Introduction
Lumbar degenerative disease is the most common cause
of lower back pain (1–3). The conventional treatment for this
disease is spinal fusion (4), a
method that was developed in the early 20th century and which has
been widely used in the treatment of lumbar degenerative disease.
Lumbar interbody fusion functions by fixing the lesioned segments
together via fusion, eliminating intercalated disc degeneration and
vertebral pathological motion, changing the biological and
mechanical environment and relieving osphyalgia (4,5).
However, a number of clinical treatments show that fusion surgery
has no evident advantages compared with non-operative treatments
(6,7). By contrast, fusion fixing may
defunctionalise the corresponding spinal segments, which may lead
to the load stress being concentrated in the adjacent segments and
abnormal activity levels increasing, thus speeding up the
degeneration of the adjacent segments (8–11).
With the extensive developments in lumbar fixed fusion and the
long-term follow-up of patients, the aggravated degeneration of the
adjacent segments subsequent to fusion gradually caught the
attention of spinal surgery doctors (12,13).
As a result, dynamic stabilisation (non-fusion technology) was
proposed as a new treatment.
Non-fusion dynamic stabilisation was introduced for
the treatment of spinal degenerative disease in a near-normal
spinal physiological environment. Dynamic stabilisation only fixes
the lumbar spine and does not fuse it. The normal stress
transmission mode of the motion segments may be recovered by
changing the load-bearing functions of the motion segments and by
limiting the range of the unstable motion segments. In this way,
the intervertebral discs that degenerate in middle age may be
spontaneously repaired, thereby relieving pain (14). Dynamic stabilisation allows the
lesion segments to retain certain abilities and reduces the effects
of stress and movement of the adjacent segment to avoid or delay
adjacent segment degeneration (15). Although the effectiveness and
indications of dynamic stabilisation have yet to be further
studied, the concepts and methods behind dynamic stabilisation have
already been accepted by the majority of spinal surgery doctors.
Dynamic stabilisation has shown good primary clinical outcomes and
increased clinical applications in artificial intervertebral discs,
prosthetic disc nuclei, the elastic pedicle system and interspinous
process fixation system. Further clinical and basic investigations
are currently being processed (16–18).
In previous years, the clinical applications of the
interspinous process fixation system have increased, with
satisfactory preliminary clinical reports at home and abroad
(19–21). In-space belongs to the dynamic
interspinous process fixation system and is a novel development and
design by Synthes (West Chester, PA, USA) for use in clinical
applications. Compared with other systems, in-space is a minimally
invasive surgery that aims to restrict lower back pain caused by
excessive stretching of the lumbar vertebrae. Therefore, in-space
is considered to perform satisfactorily in the treatment and
prevention of lumbar degeneration and instability (22). In the present study, the effects of
interspinous spacers in lumbar degenerative disease were
investigated.
Patients and methods
General data
Of the 23 patients with lumbar degenerative disease,
13 were male and 10 were female, with an age range of 20–78 years
old (mean, 43.8). The patients suffered from varying degrees of
lumbar and back pain caused by excessive lumbar stretches. These
patients experienced temporary relief in the flexed position,
however, dynamic X-ray positioning indicated lumbar instability.
The present study recorded 3 cases of lumbar lateral recess
stenosis (L4/5), 12 cases of lumbar disc herniation (10 cases of
L4/5 and 2 cases of L3/4) and 2 cases of adjacent segmental
slippage (1 case of L4/5 slippage with L3/4 instability and 1 case
of L5S1 slippage with L4/5 instability). Subsequent to 3 months of
normal conservative treatments, the physical conditions of these
patients revealed no severe osteoporosis, psychological disorders
or surgical taboos. The study was approved by the ethics committee
of Nanjing Medical University, Nanjing, China and written informed
patient consent was obtained from the patient.
Surgical methods
Patients treated with in-space alone were subjected
to surgery under local anaesthesia in the intraoperative prone
position, with an auxiliary abdominal pad to increase interspinous
spacing. Anatomical landmarks were marked on the surface of the
skin. Small joints and interspinous ligaments were narcotised in
the AP position with lateral percutaneous anaesthesia. A guidewire
was placed into the horizontal or vertical incision approximately 2
cm in the lateral direction, through interspinous under the
guidance of the perspective and always parallel to the coronal
section. Fixing the guidewire subsequently and the interspinous
spacer was placed in it. The expected opening (the inferior
endplate of the superior vertebrae parallel to the superior
endplate of the inferior vertebrae) was followed with a matched
implant placed into the sleeve, attached to the margin of the upper
and lower spinous processes. An appropriate implant was inserted
and its flank was fully unfolded in a good position.
The cases with the clinical symptoms of herniated
discs and lumbar spinal osseous stenosis were subjected to a lumbar
disc excision using a minimally invasive disc scope and
percutaneous in-space implant following full decompression of the
nerve root canal. The cases with adjacent segmental slippage that
required fixation and fusion of the adjacent segments were first
provided with pedicle screw implantations, with interspinous
decompression and fusion in the corresponding segments, followed by
in-space implantation and finally pedicle system longitudinal rod
implantation.
Effect evaluation
Pre-operative and post-operative pain assessments
were conducted using the visual analogue scale (VAS, pain scores of
0 to 10) and the Oswestry Disability Index (ODI).
Imaging evaluation
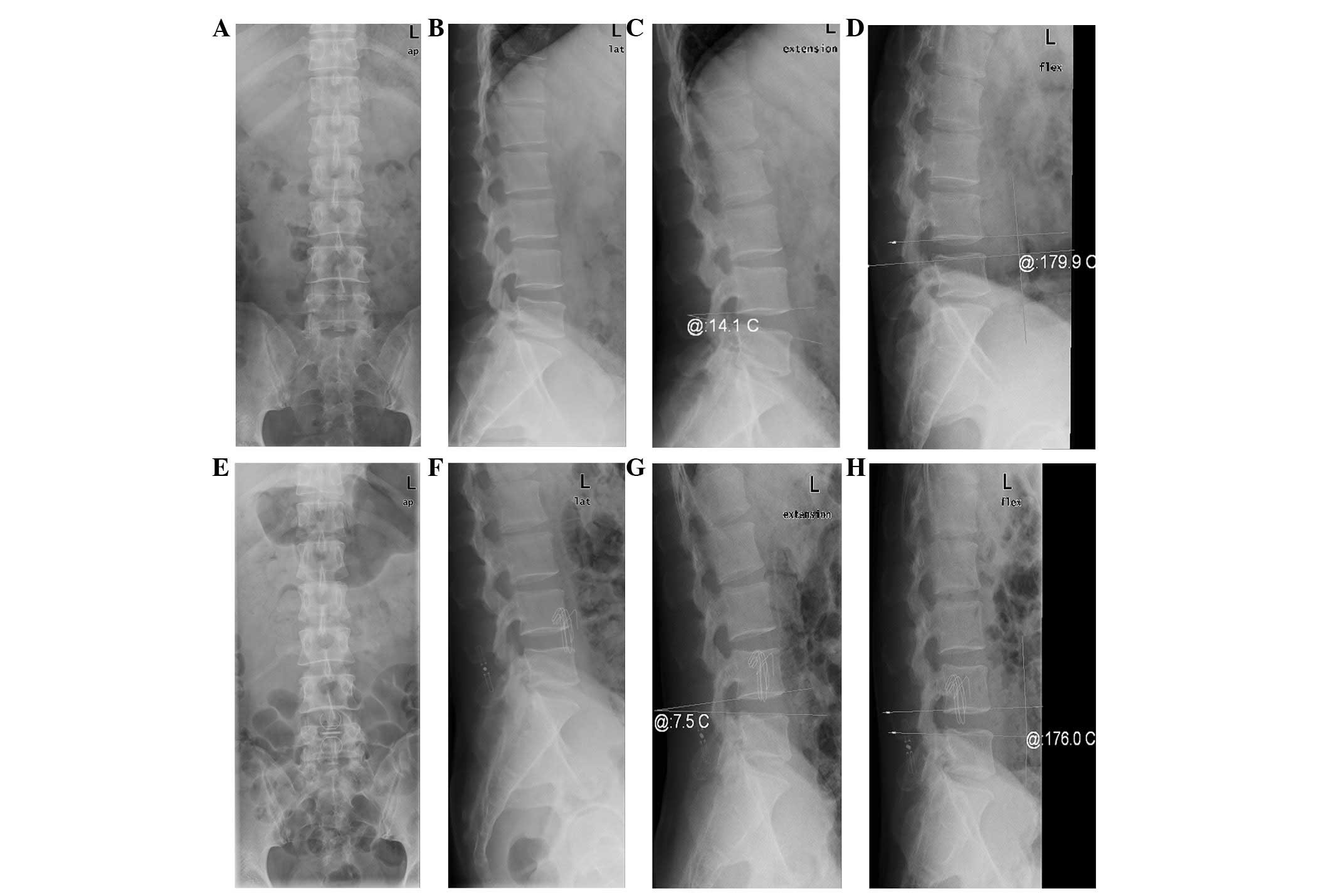
Pre-operative (1 day) and post-operative (2 week and
1, 3, 6, 12 and 18 month) lateral views were prepared in neutral,
flexion and extension positions, as well as anteroposterior views
of post-operative lumbar roentgenograms. Whether the in-space
system shifted or not was determined by observing and measuring the
distance between spinous processes, the width and height of the
intervertebral foramen, the height of the intervertebral anterior
and posterior margins, the lumbar segmental lordosis angle and the
degree of segmental mobility. After >6 months, patients without
lumbar disc excision underwent post-operative rechecks using MRI to
show the recovery of the disc lesions in the in-space implanted
segments and adjacent segments.
Statistical analysis
Pre-operative and post-operative pain VAS and ODI
scores are expressed as mean ± standard deviation. A paired t-test
was employed through statistical software SPSS 11.0. P<0.05 was
considered to indicate a statistically significant difference.
Results
Clinical effects
The surgeries were successfully performed in all
cases. The average implant time when using in-space was 19±4 min.
Only minimal bleeding occurred and the stitches were removed
subsequent to the wounds healing fully.
Final follow-up results were recorded at 6 months,
although the total follow-up period ranged from 6 to 18 months,
with a mean of 11±2.9 months. Varying degrees of improvement were
identified in the post-operative symptoms. The VAS pain scores at 1
day pre-operation, 2 weeks post-operation and the final follow-up
were 7.8±2.1, 2.9±1.3 and 1.5±0.7 out of 10, respectively. The VAS
pain scores subsequent to the surgery were lower than those
observed prior to the surgery (P<0.01). The VAS pain score at
the final follow-up was also lower than that at 1 week
post-operation (P<0.05). The ODI scores at 1 day post-operation,
2 weeks post-operationu and the final follow-up were 87.3±9.1,
54.8±6.7 and 10.6±2.1%, respectively. The ODI score at the final
follow-up was significantly lower than that at 1 day pre-operation
and 2 weeks post-operation (P<0.01). The ODI score at 2 weeks
post-operation was also lower than that at post-operation
(P<0.05).
Imaging evaluation
At 1 day pre-operation, 2 weeks post-operation and
the final follow-up, the distances between the spines were 4.2±0.5,
9.2±1.1 and 9.1±1.2 mm, respectively. Statistically significant
differences were identified between the results at 2 weeks
post-operation and 1 day pre-operation as well as between the
results at the final follow-up and 1 day pre-operation (P>0.05).
However, no significant difference was identified between the
results at 2 weeks post-operation and the final follow-up
(P<0.05). The widths and heights of the intervertebral foramen
were 8.5±1.1 and 18.7±2.1 mm at 1 day pre-operation, 10.8±1.3 and
21.4±2.3 mm at 1 week post-operation and 10.9±1.4 and 21.1±2.5 mm
at the final follow-up, respectively. Similarly, statistically
significant differences were identified between the results at 2
weeks post-operation and 1 day pre-operation as well as between the
results at the final follow-up and 1 day pre-operation (P>0.05).
However, no statistically significant differences were identified
between the results at 2 weeks post-operation and the final
follow-up (P<0.05). The heights of the intervertebral anterior
and posterior margins were 13.6±1.5 and 7.7±0.9 mm at 1 day
pre-operation, 12.7 ±1.3 and 11.3±1.4 mm at 2 weeks post-operation
and 12.9±1.5 and 11.1±1.6 mm at the final follow-up, respectively.
The anterior and posterior margin heights were significantly higher
at 2 weeks post-operation and significantly lower at the final
follow-up compared with those at 1 day pre-operation (P>0.05).
However, no statistically significant differences were identified
between the results at 2 weeks post-operation and the final
follow-up (P<0.05). The lumbar segmental lordosis angle and
segmental mobility were 14.4±1.7 and 21.6±5.8° at 1 day
pre-operation, 7.5±1.2 and 6.2±1.6° at 2 weeks post-operation and
7.9±1.4 and 6.8±1.5° at the final follow-up, respectively. A
significant improvement in the results was noted at 2 weeks
post-operation and the final follow-up compared with the results at
1 day pre-operation (P>0.05). However, no statistically
significant differences were identified between the results at 2
weeks post-operation and the final follow-up (P<0.05; Table I; Fig.
1).
 | Table I.Pre- and post-operative distances
between the spines, the widths and heights of the intervertebral
foramen, the height of the intervertebral anterior and posterior
margins, as well as the lumbar segmental lordosis angle and the
segmental mobility. |
Table I.
Pre- and post-operative distances
between the spines, the widths and heights of the intervertebral
foramen, the height of the intervertebral anterior and posterior
margins, as well as the lumbar segmental lordosis angle and the
segmental mobility.
| Variables | One day
pre-operation | Two weeks
post-operation | Last follow-up |
|---|
| Interspinous distance
(mm) | 4.2±0.5 | 9.2±1.1 | 9.1±1.2 |
| Intervertebral margin
heights (mm) | | | |
| Anterior
margin | 13.6±1.5 | 12.7±1.3 | 12.9±1.5 |
| Posterior
margin | 7.7±0.9 | 11.3±1.4 | 11.1±1.6 |
| Intervertebral/lumbar
foraminal dimensions (mm) | | | |
| Width | 8.5±1.1 | 10.8±1.3 | 10.9±1.4 |
| Height | 18.7±2.1 | 21.4±2.3 | 21.1±2.5 |
| Segmental lordosis
(°) | 14.4±1.7 | 7.5±1.2 | 7.9±1.4 |
| Segmental mobility
(°) | 21.6±5.8 | 6.2±1.6 | 6.8±1.5 |
No shifts in the in-space system or spinous
fractures were observed in any follow-up cases. After >6 months,
patients without lumbar disc excision underwent a post-operative
recheck by MRI. The results showed that the disc hydration signals
of the treated and adjacent segments at 6 months post-operation
were superior to those at day 1 pre-operation (Fig. 2).
Discussion
In the present study, the in-space system was mainly
composed of two polyetheretherketone-based cylinders connected by a
titanium alloy rod. The upper wing of the titanium alloy was opened
through the central mechanical rotating device to prevent lateral
sliding. A ‘floating’ device was formed in the interspinous process
to increase the distance between spinous processes and the
intervertebral foramen. The device was able to restrict excessive
stretching in the implanted segment, reduce pressure stress in the
interspinous process and zygapophysial joints and retain a certain
range of motility in the corresponding segment. As a result,
excessive movements that accelerate degeneration and instability
are avoided, waist pain caused by dynamic stenosis of the
intervertebral foramen is alleviated and the effect that fusion has
on the adjacent segments is prevented (23,24).
Diaz et al(25) showed that
the minimally invasive in-space system was able to effectively
prevent and treat lumbar spinal stenosis and neurogenic
claudication caused by lumbar degeneration, as well as reducing
adjacent segment degeneration and the lower back pain caused by it.
The in-space system may also be used to treat the following lumbar
degenerative diseases: i) central, lateral and intervertebral
lumbar spinal stenosis, accompanied by one-sided leg, hip and groin
pains that are relieved by flexion; ii) herniated discs,
accompanied by lower back pain; iii) facet joint symptoms caused by
inflammation on the articular surface; iv) degenerative
spondylolisthesis below the first degree with excessive lordosis;
v) degenerative disc disease with sacral migration; and vi)
interspinous pains caused by Baastrup’s syndrome (spinous process
consistent).
The patients in the present study suffered from
varying degrees of lumbar spine instabilities. Imaging indicated
dynamic spinal stenosis, which is clinically characterised by lower
back pain or radiating pains in the hyperextended position. The
patients experienced relief from this condition in the flexed
position. The elderly patients underwent routine pre-operative bone
mineral density tests, after which patients with tests showing two
or more standard deviations less than the normal were not
recommended in-space implantation. The stability of in-space
depended on the integrity of certain elements, including the
supraspinal ligament, vertebral plate, spinous process and
zygapophysial joints. Therefore, considering that the majority of
patients had herniated discs or spinal stenosis, lumbar disc
excision or spinal expansion was undertaken using minimal invasion
prior to in-space implantation to reduce any bone destruction or
ligament/muscle injuries. In-space should be implanted near the
ventral side as the basal section of the spinous process provides
stronger support. This method results in less trauma and simple
surgeries. Compared with other spinous dynamic stabilising devices,
the simple in-space system implantation used in the present study
only required local anaesthesia, took a short time and produced
minimal intraoperative bleeding. The mean surgical time for the
implantation system was 19±4 min, which may be further shortened in
the future as further experience leads to improved technical
skills. The system had almost no learning curve period, with no
special requirements in surgical corollary equipment, with the
exception of the C arm machine. Hence, this technology may be
rapidly spread and the indications are easily understood. This
machine was safely and easily used. In-space system implantation
may cause minor damage to the normal structure of the posterior
spines, only causing injuries to the interspinous ligaments but not
interfering with the canalis vertebralis or damaging the nerve
root. During the revision surgery, the surgery was safe and easy as
the first surgery retained the posterior spinal structure. No
marked peri-operative complications were observed. During the
follow-up, no system shifting or loosening and no spinous fractures
were observed. The heights and widths of the intervertebral foramen
were larger subsequent to the surgery than prior to it,
particularly in the hyperextended position. Therefore, patients
with spinal stenosis caused by hyperextension should be provided
with in-space treatment with a moderate opening of implanted gap to
restrict the back extension of the surgical segments, expand the
canalis vertebralis and nerve root canal to a limited extent and
effectively prevent spinal stenosis. In the present study, the
segment mobilities subsequent to the surgery were markedly less
than prior to the surgery. Therefore, patients with segment
instabilities should be provided with in-space implantation to
effectively prevent the excessive activities and sliding of the
segments. Also, the height of the interspinous posterior margin and
the distance between spinous processes were significantly larger
subsequent to the surgery. The patients without lumbar disc
excision underwent MRIs at 12 months post-operation and the disc
hydration signals of the treated and adjacent segments were
observed to be higher at 12 months post-operation compared with at
1 day pre-operation. This finding suggests that patients with
herniated discs should be provided with in-space implantation to
effectively alleviate the pressure in the intervertebral space and
prevent any significant increase in the stress on the adjacent
disc. Consequently, a recurrent herniated nucleus pulposus or
secondary herniated adjacent disc may be avoided.
In sumary, the research direction and goals of
clinical treatments for lumbar pain should include maintaining the
stability of the reconstruction following lumbar degeneration and
instability, keeping normal intervertebral mobility in the treated
segments and reducing the complications that may be caused by
further treatments. Using the in-space interspinous process
distraction system alone or in combination with fixation and fusion
methods in the treatment of lumbar degenerative disease is a simple
and safe treatment, with a good curative effect observed in the
initial follow-up. Therefore, the in-space system is a new
treatment option for lumbar degenerative diseases.
Acknowledgements
This study was supported by the
Foundation of Science and Technology Bureau, Changzhou (No.
CJ20112017) and the Changzhou Health Bureau Key Project (No.
ZD201103), China.
References
|
1.
|
Deyo RA and Weinstein JN: Low back pain. N
Engl J Med. 344:363–370. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Taher F, Essig D, Lebl DR, et al: Lumbar
degenerative disc disease: current and future concepts of diagnosis
and management. Adv Orthop. 2012:9707522012. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Biluts H, Munie T and Abebe M: Review of
lumbar disc diseases at Tikur Anbessa Hospital. Ethiop Med J.
50:57–65. 2012.PubMed/NCBI
|
|
4.
|
Kishen TJ and Diwan AD: Fusion versus disk
replacement for degenerative conditions of the lumbar and cervical
spine: quid est testimonium? Orthop Clin North Am. 41:167–181.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Hanley EN Jr and David SM: Lumbar
arthrodesis for the treatment of back pain. J Bone Joint Surg Am.
81:716–730. 1999.PubMed/NCBI
|
|
6.
|
Brox JI, Sørensen R, Friis A, et al:
Randomized clinical trial of lumbar instrumented fusion and
cognitive intervention and exercises in patients with chronic low
back pain and disc degeneration. Spine (Phila Pa 1976).
28:1913–1921. 2003. View Article : Google Scholar
|
|
7.
|
Gibson JN, Grant IC and Waddell G: The
Cochrane review of surgery for lumbar disc prolapse and
degenerative lumbar spondylosis. Spine (Phila Pa 1976).
24:1820–1832. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Schlegel JD, Smith JA and Schleusener RL:
Lumbar motion segment pathology adjacent to thoracolumbar, lumbar
and lumbosacral fusions. Spine (Phila Pa 1976). 21:970–81. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Fan SW, Zhou ZJ, Hu ZJ, Fang XQ, Zhao FD
and Zhang J: Quantitative MRI analysis of the surface area, signal
intensity and MRI index of the central bright area for the
evaluation of early adjacent disc degeneration after lumbar fusion.
Eur Spine J. 21:1709–1715. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Lund T and Oxland TR: Adjacent level disk
disease - is it really a fusion disease? Orthop Clin North Am.
42:529–541. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Shono Y, Kaneda K, Abumi K, et al:
Stability of posterior spinal instrumentation and its effects on
adjacent motion segments in the lumbosacral spine. Spine (Phila Pa
1976). 23:1550–1558. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Gillet P: The fate of the adjacent motion
segments after lumbar fusion. J Spinal Disord Tech. 16:338–345.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Harrop JS, Youssef JA, Maltenfort M, et
al: Lumbar adjacent segment degeneration and disease after
arthrodesis and total disc arthroplasty. Spine (Phila Pa 1976).
33:1701–1707. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Christie SD, Song JK and Fessler RG:
Dynamic interspinous process technology. Spine (Phila Pa 1976).
30(16 Suppl): S73–S78. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Wiseman CM, Lindsey DP, Fredrick AD and
Yerby SA: The effect of an interspinous process implant on facet
loading during extension. Spine (Phila Pa 1976). 30:903–907. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Matějka J, Zeman J, Matějka T, Nepraš P
and Belatka J: Lumbar total disc replacement. Short-term results.
Acta Chir Orthop Traumatol Cech. 79:37–40. 2012.(In Czech).
|
|
17.
|
Di Silvestre M, Lolli F and Bakaloudis G:
Degenerative lumbar scoliosis in elderly patients: dynamic
stabilization without fusion versus posterior instrumented fusion.
Spine J. Dec 17–2012.[Epub ahead of print].
|
|
18.
|
Villarejo F, Carceller F, de la Riva AG
and Budke M: Experience with coflex interspinous implant. Acta
Neurochir Suppl. 108:171–175. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Sénégas J, Vital JM, Pointillart V and
Mangione P: Long-term actuarial survivorship analysis of an
interspinous stabilization system. Eur Spine J. 16:1279–1287.
2007.
|
|
20.
|
Chi DM and Zhu Y: Non-fusion in
degenerative lumbar diseases. Zhonghua Gu Ke Za Zhi. 10:622–624.
2005.(In Chinese).
|
|
21.
|
Lin Y, Li F and Chen AM: Early evaluation
of posterior dynamic stabilization system in lumbar disc
herniation. Orthopaedic Biomechanics Materials And Clinical Study.
5:12–14. 2008.(In Chinese).
|
|
22.
|
Bono CM and Vaccaro AR: Interspinous
process devices in the lumbar spine. J Spinal Disord Tech.
20:255–261. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Lazaro BC, Brasiliense LB, Sawa AG, et al:
Biomechanics of a novel minimally invasive lumbar interspinous
spacer: effects on kinematics, facet loads, and foramen height.
Neurosurgery. 66(3 Suppl Operative): 126–133. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Park SW, Lim TJ and Park J: A
biomechanical study of the instrumented and adjacent lumbar levels
after in-Space interspinous spacer insertion. J Neurosurg Spine.
12:560–569. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Diaz RC, Berbeo ME, Mora C and Esteban E:
Early clinical and radiological results of a novel minimal invasive
percutaneous interspinous device (in-space) in the management of
degenerative lumbar spine disease. One year follow-up study.
Congress of Neurological Surgeons. In: Annual Meeting; Florida.
2008
|