Introduction
Immediate reperfusion in an infarct-related artery
is associated with a favorable outcome in the treatment of acute
myocardial infarction (AMI). However, specific patients continue to
have poor cardiac function following this procedure, which leads to
a poor prognosis (1).
Angiopoietin (Ang)-1, a secreted 70-kDa glycoprotein
constitutively expressed by pericytes and vascular smooth muscle
cells, is a major agonist for the tyrosine kinase receptor Tie-2
(2). Binding of Ang-1 to Tie-2
promotes vessel integrity, inhibits vascular leakage and suppresses
inflammatory gene expression (3).
Ang-2 is also a secreted 70-kDa glycoprotein and is exclusively
expressed by endothelial cells and acts as an antagonist for Tie-2
(4). Ang-2 has been reported to
completely disrupt protective Tie-2 signaling in numerous studies
(3). Since Ang-1 and Ang-2 are
antagonistic ligands, the Ang-2/Ang-1 ratio (Ang-2/Ang-1) reflects
the imbalance between them.
Ang-1 and Ang-2 have been revealed to participate in
a number of cardiovascular diseases (5–13).
Atherosclerotic plaque microvessel density is associated with
plaque hemorrhage and rupture (14). In plaques with high microvessel
density, the balance between Ang-1 and Ang-2 is in favor of Ang-2,
indicating a role for Ang-2 in the development of unstable plaques
(5). Clinical studies have
reported that higher plasma Ang-2 levels are predictive of
myocardial infarction (6,7) and stroke recurrence (8) and are independent of traditional risk
factors. Our previous study (9)
demonstrated that serum Ang-1, Ang-2 and Ang-2/Ang-1 are elevated
in AMI patients. The extent of myocardial damage may correlate with
serum Ang-2 and Ang-2/Ang-1, indicating that Ang-2 and Ang-2/Ang-1
are potential biomarkers of the severity of the disease. However,
little is known regarding the role of Ang-1, Ang-2 and Ang-2/Ang-1
in predicting outcomes in AMI patients.
Previous studies revealed that Ang-1 binds integrins
on cardiomyocytes and plays a cardioprotective role in cardiac
remodeling (15,16). In addition, circulating Ang-2
levels have been identified to be elevated in heart failure (HF)
patients (17,18), indicating that Ang-2 may
participate in the progression of HF. However, whether circulating
Ang-1, Ang-2 and Ang-2/Ang-1 are predictive of HF in AMI patients
remains unknown. Therefore, the aim of this study was to
investigate the association of serum Ang-1, Ang-2 and Ang-2/Ang-1
with HF in AMI patients during hospitalization.
Materials and methods
Patient selection
This study included 103 consecutive patients (88
males and 15 females). The average age was 57.6±12.1 years old.
Patients were admitted to the Department of Cardiology, Peking
University Third Hospital with first ST-segment elevation
myocardial infarction (STEMI) between October 2010 and January
2012. STEMI was diagnosed according to the 2004 guidelines of the
American College of Cardiology/American Heart Association. All
patients received primary percutaneous coronary intervention (PCI)
within 12 h from symptom onset. The exclusion criteria were: age
>80 years, previous history of myocardial infarction,
significant valvular heart disease, peripheral vascular disease,
chronic HF, chronic inflammatory diseases, significant kidney or
hepatic diseases or tumor. Heart failure was defined as Killip
Class ≥II during hospitalization. This study was conducted in
accordance with the Declaration of Helsinki and with approval from
the Ethics Committee of Peking University Health Science Center.
Written informed consent was obtained from all participants.
Laboratory assays
Venous blood samples were obtained from all subjects
at admission to hospital. All samples were collected in vacuum
blood collection tubes with clot activator and were immediately
placed in 4°C refrigerators. Within 30 min following collection,
samples were centrifuged at 3,000 rpm for 10 min at 4°C, divided
into aliquots and stored at −80°C until analysis. Repeated
freeze-thaw cycles were avoided.
Serum Ang-1 and Ang-2 levels were measured by
enzyme-linked immunosorbent assay (ELISA) according to the
manufacturer’s instructions (R&D Systems, Minneapolis, MN,
USA). The minimal detection limit was 156 pg/ml. These assays were
performed by an investigator blinded to the sample sources. The
Ang-2/Ang-1 ratio was calculated.
A previous study revealed that N-terminal pro-B-type
natriuretic peptide (NT-proBNP) levels in STEMI patients increased
markedly within 24 h following successful primary PCI and then
decreased (19). Therefore,
NT-proBNP was measured 24 h following PCI, using an E170 Elecsys
Modular Analytics assay (Roche Diagnostics GmbH, Mannheim,
Germany).
Echocardiography
Each patient underwent echocardiography 24 h
following primary PCI, using a GE-Vingmed V echocardiographic
machine (Vivid 7; GE Healthcare, Wauwatosa, WI, USA) with a 3.3-MHz
multiphase array probe. Left ventricular ejection fraction (LVEF)
was obtained using a modified biplane version of Simpson’s method
with apical two- and four-chamber views. These examinations were
performed by experienced cardiologists.
Statistical analysis
Comparisons between groups were conducted by
Student’s unpaired t-test, Mann-Whitney U test or Chi-square test.
For correlation analysis, the NT-proBNP level was transformed to
the natural logarithm (Ln). The Spearman or Pearson correlation was
used to identify the bivariate correlations. Multivariable logistic
regression analyses were performed to determine whether Ang-1,
Ang-2 and Ang-2/Ang-1 were independently associated with HF during
hospitalization. P<0.05 was considered to indicate a
statistically significant difference. All analyses were performed
using SPSS for Windows v15.0 (SPSS, Inc., Chicago, IL, USA).
Results
Clinical characteristics and laboratory
observations
Among the 103 patients included in this study, 20
developed HF during hospitalization (Killip class II, n=16; III,
n=3; and IV, n=1).
Table I summarizes
the clinical characteristics and laboratory observations of
patients with or without HF. Serum Ang-2 levels and Ang-2/Ang-1
ratio were observed to be significantly higher in patients with HF
than in patients without HF (2,203.1±122.0 vs. 2,102.3±114.4 pg/ml,
P=0.001 and 11.4±1.6×10−2 vs. 10.6±1.1×10−2,
P=0.007). Other variables associated with HF in univariate analysis
included gender (male; 70.0 vs. 89.2%, P=0.029), age (64.9±11.7 vs.
55.9±11.6 years, P=0.002), LVEF (48.1±7.3 vs. 53.3±7.6%, P=0.007),
NT-proBNP [3,369 (1,112–4,778) vs. 829 (375–1,379) pg/ml,
P<0.001] and peak cTnT [6.9 (3.3–10) vs. 3.1 (2.0–5.3) ng/ml,
P=0.002].
 | Table I.Clinical characteristics and
laboratory observations. |
Table I.
Clinical characteristics and
laboratory observations.
| All patients
(n=103) | HF (n=20) | No HF (n=83) | P-value |
|---|
| Male, n (%) | 88 (85.4) | 14 (70.0) | 74 (89.2) | 0.029 |
| Age, years | 57.6±12.1 | 64.9±11.7 | 55.9±11.6 | 0.002 |
| Hypertension, n
(%) | 49 (47.6) | 12 (60.0) | 37 (44.6) | 0.319 |
| Diabetes mellitus, n
(%) | 24 (23.3) | 5 (25.0) | 19 (22.9) | 0.842 |
| Hyperlipidemia, n
(%) | 46 (44.7) | 8 (40.0) | 38 (45.8) | 0.803 |
| Current smokers, n
(%) | 80 (77.7) | 13 (65.0) | 67 (80.7) | 0.103 |
| Systolic blood
pressure, mmHg | 137.4±29.9 | 136.0±38.7 | 137.8±27.6 | 0.819 |
| Heart rate,
beats/minute | 74.4±14.9 | 74.4±17.4 | 74.4±14.3 | 1.000 |
| Time from symptom
onset to reperfusion, min | 240 (193–367) | 285 (220–400) | 230 (180–365) | 0.168 |
| Culprit vessel | | | | |
| LAD, n (%) | 57 (55.3) | 12 (60.0) | 45 (54.2) | 0.803 |
| Other vessels, n
(%) | 46 (44.7) | 8 (40.0) | 38 (45.8) | |
| LVEF, % | 52.2±7.8 | 48.1±7.3 | 53.3±7.6 | 0.007 |
| NT-proBNP, pg/ml | 1,027
(450–1,941) | 3,369
(1,112–4,778) | 829 (375–1,379) | <0.001 |
| Peak cTnT, ng/ml | 3.8 (2.0–6.8) | 6.9 (3.3–10) | 3.1 (2.0–5.3) | 0.002 |
| Hs-CRP, mg/l | 6.3 (2.5–15.0) | 7.0 (4.7–31.2) | 6.2 (2.1–13.2) | 0.088 |
| WBC count,
109/l | 10.6±3.5 | 10.2±3.5 | 10.7±3.5 | 0.586 |
| Hb, g/L | 147.3±16.5 | 141.8±21.3 | 148.7±14.9 | 0.092 |
| Serum creatinine,
umol/l | 75.8±13.7 | 76.6±11.6 | 75.6±14.2 | 0.782 |
| Ang-1, pg/ml | 19,885.0±1,891.3 | 19,575.5±2,425.5 | 19,959.6±1,748.5 | 0.418 |
| Ang-2, pg/ml | 2,121.9±122.1 | 2,203.1±122.0 | 2,102.3±114.4 | 0.001 |
| Ang-2/Ang-1,
10−2 | 10.8±1.2 | 11.4±1.6 | 10.6±1.1 | 0.007 |
Association between serum angiopoietins
and clinical variables
Serum Ang-2 levels and Ang-2/Ang-1 ratio were
negatively correlated with LVEF (r=−0.352, P<0.001 and r=−0.365,
P<0.001, respectively; Fig. 1)
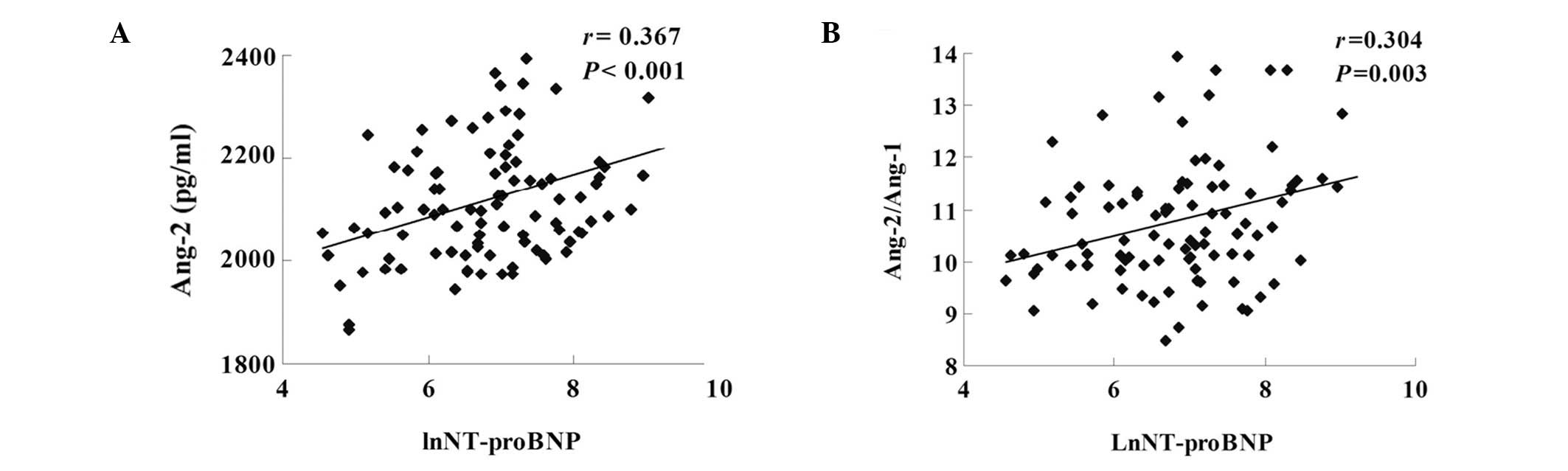
and positively correlated with LnNT-proBNP (r=0.367, P<0.001 and
r=0.304, P=0.003, respectively; Fig.
2) and peak cTnT (r=0.421, P<0.001 and r=0.278, P=0.009,
respectively; Fig. 3). However,
serum Ang-1 levels were not significantly correlated with LVEF
(r=0.194, P=0.05), LnNT-proBNP (r=−0.116, P=0.266) or peak cTnT
(r=−0.056, P=0.607). Serum Ang-1, Ang-2 and Ang-2/Ang-1 were not
observed to be significantly associated with age, gender,
comorbidities (hypertension, diabetes mellitus or hyperlipidemia),
smoking status, time from symptom onset to reperfusion, hemodynamic
variables (systolic blood pressure or heart rate) or laboratory
observations other than LnNT-proBNP and peak cTnT (data not
shown).
Association between serum angiopoietins
and heart failure
In the multivariable logistic regression analysis,
Ang-2 (P=0.031), Ang-2/Ang-1 (P=0.018) and NT-proBNP (P=0.001) were
independently associated with HF, following adjustment for age,
gender, hypertension, diabetes mellitus, time from symptom onset to
reperfusion, LVEF, serum creatinine, hemoglobin (Hb) level and peak
cTnT (Table II).
 | Table II.Multivariable predictors of heart
failure. |
Table II.
Multivariable predictors of heart
failure.
| OR | 95% CI | P-value |
|---|
| NT-proBNP | 1.001 | 1.000–1.002 | 0.001 |
| Ang-2 | 1.011 | 1.003–1.019 | 0.031 |
| Ang-2/1 | 4.306 | 1.287–14.406 | 0.018 |
Discussion
Ang-1 and Ang-2 are well known to be associated with
several forms of cardiovascular (6–14)
and inflammatory diseases (20,21).
Previous studies revealed that circulating Ang-2 levels and the
Ang-2/Ang-1 ratio may be suitable biomarkers of inflammatory
diseases. In patients with severe sepsis, circulating Ang-2 levels
correlate with markers of endothelial cell activation and 28-day
mortality (20). In acute lung
injury patients, Ang-2/Ang-1 is an independent predictor of
mortality (21).
AMI is also characterized by inflammatory processes
and endothelial dysfunction (22).
The prognosis for AMI and the preservation of cardiac function have
been significantly improved by PCI and proper cardiac care unit
management. However, specific patients still exhibit poor cardiac
function and clinical course (1).
Our previous study (9)
demonstrated that serum Ang-1, Ang-2 and Ang-2/Ang-1 are elevated
in AMI patients. However, whether circulating Ang-1, Ang-2 and
Ang-2/Ang-1 are predictive of HF in AMI patients remains unknown.
The current study is the first to investigate the predictive value
of Ang1, Ang-2 and Ang2/Ang-1 in AMI patients.
This study identified that the serum Ang-2 levels
and the Ang-2/Ang-1 ratio were positively correlated with
LnNT-proBNP and negatively correlated with LVEF, indicating that
higher Ang-2 and Ang-2/Ang-1 were associated with poor cardiac
function in AMI patients. In the multivariable logistic regression
analysis, Ang-2 and Ang-2/Ang-1 were independently associated with
HF in AMI patients during hospitalization.
First, the extent of myocardial damage may be linked
to the level of circulating Ang-2 and the Ang-2/Ang-1 ratio.
Myocardial ischemia and reperfusion may not only injure
cardiomyocytes, but also cause endothelial activation and injury,
which increase vascular permeability and the recruitment of
inflammatory cells (22). Ang-1
and Ang-2 play divergent roles in vascular quiescence and
inflammation. Ang-2 mediates vascular leakage (23) and inflammation (24), while Ang-1 has anti-permeability
(23), anti-inflammatory (25) and cardioprotective effects
(15). In an animal myocardial
infarction model, adenoviral vectors carrying Ang-2 have been
demonstrated to increase infarct size (26), while the administration of
adenovirus expressing Ang-1 in an animal ischemia/reperfusion model
prevented vascular leakage and cardiomyocyte death and enhanced
cardiac function (27).
Second, Ang-1 and Ang-2 may participate in the
progression of HF. A previous study by Chong et al which
included 39 patients with acute HF, 40 patients with chronic HF and
17 healthy controls, identified elevated plasma Ang-2 levels in all
HF patients and a significant correlation between Ang-2 and LVEF
(17). Eleuteri et al
revealed that Ang-2 levels are inversely correlated with the 6-min
walking test (r=−0.65, P<0.0001) and peak oxygen consumption
(r=−0.57, P=0.0002) in patients with chronic HF, indicating that
Ang-2 levels progressively increase with hemodynamic and functional
decline in these patients (18).
In animal cardiac hypertrophy models, Ang-1 reduced the left
ventricle/tibia ratio and fibrosis, indicating a cardioprotective
role in cardiac remodeling (16).
The present study did not identify a significant
correlation between the level of Ang-1 and cardiac function in AMI
patients. Consistent with the findings of Chong et
al(17), plasma Ang-1 levels
were not observed to differ significantly between the patients with
HF and the healthy controls. We hypothesized that the protective
effect of Ang-1 may be overcome by Ang-2.
The sample size of this study is relatively small.
Therefore, future studies are required to confirm our findings.
Ang-1 binds to integrins on cardiomyocytes and plays a
cardioprotective role in cardiac remodeling (16). However, the effect of Ang-2 on
cardiac remodeling remains unknown. Further investigation is also
required to determine whether Ang-1, Ang-2 and Ang-2/Ang-1 are
associated with HF in AMI patients in the chronic phase.
In the present study, serum Ang-1 and Ang-2 were
observed to be associated with development of HF in AMI patients in
the acute phase. Serum Ang-2 levels and the Ang-2/Ang-1 ratio were
identified to be independent predictors of HF during
hospitalization.
Acknowledgements
This study was supported by the
National Natural Sciences Foundation of China (nos. 81070260 and
31070948) and the Beijing Natural Sciences Foundation (nos. 7102099
and 7122200).
References
|
1.
|
Task Force on the management of ST-segment
elevation acute myocardial infarction of the European Society of
Cardiology (ESC); Steg PG, James SK, Atar D, et al: ESC Guidelines
for the management of acute myocardial infarction in patients
presenting with ST-segment elevation. Eur Heart J. 33:2569–2619.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Davis S, Aldrich TH, Jones PF, et al:
Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by
secretion-trap expression cloning. Cell. 87:1161–1169. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Thomas M and Augustin HG: The role of the
Angiopoietins in vascular morphogenesis. Angiogenesis. 12:125–137.
2009. View Article : Google Scholar
|
|
4.
|
Maisonpierre PC, Suri C, Jones PF, et al:
Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo
angiogenesis. Science. 277:55–60. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Post S, Peeters W, Busser E, et al:
Balance between angiopoietin-1 and angiopoietin-2 is in favor of
angiopoietin-2 in atherosclerotic plaques with high microvessel
density. J Vasc Res. 45:244–250. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Patel JV, Lim HS, Varughese GI, Hughes EA
and Lip GY: Angiopoietin-2 levels as a biomarker of cardiovascular
risk in patients with hypertension. Ann Med. 40:215–222. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Iribarren C, Phelps BH, Darbinian JA, et
al: Circulating angiopoietins-1 and -2, angiopoietin receptor Tie-2
and vascular endothelial growth factor-A as biomarkers of acute
myocardial infarction: a prospective nested case-control study. BMC
Cardiovasc Disord. 11:312011. View Article : Google Scholar
|
|
8.
|
Chen J, Yu H, Sun K, et al: Promoter
variant of angiopoietin-2 and plasma angiopoietin-2 are associated
with risk of stroke recurrence in lacunar infarct patients. Biochem
Biophys Res Commun. 398:212–216. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Chen S, Guo L, Cui M, Sun L and Mi L:
Dynamic changes in serum angiopoietin-1, angiopoietin-2 and
angiopoietin-2/angiopoietin-1 ratio in acute myocardial infarction
patients treated with primary percutaneous coronary intervention.
Biomarkers. 17:441–446. 2012. View Article : Google Scholar
|
|
10.
|
Lee KW, Lip GY and Blann AD: Plasma
angiopoietin-1, angiopoietin-2, angiopoietin receptor Tie-2 and
vascular endothelial growth factor levels in acute coronary
syndromes. Circulation. 110:2355–2360. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Nadar SK, Blann A, Beevers DG and Lip GY:
Abnormal angiopoietins 1&2, angiopoietin receptor Tie-2 and
vascular endothelial growth factor levels in hypertension:
relationship to target organ damage [a sub-study of the
Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT)]. J Intern Med.
258:336–343. 2005.
|
|
12.
|
Findley CM, Mitchell RG, Duscha BD, Annex
BH and Kontos CD: Plasma levels of soluble Tie2 and vascular
endothelial growth factor distinguish critical limb ischemia from
intermittent claudication in patients with peripheral arterial
disease. J Am Coll Cardiol. 52:387–393. 2008. View Article : Google Scholar
|
|
13.
|
Lim HS, Blann AD, Chong AY, Freestone B
and Lip GY: Plasma vascular endothelial growth factor,
angiopoietin-1 and angiopoietin-2 in diabetes: implications for
cardiovascular risk and effects of multifactorial intervention.
Diabetes Care. 27:2918–2924. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Di Stefano R, Felice F and Balbarini A:
Angiogenesis as risk factor for plaque vulnerability. Curr Pharm
Des. 15:1095–1106. 2009.PubMed/NCBI
|
|
15.
|
Dallabrida SM, Ismail N, Oberle JR, Himes
BE and Rupnick MA: Angiopoietin-1 promotes cardiac and skeletal
myocyte survival through integrins. Circ Res. 96:e8–e24. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Jeansson M, Gawlik A, Anderson G, et al:
Angiopoietin-1 is essential in mouse vasculature during development
and in response to injury. J Clin Invest. 121:2278–2289. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Chong AY, Caine GJ, Freestone B, Blann AD
and Lip GY: Plasma angiopoietin-1, angiopoietin-2 and angiopoietin
receptor tie-2 levels in congestive heart failure. J Am Coll
Cardiol. 43:423–438. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Eleuteri E, Di Stefano A, Tarro Genta F,
et al: Stepwise increase of angiopoietin-2 serum levels is related
to haemodynamic and functional impairment in stable chronic heart
failure. Eur J Cardiovasc Prev Rehabil. 18:607–614. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Buchner S, Debl K, Barlage S, et al:
Dynamic changes in N-terminal pro-brain natriuretic peptide in
acute coronary syndromes treated with percutaneous coronary
intervention: a marker of ischemic burden, reperfusion and outcome.
Clin Chem Lab Med. 48:875–881. 2010. View Article : Google Scholar
|
|
20.
|
Ricciuto DR, dos Santos CC, Hawkes M, et
al: Angiopoietin-1 and angiopoietin-2 as clinically informative
prognostic biomarkers of morbidity and mortality in severe sepsis.
Crit Care Med. 39:702–710. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Ong T, McClintock DE, Kallet RH, Ware LB,
Matthay MA and Liu KD: Ratio of angiopoietin-2 to angiopoietin-1 as
a predictor of mortality in acute lung injury patients. Crit Care
Med. 38:1845–1851. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Turer AT and Hill JA: Pathogenesis of
myocardial ischemia-reper-fusion injury and rationale for therapy.
Am J Cardiol. 106:360–368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
van der Heijden M, van Nieuw Amerongen GP,
van Bezu J, Paul MA, Groeneveld AB and van Hinsbergh VW: Opposing
effects of the angiopoietins on the thrombin-induced permeability
of human pulmonary microvascular endothelial cells. PLoS One.
6:e234482011.PubMed/NCBI
|
|
24.
|
Fiedler U, Reiss Y, Scharpfenecker M, et
al: Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and
has a crucial role in the induction of inflammation. Nat Med.
12:235–239. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Gu H, Cui M, Bai Y, et al:
Angiopoietin-1/Tie2 signaling pathway inhibits
lipopolysaccharide-induced activation of RAW264.7 macrophage cells.
Biochem Biophys Res Commun. 392:178–182. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Tuo QH, Zeng H, Stinnett A, et al:
Critical role of angiopoietins/Tie-2 in hyperglycemic exacerbation
of myocardial infarction and impaired angiogenesis. Am J Physiol
Heart Circ Physiol. 294:2547–2557. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Lee SW, Won JY, Lee HY, et al:
Angiopoietin-1 protects heart against ischemia/reperfusion injury
through VE-cadherin dephosphorylation and myocardiac
integrin-β1/ERK/caspase-9 phosphorylation cascade. Mol Med.
17:1095–1106. 2011.PubMed/NCBI
|