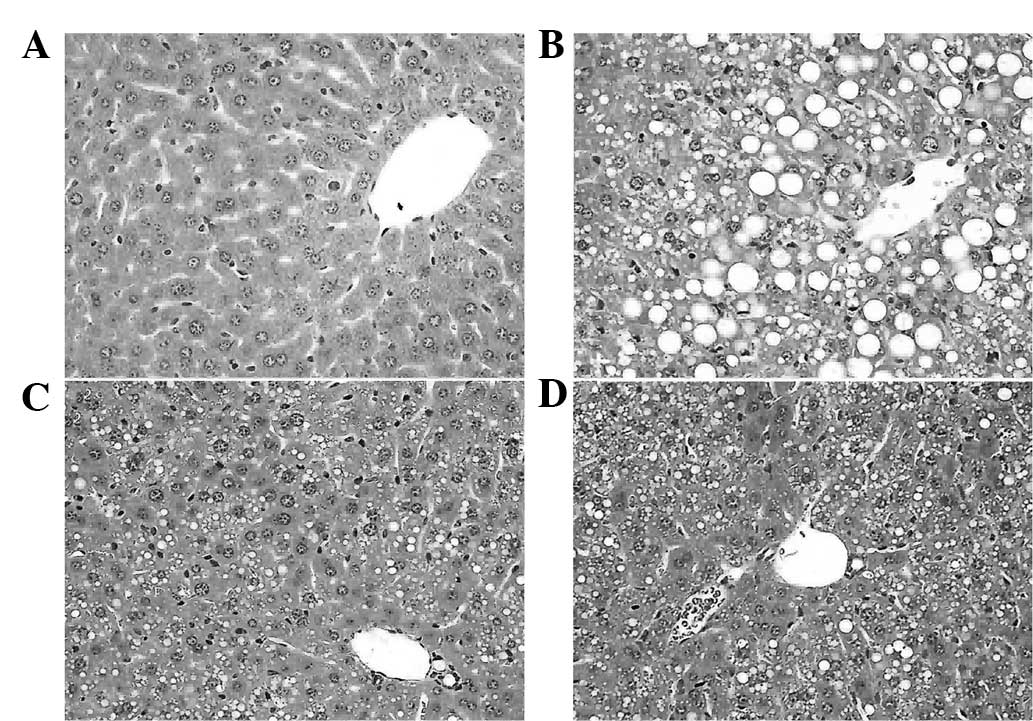
Histopathological evaluation
The histopathology of the livers was analyzed to
determine whether curcumin and puerarin prevented liver
destruction. As shown in Fig. 5B,
typical steatosis (balloon degeneration in hepatocytes and evident
infiltration with inflammatory cells in the intercellular
substance) was observed in the liver tissues of the MCD group
compared with that of the normal control mice (Fig. 5A). However, curcumin and puerarin
treatments alleviated those changes of pathology (Fig. 5C and D), which indicated the
therapeutic effects of curcumin and puerarin. Furthermore, Oil red
O staining was used to detect the quantity of lipids in the
hepatocytes. The results showed that the MCD diet significantly
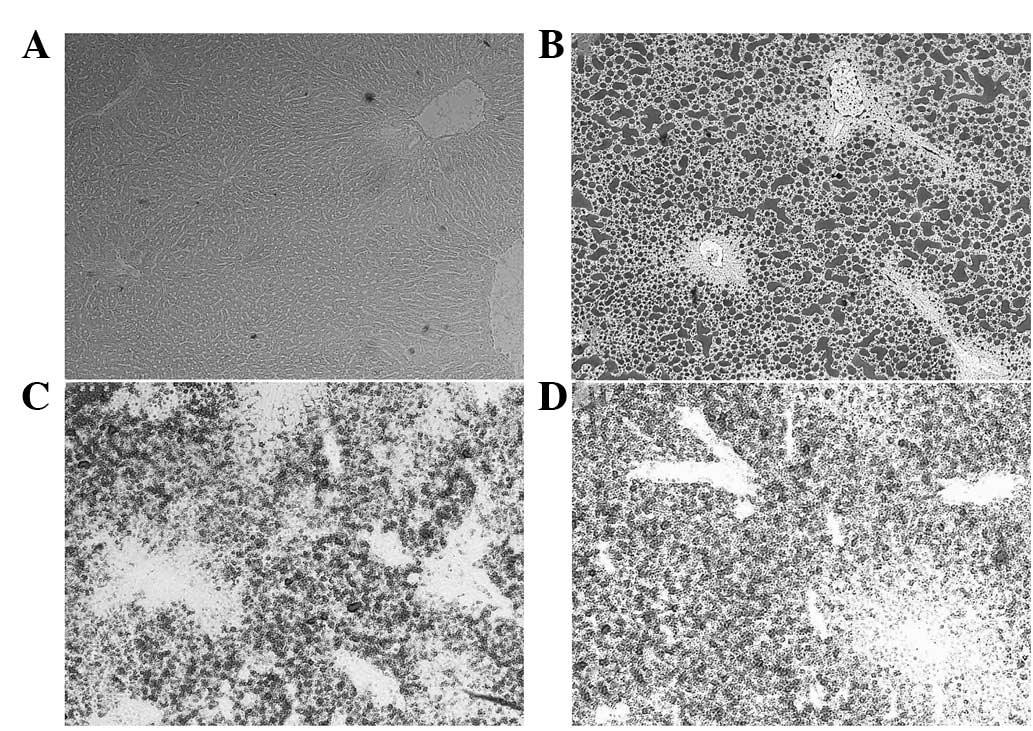
increased lipidosis in hepatocytes (Fig. 6A and B), but curcumin and puerarin
treatments markedly restrained the deposition of lipid droplets in
the hepatocytes (Fig. 6C and
D).
Curcumin and puerarin regulate PPARγ and
NF-κB expression in MCD diet-induced mice
It was investigated whether curcumin and puerarin
had a regulatory effect on PPARγ and NF-κB expression. As shown in
Fig. 7 the PPARγ level was
significantly decreased in the MCD group compared with that of the
MCS group (P<0.01). Notably, curcumin and puerarin induced
significant elevations of the PPARγ level compared with that in the
MCD group (P<0.05). As shown in Fig. 8, NF-κB levels increased
significantly in the MCD group compared with that of the MCS group
(P<0.05). However, the elevation of the NF-κB level was markedly
attenuated in the curcumin-treated group compared with that of the
MCD group (P<0.05).
Discussion
Nonalcoholic fatty liver disease (NAFLD) is a
multi-factorial disorder resulting from a variety of genetic and
environmental factors. At present, the pathogenesis of NAFLD is not
fully understood and therapeutic clinical trials are ongoing
(22). Although lipid accumulation
in the liver is the major hallmark of NAFLD, the mechanisms
resulting in steatohepatitis remain elusive (23). Researchers have suggested the
‘2-hit’ hypothesis to explain NAFLD pathogenesis (24,4).
Briefly, the ‘first hit’ involves hepatic TG accumulation or
steatosis. The ‘second hit’ relates to the induction of
inflammatory cytokines and oxidative stress. Hepatic TG
accumulation may occur as a result of increased fat synthesis,
increased fat delivery, decreased fat export and/or decreased fat
oxidation (25). To a certain
degree, PPARγ is important for the ‘first hit’ stage (25). Inflammatory cytokines, such as
TNF-α and IL-6, are involved in the ‘second hit’ stage, which
mediates steatohepatitis in patients with NAFLD (26). TNF-α, a pro-inflammatory cytokine,
not only promotes insulin resistance, but also mediates cholesterol
and TG metabolism (27).
Similarly, the involvement of IL-6 has also been identified in
animal models of and patients with NAFLD, with elevated serum
levels of IL-6 being correlated with increasing steatohepatitis
(28). It is worth stressing that
the elevated expression levels of TNF-α and IL-6 were mediated by
activation of the NF-κB signaling pathway (29). NF-κB, a critical transcription
factor, may promote hepatic steatosis, hepatic injury and fibrosis
by upregulating serum and hepatic levels of TNF-α and IL-6
(30). An increasing number of
studies have suggested that PPARγ links the NF-κB signaling pathway
through being mediated by regulating adipokines via controlled by
suppressing TNF-alpha and IL-6, which closely connect to NF-κB and
is critical for the treatment of NAFLD (6,7,31).
In the present study, mice models of steatohepatitis
induced by a MCD diet were employed to compare the efficacies of
puerarin and curcumin against steatohepatitis. The MCD diet-induced
animal model is an internationally-recognized model for the study
of the inflammation and fibrosis associated with NAFLD (32). Methionine and choline are
precursors of phosphatidylcholine (PC). PC is an essential
substrate for very low density lipoproteins (VLDL). Deficiency of
methionine and choline may reduce VLDL production or secretion,
which limits lipid packaging and export (33,34).
As has been observed in previous studies (32,35),
the MCD was noted to increased plasma TNF-α and IL-6 levels,
increase hepatic tissue NF-κB expression, but decrease hepatic
tissue PPARγ expression. The present study also demonstrated that
there were no noticeable changes in the plasma TG levels of mice
fed an MCD (32). However, the
present study identified that puerarin and curcumin had promising
hepatoprotective and anti-steatohepatitis activities. In addition,
curcumin significantly reduced serum levels of TNF-α and IL-6, and
hepatic tissue levels of NF-κB and PPARγ. The effects of puerarin
differed from those of curcumin. Puerarin downregulated the serum
levels of TNF-α and hepatic tissue levels of PPARγ, but
demonstrated no significant effects on the levels of IL-6 and
NF-κB. Moreover, compared with curcumin, puerarin indicated notable
anti-hyperlipidemic effects (Fig. 2A
and B). These results suggest that curcumin and puerarin affect
NAFLD by different mechanisms. It is hypothesized that puerarin may
be involved in the early pathological stage (first hit) through
regulating the lipid metabolism mediated by PPARγ. By contrast,
curcumin may impact multiple nodes, particularly the inflammatory
response stage (second hit). These results suggest a novel strategy
for preventing NAFLD and for the development of novel agents with
anti-steatohepatitis effects.
In conclusion, curcumin and puerarin induce
favorable effects on steatohepatitis through different mechanisms.
Puerarin may regulate lipid metabolism in the ‘first hit’ stage
through the PPARγ pathway, whereas curcumin may inhibit the
inflammatory response in the ‘second hit’ stage through the
PPARγ/NF-κB pathway. Further experiments focusing on the molecular
mechanisms of curcumin and puerarin using different blocking agents
and advanced experimental techniques are required.
Acknowledgements
This study was supported by the Innovation team of
Beijing University of Chinese Medicine (grant no. 2011-CXTD-24);
the self-selected topic of Beijing University of Chinese Medicine
(grant no. JYBZZ-JS004); and the Natural Science Foundation of
Beijing (grant no. 7102094).
References
|
1
|
Williamson RM, Price JF, Glancy S, et al;
Edinburgh Type 2 Diabetes Study Investigators. Prevalence of and
risk factors for hepatic steatosis and nonalcoholic fatty liver
disease in people with type 2 diabetes: the Edinburgh Type 2
Diabetes Study. Diabetes Care. 34:1139–1144. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Valantinas J, Apanaviciene DA, Maroziene L
and Sveikata A: The prevalence of metabolic risk factors among
outpatients with diagnosed nonalcoholic fatty liver disease in
Lithuania. Med Sci Monit. 18:PH57–PH62. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chitturi S and Farrell GC:
Etiopathogenesis of nonalcoholic steatohepatitis. Semin Liver Dis.
21:27–41. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reid AE: Nonalcoholic steatohepatitis.
Gastroenterology. 121:710–723. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kallwitz ER, McLachlan A and Cotler SJ:
Role of peroxisome proliferators-activated receptors in the
pathogenesis and treatment of nonalcoholic fatty liver disease.
World J Gastroenterol. 14:22–28. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu CW, Chu ES, Lam CN, et al: PPARgamma is
essential for protection against nonalcoholic steatohepatitis. Gene
Ther. 17:790–798. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yuhas Y, Berent E, Cohen R and Ashkenazi
S: Roles of NF-kappaB activation and peroxisome
proliferator-activated receptor gamma inhibition in the effect of
rifampin on inducible nitric oxide synthase transcription in human
lung epithelial cells. Antimicrob Agents Chemother. 53:1539–1545.
2009. View Article : Google Scholar
|
|
8
|
Sanyal AJ, Mofrad PS, Contos MJ, et al: A
pilot study of vitamin E versus vitamin E and pioglitazone for the
treatment of nonalcoholic steatohepatitis. Clin Gastroenterol
Hepatol. 2:1107–1115. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Promrat K, Lutchman G, Uwaifo GI, et al: A
pilot study of pioglitazone treatment for nonalcoholic
steatohepatitis. Hepatology. 39:188–196. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Elasy TA and Griffin M: Thiazolidinedione
use, fluid retention, and congestive heart failure: a consensus
statement from the American Heart Association and American Diabetes
Association: response to Nesto. Diabetes Care. 27:20962004.
View Article : Google Scholar
|
|
11
|
Nesto RW, Bell D, Bonow RO, et al:
Thiazolidinedione use, fluid retention, and congestive heart
failure: a consensus statement from the American Heart Association
and American Diabetes Association. Diabetes Care. 27:256–263. 2004.
View Article : Google Scholar
|
|
12
|
Parulkar AA, Pendergrass ML, Granda-Ayala
R, Lee TR and Fonseca VA: Nonhypoglycemic effects of
thiazolidinediones. Ann Intern Med. 134:61–71. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Seeff LB, Lindsay KL, Bacon BR, Kresina TF
and Hoofnagle JH: Complementary and alternative medicine in chronic
liver disease. Hepatology. 34:595–603. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ghosh N, Ghosh R, Mandal V and Mandal SC:
Recent advances in herbal medicine for treatment of liver diseases.
Pharm Biol. 49:970–988. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yao QH, Wang DQ, Cui CC, et al: Curcumin
ameliorates left ventricular function in rabbits with pressure
overload: inhibition of the remodeling of the left ventricular
collagen network associated with suppression of myocardial tumor
necrosis factor-alpha and matrix metalloproteinase-2 expression.
Biol Pharm Bull. 27:198–202. 2004.
|
|
16
|
Vizzutti F, Provenzano A, Galastri S, et
al: Curcumin limits the fibrogenic evolution of experimental
steatohepatitis. Lab Invest. 90:104–115. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tang Y, Zheng S and Chen A: Curcumin
eliminates leptin’s effects on hepatic stellate cell activation via
interrupting leptin signaling. Endocrinology. 150:3011–3020.
2009.
|
|
18
|
Xiao C, Li J, Dong X, et al:
Anti-oxidative and TNF-α suppressive activities of puerarin
derivative (4AC) in RAW264.7 cells and collagen-induced arthritic
rats. Eur J Pharmacol. 666:242–250. 2011.
|
|
19
|
Zheng P, Ji G, Ma Z, et al: Therapeutic
effect of puerarin on non-alcoholic rat fatty liver by improving
leptin signal transduction through JAK2/STAT3 pathways. Am J Chin
Med. 37:69–83. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chung MJ, Sung NJ, Park CS, et al:
Antioxidative and hypocholesterolemic activities of water-soluble
puerarin glycosides in HepG2 cells and in C57 BL/6J mice. Eur J
Pharmacol. 578:159–170. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xiao LZ, Gao LJ and Ma SC: Comparative
study on effects of puerarin and granulocyte colony-stimulating
factor in treating acute myocardial infarction. Zhongguo Zhong Xi
Yi Jie He Za Zhi. 25:210–213. 2005.(In Chinese).
|
|
22
|
Dowman JK, Tomlinson JW and Newsome PN:
Pathogenesis of non-alcoholic fatty liver disease. QJM. 103:71–83.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fon Tacer K and Rozman D: Nonalcoholic
Fatty liver disease: focus on lipoprotein and lipid deregulation. J
Lipids. 2011:7839762011.PubMed/NCBI
|
|
24
|
Day CP and James OF: Steatohepatitis: a
tale of two ‘hits’? Gastroenterology. 114:842–845. 1998.
|
|
25
|
Postic C and Girard J: Contribution of de
novo fatty acid synthesis to hepatic steatosis and insulin
resistance: lessons from genetically engineered mice. J Clin
Invest. 118:829–838. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kugelmas M, Hill DB, Vivian B, Marsano L
and McClain CJ: Cytokines and NASH: a pilot study of the effects of
lifestyle modification and vitamin E. Hepatology. 38:413–419. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tilg H and Diehl AM: Cytokines in
alcoholic and nonalcoholic steatohepatitis. N Engl J Med.
343:1467–1476. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tarantino G, Conca P, Pasanisi F, et al:
Could inflammatory markers help diagnose nonalcoholic
steatohepatitis? Eur J Gastroenterol Hepatol. 21:504–511. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cai D, Yuan M, Frantz DF, et al: Local and
systemic insulin resistance resulting from hepatic activation of
IKK-beta and NF-kappaB. Nat Med. 11:183–190. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gaemers IC and Groen AK: New insights in
the pathogenesis of non-alcoholic fatty liver disease. Curr Opin
Lipidol. 17:268–273. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Reddy JK: Nonalcoholic steatosis and
steatohepatitis. III Peroxisomal beta-oxidation, PPAR alpha, and
steatohepatitis. Am J Physiol Gastrointest Liver Physiol.
281:G1333–G1339. 2001.PubMed/NCBI
|
|
32
|
Anstee QM and Goldin RD: Mouse models in
non-alcoholic fatty liver disease and steatohepatitis research. Int
J Exp Pathol. 87:1–16. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vance JE and Vance DE: The role of
phosphatidylcholine biosynthesis in the secretion of lipoproteins
from hepatocytes. Can J Biochem Cell Biol. 63:870–881. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yao ZM and Vance DE: The active synthesis
of phosphatidylcholine is required for very low density lipoprotein
secretion from rat hepatocytes. J Biol Chem. 263:2998–3004.
1988.PubMed/NCBI
|
|
35
|
Gyamfi MA, Damjanov I, French S and Wan
YJ: The pathogenesis of ethanol versus methionine and choline
deficient diet-induced liver injury. Biochem Pharmacol. 75:981–995.
2008. View Article : Google Scholar : PubMed/NCBI
|