Introduction
Alzheimer’s disease (AD) is the most common form of
dementia. Currently, there is no cure for the disease, which
worsens as it progresses and eventually leads to mortality. The
cause of the majority of Alzheimer’s cases remains unknown. The
most significant hypothesis attempting to explain the cause of the
disease is the cholinergic hypothesis (1), which proposes that AD is caused by
reduced synthesis of the neurotransmitter acetylcholine.
Acetylcholinesterase inhibitors (AChEIs), including
tacrine, rivastigmine, galantamine and donepezil, are currently
used to treat the cognitive manifestations of AD (2). However, AChEIs may cause a broad
spectrum of adverse events in the gastrointestinal system,
including nausea, vomiting and diarrhea (3,4).
These side-effects arise in ∼10–20% of users and are mild to
moderate in severity. These adverse events, which force a number of
patients to stop taking AChEI agents, are generally recognized to
be a result of parasympathetic nervous system activity. AChEIs
ameliorate dementia by inhibiting acetylcholinesterase (AChE) in
the central nervous system (5,6).
The AChEI huperzine A, an alkaloid isolated from
Huperzia serrata, has been used in the treatment of the
cognitive deterioration associated with AD in China (7). It also results in nausea, vomiting
and diarrhea, similar to other AChEIs. To date, it is not known how
quickly these side-effects become tolerated. The present study
aimed to observe the effects of huperzine A on gastrointestinal
motility and AChE activity in mice, following varying periods of
administration, to provide guidance to doctors on how to use
huperzine A so as to attenuate adverse events.
Materials and methods
Chemicals and instruments
Huperzine A tablets were obtained from Henan Tailong
Pharmaceutical Co., Ltd. (Henan, China). Loperamide hydrochloride
capsules were obtained from Xian Janssen Pharmaceutical Ltd. (Xian,
China). All other chemicals and reagents used in this study were of
analytical grade.
Animals
Male Swiss mice weighing 20±2 g were obtained from
the Experimental Animal Center of Luye Pharmaceutical Company
(Shandong, China). All experimental procedures carried out in this
study were performed in accordance with the guidelines for the care
and use of laboratory animals of Yantai University and were
approved by the Ethics Committee of the university. All mice were
housed in diurnal lighting conditions (12 h/12 h) and allowed free
access to food and water.
Gastrointestinal motility
Fifty mice were randomly divided into five groups
(10 animals per group): a vehicle group, a loperamide group (Lop),
a loperamide + 0.05 mg/kg huperzine A group (Lop+Hup A 0.05), a
loperamide + 0.1 mg/kg huperzine A group (Lop+Hup A 0.1) and a
loperamide + 0.2 mg/kg huperzine A group (Lop+Hup A 0.2). The
animals in the vehicle and Lop groups received intragastric
administration of solvent, while huperzine A was administered to
the animals in the Lop+Hup A groups. Each mouse was fasted for 12 h
prior to the gastrointestinal motility test. After single and
multiple dosing (7 or 28 doses, one dose per day), the mice
received an oral administration of 4 mg/kg loperamide, 1 h after
the last administration of huperzine A. Thirty minutes later, each
mouse received an oral administration of 0.2 ml charcoal meal.
After 15 min, each animal was sacrificed and the intestinal
distance of movement of the charcoal meal from the pylorus was
measured and expressed as a percentage of the distance from the
pylorus to the cecum.
AChE activity assays
Following the gastrointestinal motility test, the
brain, stomach and duodenum of mice in each group were separated on
ice and homogenized with ice-cold saline to form a 10% (w/v)
homogenate. AChE activity was determined based on the methods of
Ellman et al(8). Briefly, a
reaction mixture containing 955 μl sodium phosphate (0.1 M,
pH 7.4), 25 μl 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB;
final concentration, 0.5 mM) and 10 μl homogenate was
incubated for 5 min at 37°C, then 10 μl 0.1 M acetylcholine
iodide (final concentration, 1 mM) was added. After incubation for
15 min at 37°C, the absorbance was measured at 412 nm at room
temperature. AChE activity was expressed as U/g protein.
Statistical analysis
Data were analyzed using one-way analysis of
variance (ANOVA) with Bonferroni post hoc test for multiple
t-tests. A value of P<0.05 was considered to indicate a
statistically significant difference. All data in this study were
expressed as mean ± standard deviation.
Results
Effects of huperzine A on
gastrointestinal motility
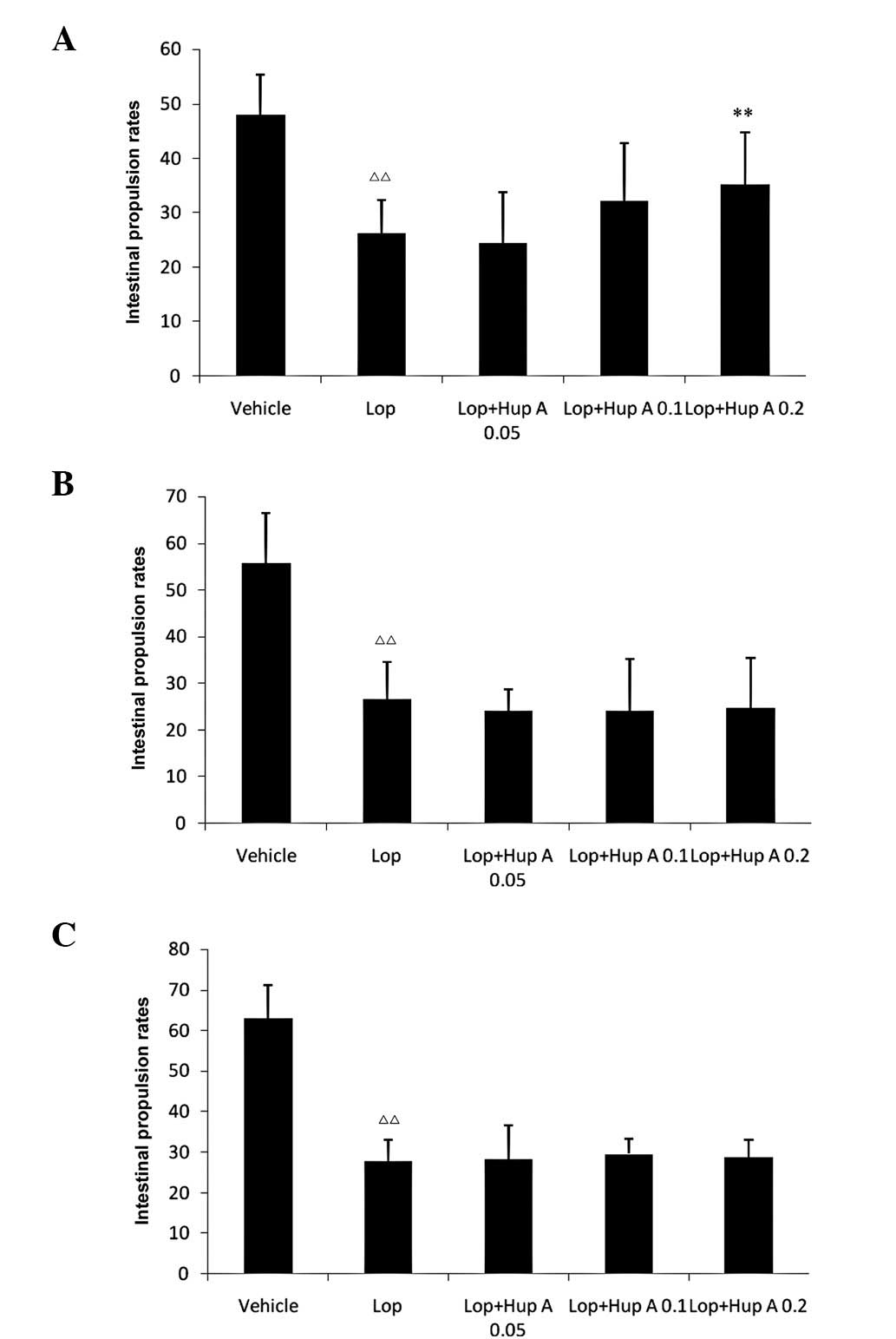
Following a single dose of huperzine A, the
intestinal propulsion rates were significantly increased; however,
following the administration of multiple doses (7 or 28 doses, one
dose per day), no significant differences in intestinal propulsion
rates were observed compared with those in the Lop group (Fig. 1).
Effects of huperzine A on AChE activity
of gastrointestinal tissues in mice
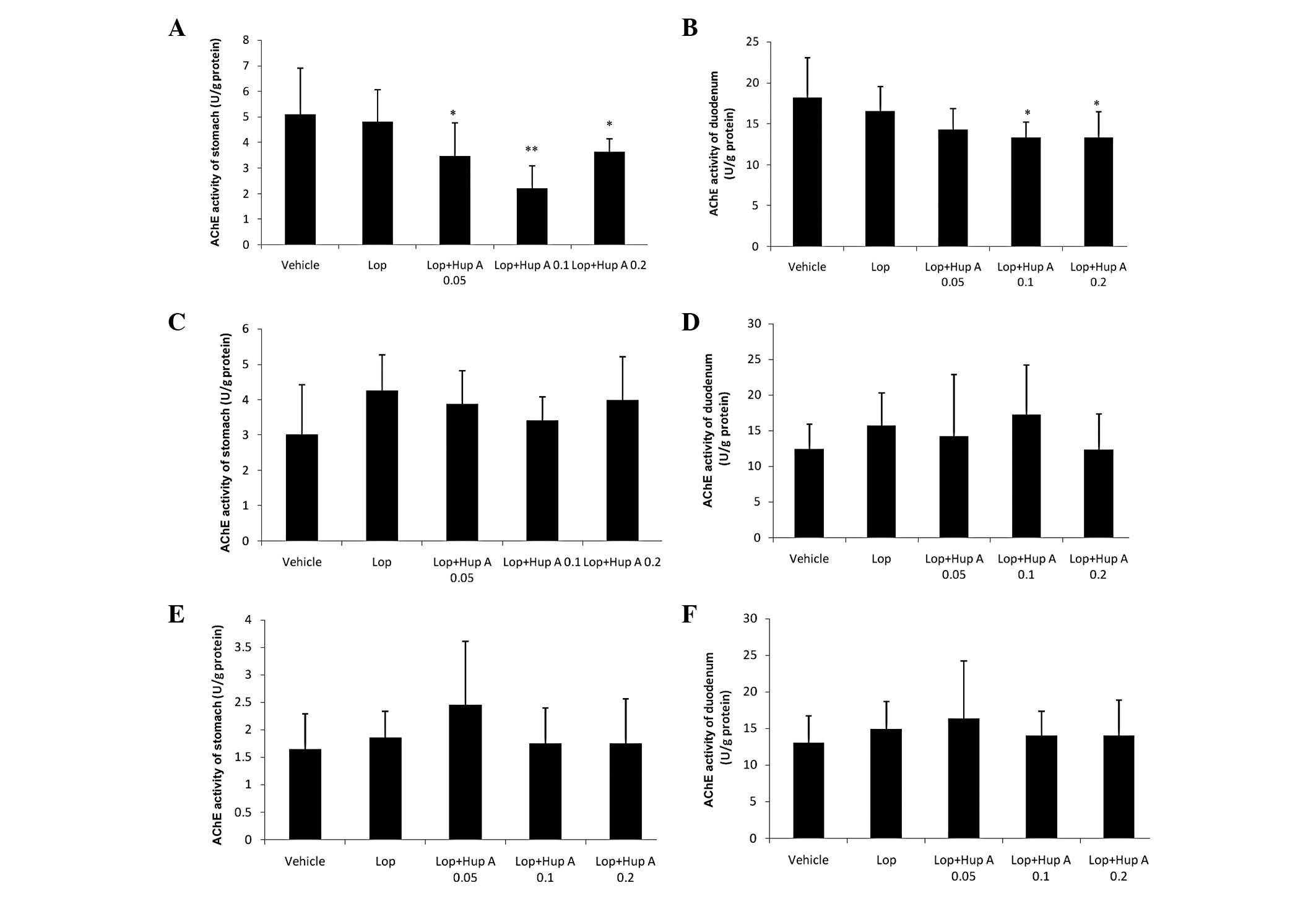
Following a single dose of huperzine A, AChE
activity in the stomach and duodenum was significantly inhibited;
however, following the administration of multiple doses (7 or 28
doses, one dose per day), no significant differences in AChE
activity were observed compared with those in the Lop group
(Fig. 2).
Effects of huperzine A on AChE activity
in the brain
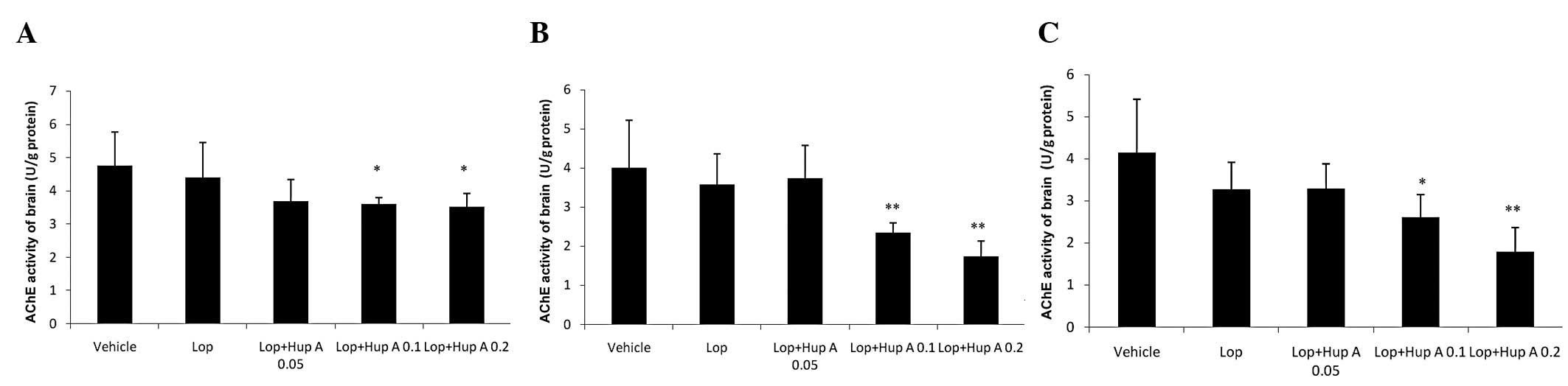
Following single- or multiple-dose administration of
huperzine A, the AChE activities in the brains of the mice were
significantly inhibited compared with that in the Lop group
(Fig. 3).
Discussion
AChEIs have been approved for the symptomatic
treatment of AD for approximately twenty years. However, the
side-effects associated with increased cholinergic activity,
particularly in the gastrointestinal system, prevent patients from
receiving effective doses of the drug. In addition, the advanced
age and frail nature of many patients with AD mean that poor
tolerability is a serious concern.
Gastrointestinal motor activity is mainly regulated
by the neural and hormonal systems (9). Cholinergic neurons are considered to
be the major excitatory neurons involved in gastrointestinal motor
activity since the majority of gastrointestinal contractions are
markedly inhibited by atropine, a muscarinic receptor antagonist
(10,11). Acetylcholine (ACh) is an important
regulator of gastrointestinal motility and the inhibition of AChE
activity has been reported to enhance gastrointestinal motility
(12,13).
In the present study we investigated
gastrointestinal motility and AChE activity in the stomach and
duodenum following single- and multiple-dose oral administration of
huperzine A at therapeutic doses in mice (14,15).
In order to enhance the detection sensitivity of
huperzine A on gastrointestinal motility, mice were administered
loperamide, an opioid-receptor agonist often used against diarrhea,
to slow down the gastrointestinal motility. The results revealed
that the AChE activities in the brains of mice receiving single-
and multiple-dose huperzine A treatment were significantly
reducted, which indicates that the dosage of huperzine A
administered would be effective for AD. After a single dose of
huperzine A, the gastrointestinal AChE activity was reduced and
intestinal propulsion rate was significantly increased, which
demonstrates that gastrointestinal side-effects are likely to occur
during the initial period of treatment with huperzine A. However,
after multiple-dose (7 or 28 doses, one dose per day)
administration, no significant differences in gastrointestinal AChE
activity and intestinal propulsion rates were observed. These
results indicate that huperzine A affects gastrointestinal motility
by inhibiting AChE activity, but after multiple-dose administration
it is well-tolerated in the gastrointestinal system of mice. The
molecular mechanisms explaining how gastrointestinal motility and
AChE activity are unaffected by multiple-dose administration
require further study.
These findings indicate that the gastrointestinal
adverse effects of huperzine A may be well-tolerated relatively
quickly and that patients with AD are likely to have minimal
gastrointestinal side-effects after taking multiple doses of
huperzine A.
Acknowledgements
This study was supported by the
Taishan Scholar Project, a project of Shandong Province Higher
Educational Science and Technology Program (grant no. J10LF76) and
the Foundation for Outstanding Middle-aged and Young Scientists
(grant no. BS2011YY061).
References
|
1.
|
Francis PT, Palmer AM, Snape M and Wilcock
GK: The cholinergic hypothesis of Alzheimer’s disease: a review of
progress. J Neurol Neurosurg Psychiatry. 66:137–147. 1999.
|
|
2.
|
Pohanka M: Cholinesterases, a target of
pharmacology and toxicology. Biomed Pap Med Fac Univ Palacky
Olomouc Czech Repub. 155:219–229. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Birks J: Cholinesterase inhibitors for
Alzheimer’s disease. Cochrane Database Syst Rev.
25:CD0055932006.
|
|
4.
|
Mimica N and Presecki P: Side effects of
approved antidementives. Psychiatr Danub. 21:108–113. 2009.
|
|
5.
|
Farlow M, Veloso F, Moline M, et al:
Safety and tolerability of donepezil 23 mg in moderate to severe
Alzheimer’s disease. BMC Neurol. 11:572011.
|
|
6.
|
Alva G and Cummings JL: Relative
tolerability of Alzheimer’s disease treatments. Psychiatry
(Edgmont). 5:27–36. 2008.
|
|
7.
|
Wang R, Yan H and Tang XC: Progress in
studies of huperzine A, a natural cholinesterase inhibitor from
Chinese herbal medicine. Acta Pharmacol Sin. 27:1–26. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Ellman GL, Courtney KD, Andres V Jr and
Featherstone RM: A new and rapid colorimetric determination of
acetylcholinesterase activity. Biochem Pharmacol. 7:88–95. 1961.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Rogers RC, McTigue DM and Hermann GE:
Vagal control of digestion: modulation by central neural and
peripheral endocrine factors. Neurosci Biobehav Rev. 20:57–66.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Shiba Y, Mizumoto A, Inatomi N, Haga N,
Yamamoto O and Itoh Z: Stimulatory mechanism of EM523-induced
contractions in postprandial stomach of conscious dogs.
Gastroenterology. 109:1513–1521. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Furuichi A, Makimoto N, Ogishima M, et al:
In vivo assessment of the regulatory mechanism of cholinergic
neuronal activity associated with motility in dog small intestine.
Jpn J Pharmacol. 86:73–78. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Iwanaga Y, Miyashita N, Morikawa K,
Mizumoto A, Kondo Y and Itoh Z: A novel water-soluble dopamine-2
antagonist with anticholinesterase activity in gastrointestinal
motor activity. Comparison with domperidone and neostigmine.
Gastroenterology. 99:401–408. 1990.
|
|
13.
|
Ueki S, Seiki M, Yoneta T, et al:
Gastroprokinetic activity of nizatidine, a new H2-receptor
antagonist, and its possible mechanism of action in dogs and rats.
J Pharmacol Exp Ther. 264:152–157. 1993.PubMed/NCBI
|
|
14.
|
Wang Y, Tang XC and Zhang HY: Huperzine A
alleviates synaptic deficits and modulates amyloidogenic and
nonamyloidogenic pathways in APPswe/PS1dE9 transgenic mice. J
Neurosci Res. 90:508–517. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Zhu XD and Tang XC: Improvement of
impaired memory in mice by huperzine A and huperzine B. Zhongguo
Yao Li Xue Bao. 9:492–497. 1988.(In Chinese).
|