Introduction
Lung cancer remains the most common human cancer in
the worldwide, with non-small cell lung cancer (NSCLC) accounting
for ∼80% of cases (1,2). Despite great achievements made over
the past decades in surgery, radiotherapy and chemotherapy, the
5-year survival rate of lung cancer in many countries is <15%
(3). Chemotherapy remains the
mainstay of treatments of lung cancer and cisplatin is one of the
most widely used first-line chemotherapeutic agents for NSCLC
treatment (4). However, these
therapeutic strategies are unsatisfactory due to side effects
experienced and drug resistance. Thus, identification of new
therapeutic agents that exert combination effects with cisplatin
for the treatment of NSCLC is necessary.
Osthole (Fig. 1), a
natural compound, is extracted from various medicinal plants such
as Cnidium monnieri (L.) Cusson. In recent years,
numerous studies have focused on the potential of osthole in cancer
therapy. Osthole reportedly possesses anticancer effects by
inhibiting cancer cell growth, metastasis and inducing cell
apoptosis (5–9). Recently, we reported that osthole
induces G2/M arrest and apoptosis by modulating the PI3K/Akt
pathway and suppresses migration and invasion through inhibition of
matrix metalloproteinase-2 and matrix metallopeptidase-9 in human
lung adenocarcinoma A549 cells (10,11).
However, the combination effects of osthole and cisplatin on human
lung cancer cells remain unclear.
The aim of the present study was to investigate the
anti-cancer activity of combining osthole with cisplatin in human
large cell lung carcinoma NCI-H460 cells in vitro. The
results demonstrated that the combination of osthole and cisplatin
resulted in greater efficacy in growth inhibition and apoptosis
induction in NCI-H460 lung cancer cells.
Materials and methods
Reagents and chemicals
Osthole and cisplatin were purchased from the
National Institute for the control of Pharmaceutical and Biological
Products (Beijing, China). Cell culture RPMI-1640 medium,
antibiotics and trypsin were purchased from Biological Industries
(Kibutz Beit Haemek, Israel). Fetal bovine serum (FBS) was
purchased from Solarbio Science & Technology Co., Ltd.
(Beijing, China). 3-(4,5-dimethyl
thiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT), dimethyl
sulfoxide (DMSO) and Hoechst 33342 were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Annexin V-FITC and PI double
staining kit were purchased from Key Gene (Nanjing, China).
Antibodies were purchased from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA). The other reagents were procured
locally.
Cell culture
Human large cell lung carcinoma NCI-H460 cells were
obtained from the China Center for Type Culture Collection (Wuhan,
China) and maintained in RPMI-1640 supplemented with 10% FBS, 100
U/ml penicillin and 100 μg/ml streptomycin at 37°C in a
humidified atmosphere of 5% CO2.
Cell viability assay
The effect of osthole, cisplatin and their
combination on the proliferation of NCI-H460 cells was measured by
MTT assay. Briefly, NCI-H460 cells were plated at a density of
1×104 cells per well in 96-well plates overnight and
then treated with 0, 50, 100 and 200 μmol/l osthole; 1.5,
3.0 and 6.0 μmol/l cisplatin or their combination (50
μmol/l osthole plus 1.5 μmol/l cisplatin ) for 48 h.
Twenty microliters of MTT solution (2 mg/ml in PBS) were added to
each well and the cells were cultured for another 4 h at 37°C. The
medium was completely removed and 150 μl DMSO was added to
solubilize MTT formazan crystals. The plates were then agitated and
the optical density was determined at 570 nm (OD570) using an ELISA
plate reader (Model 550; Bio-Rad, Hercules, CA, USA). At least
three independent experiments were performed.
Annexin V/PI flow cytometric
analysis
Apoptotic rates were determined by flow cytometry
using an Annexin V-FITC apoptosis kit. Briefly, NCI-H460 cells were
seeded at a density of 1×106 cells per well in 6-well
plates overnight and then treated with 50 μmol/l osthole,
1.5 μmol/l cisplatin or their combination for 48 h. Cells
(1×106) were collected by centrifugation (326 × g) and
washed twice with cold PBS. Staining was performed according to the
manufacturer’s instructions and the cells were analyzed using a
FACScan flow cytometer and analyzed using CellQuest software
(Becton-Dickinson, Redlands, CA, USA). At least three independent
experiments were performed.
Fluorescence microscopy
NCI-H460 cells (1×106) were seeded in
6-well plates overnight and then treated with 50 μmol/l
osthole, 1.5 μmol/l cisplatin or their combination for 48 h.
Cells were washed twice with PBS and fixed with cold methanol and
acetic acid (3/1, v/v) overnight, then stained with Hoechst 33342
(1 mg/ml) for 30 min in the dark. Stained cells were observed with
a fluorescence microscope (magnification, ×400) (Nikon, Tokyo,
Japan).
Western blot analysis
NCI-H460 cells (1×106) were seeded in
6-well plates overnight. Cells were treated as described above.
Cells were then washed twice with ice-cold PBS. The total proteins
were solubilized and extracted with lysis buffer (20 mM HEPES, pH
7.9, 20% glycerol, 200 mM KCl, 0.5 mM EDTA, 0.5% NP40, 0.5 mM DTT,
1% protease inhibitor cocktail). Protein concentration was
determined by bicinchoninic acid protein assay. The samples were
separated by SDS-PAGE, transferred to PVDF membranes by
electroblotting and probed with anti-Bax, anti-Bcl-2 and
anti-actin. Membranes were then incubated with horseradish
peroxidase-conjugated secondary antibodies. Blots were developed
using an ECL kit.
Statistical analysis
The experiments were performed for three times. Data
were expressed as the mean ± standard deviation (SD). Statistical
correlation of data was checked for significance by ANOVA and
Student’s t test. P<0.05 was considered to indicate a
statistically significant difference. These analyses were performed
using SPSS 13.0 software.
Results
Combined effect of osthole and cisplatin
on NCI-H460 cell viability
To investigate the combined effect of osthole and
cisplatin on the cell viability of NCI-H460 lung cancer cells, the
cells were treated with various doses of osthole and cisplatin for
48 h, respectively. Cell viability was determined by MTT assay. It
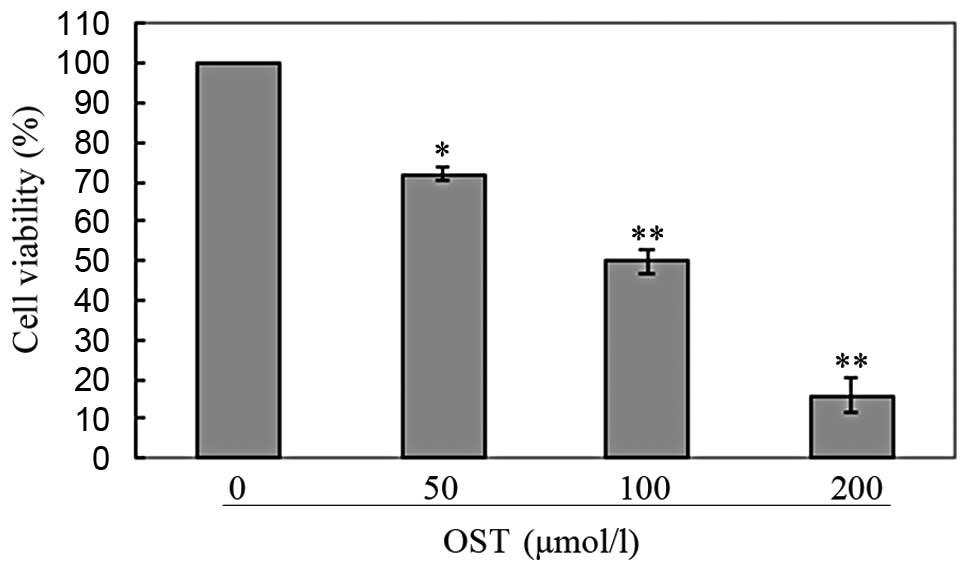
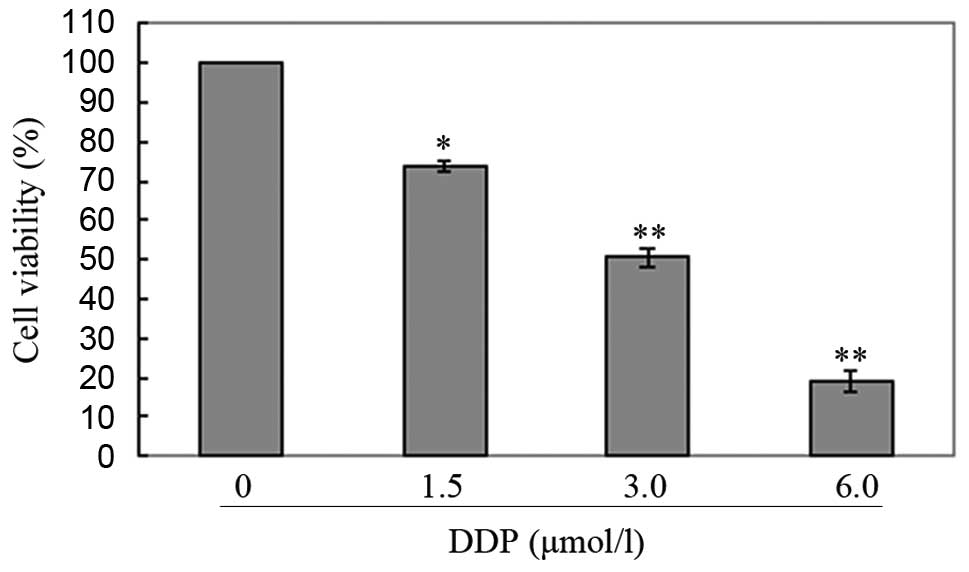
was noted that both osthole (Fig.
2) and cisplatin (Fig. 3)
inhibited cell proliferation in a dose-dependent manner. Based on
these results, we selected a moderate concentration (50
μmol/l osthole plus 1.5 μmol/l cisplatin) for
combination treatment. Compared with monotherapy, combination
treatment inhibited cell proliferation more significantly (Fig. 4).
Combined effect of osthole and cisplatin
on NCI-H460 cell apoptosis
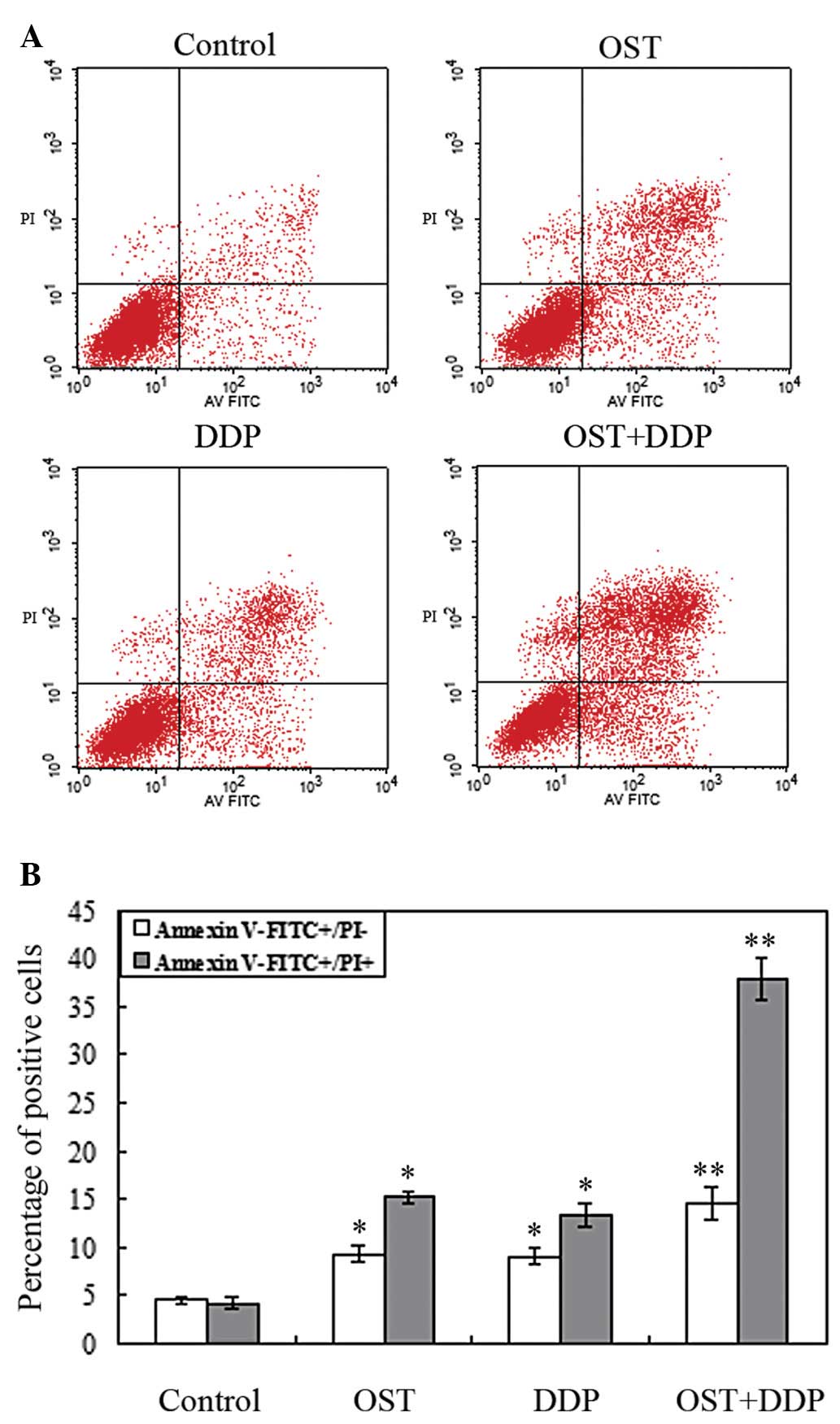
The apoptosis-inducing effect of osthole, cisplatin
and their combination was evaluated by Annexin V/PI staining.
NCI-H460 cells were treated with 50 μmol/l osthole, 1.5
μmol/l cisplatin or their combination for 48 h and were
analyzed by flow cytometry to analyze apoptosis. As shown in
Fig. 5A and B, osthole or
cisplatin alone induced NCI-H460 cell apoptosis. The percentage of
early and late apoptotic cells was increased compared to the
control group.
Compared with monotherapy, the percentage of
apoptotic cells induced by their combination was significantly
higher.
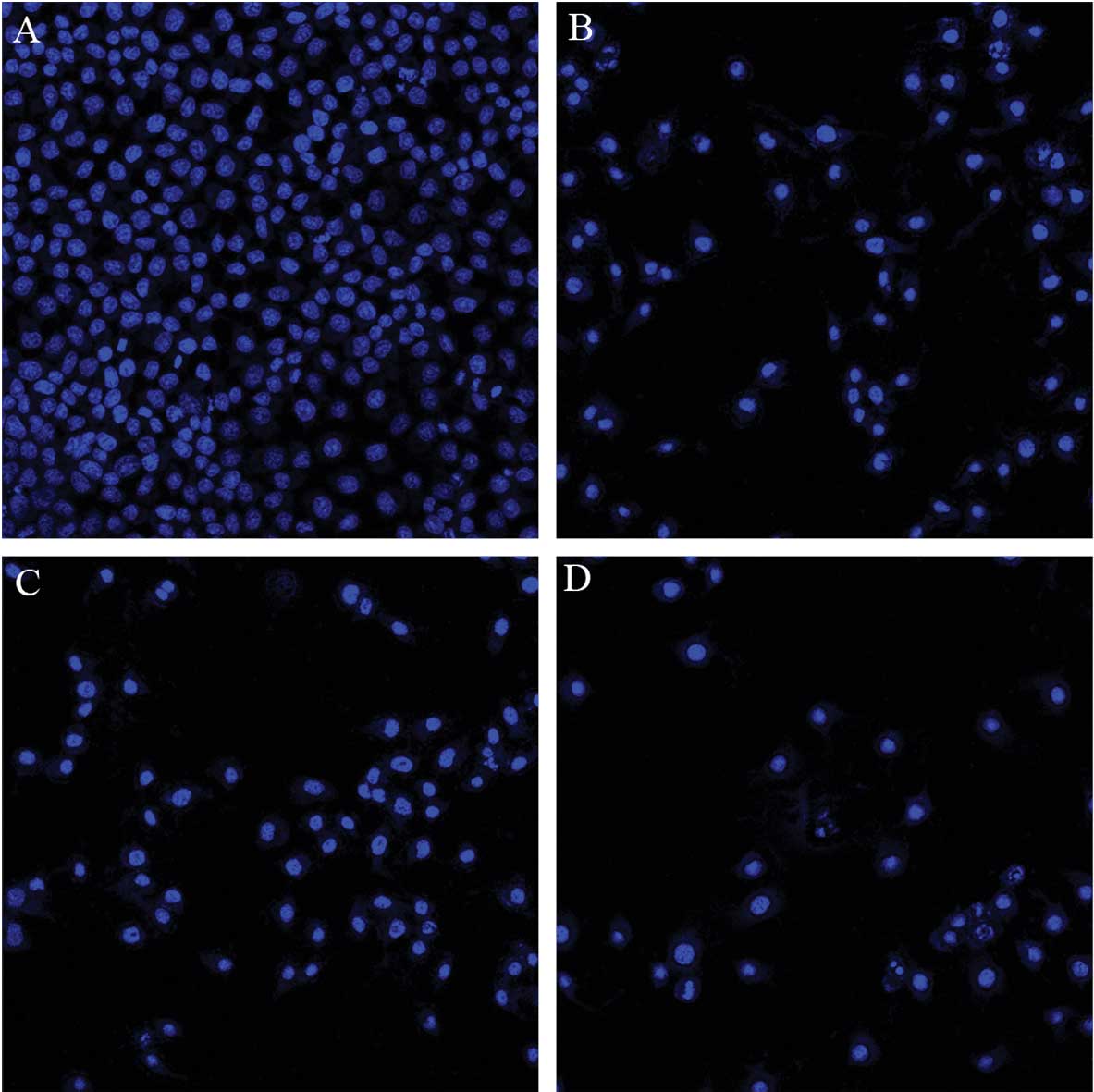
Following incubation with 50 μmol/l osthole,
1.5 μmol/l cisplatin or their combination for 48 h, the
cells were examined by fluorescence microscopy. Condensation of
chromatin, nuclear fragmentations and apoptotic bodies were clearly
identified in treated cells (Fig.
6). Compared with monotherapy, apoptotic cells significantly
increased in the combination treatment.
Combined effect of osthole and cisplatin
on the expression of Bcl-2 family proteins
To investigate the molecular mechanism of the
combined anticancer effect of osthole with cisplatin, we tested the
effect of osthole, cisplatin or their combination on the expression
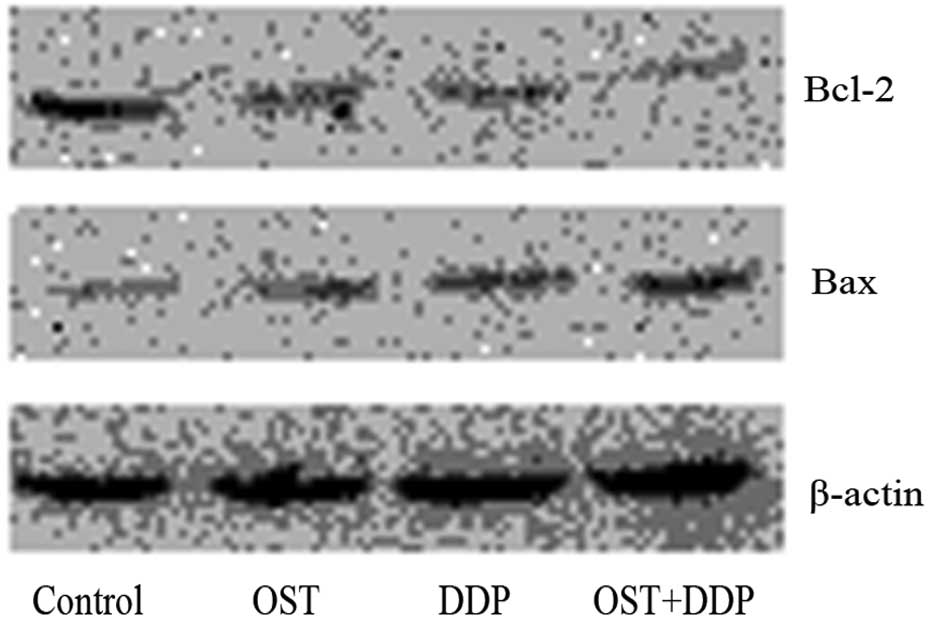
of Bcl-2 and Bax. As shown in Fig.
7, western blot analysis revealed that treatment with either
osthole or cisplatin decreased Bcl-2 expression and increased Bax
expression. Compared with monotherapy, combination treatment with
osthole and cisplatin decreased Bcl-2 expression and increased Bax
expression more significantly.
Discussion
Drug combination therapies are common practice in
the treatment of cancer. At present, cisplatin is the most active
chemotherapeutic agent for the treatment of NSCLC and is usually
combined with other agents such as docetaxel, gemcitabine and
paclitaxel (4). However, its use
is limited due to severe side effects such as anemia,
neurotoxicity, nephrotoxicity and the acquisition of drug
resistance (12,13). To address these problems, attention
has been focused on identifying novel agents that can be combined
with cisplatin to increase the therapeutic efficacy and decrease
side effects.
Recently, several studies reported that some natural
agents combined with cisplatin enhanced the anticancer effects of
various types of cancer (14–18).
The combinatorial use of conventional chemotherapeutic agents and
these natural compounds exert enhanced anticancer activity through
combination effects. Combination treatment may also decrease the
side effects since efficacy can be achieved with lower doses. In
the present study, we have studied the effect of combining osthole,
a natural compound extracted from various medicinal plants, with
cisplatin on NCI-H460 lung cancer cells.
The mechanism of action of cisplatin includes the
inhibition of cell proliferation and induction of cell apoptosis
(19). To determine whether
osthole enhances the anticancer effect of cisplatin, NCI-H460 cells
were treated with osthole alone or in combination with cisplatin
and cell growth and apoptosis were evaluated using MTT assay, flow
cytometry assay and fluorescence microscopy. We found that in
comparison with single agent treatment, the combination of osthole
and cisplatin produced greater efficacy in growth inhibition and
apoptosis induction, suggesting that osthole plays a combination
role in cisplatin-induced apoptosis and growth inhibition in lung
cancer cells.
Bcl-2 family proteins are key regulators of the
apoptotic pathway (20). To
elucidate the molecular mechanisms of the combined anticancer
effect of osthole with cisplatin, we investigated the effects of
osthole alone and in combination with cisplatin on Bcl-2 family
proteins in NCI-H460 cells using western blot analysis. Our results
showed that treatment of NCI-H460 cells with osthole in combination
with cisplatin significantly decreased Bcl-2 expression and
increased Bax expression, indicating that these agents induced
apoptosis through regulation of the expression of Bcl-2 family
proteins.
In conclusion, findings of the present study have
shown that osthole enhanced the anticancer effect of cisplatin on
human lung cancer cells in vitro. This investigation
suggests that the combination of osthole and cisplatin may serve as
an important potential clinical chemotherapeutic approach in human
lung cancer.
References
|
1.
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2.
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar
|
|
3.
|
Erridge SC, Moller H, Price A and Brewster
D: International comparisons of survival from lung cancer: pitfalls
and warnings. Nat Clin Pract Oncol. 4:570–577. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Schiller JH, Harrington D, Belani CP,
Langer C, Sandler A, Krook J, Zhu J and Johnson DH: Comparison of
four chemotherapy regimens for advanced non-small-cell lung cancer.
N Engl J Med. 346:92–98. 2002. View Article : Google Scholar
|
|
5.
|
Hung CM, Kuo DH, Chou CH, Su YC, Ho CT and
Way TD: Osthole suppresses hepatocyte growth factor (HGF)-induced
epithelial-mesenchymal transition via repression of the
c-Met/Akt/mTOR pathway in human breast cancer cells. J Agric Food
Chem. 59:9683–9690. 2011. View Article : Google Scholar
|
|
6.
|
Zhang L, Jiang G, Yao F, He Y, Liang G,
Zhang Y, Hu B, Wu Y, Li Y and Liu H: Growth inhibition and
apoptosis induced by osthole, a natural coumarin, in hepatocellular
carcinoma. PLoS One. 7:e378652012. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Chou SY, Hsu CS, Wang KT, Wang MC and Wang
CC: Antitumor effects of Osthol from Cnidium monnieri: an in vitro
and in vivo study. Phytother Res. 21:226–230. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Kao SJ, Su JL, Chen CK, Yu MC, Bai KJ,
Chang JH, Bien MY, Yang SF and Chien MH: Osthole inhibits the
invasive ability of human lung adenocarcinoma cells via suppression
of NF-κB-mediated matrix metalloproteinase-9 expression. Toxicol
Appl Pharmacol. 261:105–115. 2012.PubMed/NCBI
|
|
9.
|
Yang D, Gu T, Wang T, Tang Q and Ma C:
Effects of osthole on migration and invasion in breast cancer
cells. Biosci Biotechnol Biochem. 74:1430–1434. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Xu X, Zhang Y, Qu D, Jiang T and Li S:
Osthole induces G2/M arrest and apoptosis in lung cancer A549 cells
by modulating PI3K/Akt pathway. J Exp Clin Cancer Res. 30:332011.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Xu XM, Zhang Y, Qu D, Feng XW, Chen Y and
Zhao L: Osthole suppresses migration and invasion of A549 human
lung cancer cells through inhibition of matrix metalloproteinase-2
and matrix metallopeptidase-9 in vitro. Mol Med Rep.
6:1018–1022. 2012.PubMed/NCBI
|
|
12.
|
Douillard JY, Eckardt J and Scagliotti GV:
Challenging the platinum combinations in the chemotherapy of NSCLC.
Lung Cancer. 38(Suppl 4): 21–28. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Stewart DJ: Mechanisms of resistance to
cisplatin and carboplatin. Crit Rev Oncol Hematol. 63:12–31. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Zhang Y, Wang C, Wang H, Wang K, Du Y and
Zhang J: Combination of Tetrandrine with cisplatin enhances
cytotoxicity through growth suppression and apoptosis in ovarian
cancer in vitro and in vivo. Cancer Lett. 304:21–32. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Duarte VM, Han E, Veena MS, Salvado A, Suh
JD, Liang LJ, Faull KF, Srivatsan ES and Wang MB: Curcumin enhances
the effect of cisplatin in suppression of head and neck squamous
cell carcinoma via inhibition of IKKβ protein of the NFκB pathway.
Mol Cancer Ther. 9:2665–2675. 2010.PubMed/NCBI
|
|
16.
|
Jafri SH, Glass J, Shi R, Zhang S, Prince
M and Kleiner-Hancock H: Thymoquinone and cisplatin as a
therapeutic combination in lung cancer: In vitro and in vivo. J Exp
Clin Cancer Res. 29:872010. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Li H, Zhu X, Zhang Y, Xiang J and Chen H:
Arsenic trioxide exerts synergistic effects with cisplatin on
non-small cell lung cancer cells via apoptosis induction. J Exp
Clin Cancer Res. 28:1102009. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Ji NF, Yao LS, Li Y, He W, Yi KS and Huang
M: Polysaccharide of Cordyceps sinensis enhances cisplatin
cytotoxicity in non-small cell lung cancer H157 cell line. Integr
Cancer Ther. 10:359–367. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Yoshizumi N, Fujiwara J, Yoshizaki A, Sato
M, Sakai R and Nishiya I: Cytokinetic effects of carboplatin and
cisplatin on a human ovarian cancer cell line. Hum Cell. 1:301–307.
1988.(In Japanese).
|
|
20.
|
Thees S, Hubbard GB, Winckler J, Schultz C
and Rami A: Specific alteration of the Bax/Bcl2 ratio and
cytochrome c without execution of apoptosis in the hippocampus of
aged baboons. Restor Neurol Neurosci. 23:1–9. 2005.PubMed/NCBI
|