Introduction
Interleukin-8 (IL-8) or CXCL8 is a significant
regulator of leukocyte trafficking and activation that results from
the interaction with the cell surface receptors, CXCR1 and CXCR2
(1). In the field of vascular
biology, monocytes/macrophages serve as the main source and primary
target of IL-8. However, each cellular component of the vascular
wall is able to produce IL-8 (1–3).
The renin-angiotensin system plays an important role
in the initiation and progression of atherosclerosis (4). Angiotensin II (Ang II), the most
active component of the renin-angiotensin system, has significant
pre-inflammatory functions in the vascular wall, including the
production of inflammatory cytokines and adhesion molecules
(5,6).
The impact of Ang II on monocyte/macrophage-derived
IL-8 has yet to be thoroughly investigated. We proposed that the
pre-inflammatory properties of Ang II are not limited to vascular
endothelium but are further expanded in circulating mononuclear
cells. Thus, we hypothesized that Ang II significantly affects IL-8
production and/or significantly alters the CXCR1/CXCR2 phenotype of
human monocytes/macrophages. To support our hypothesis, THP-1
monocytes were utilized to detect alterations of IL-8 production
and CXCR1/CXCR2 surface expression in naïve cells and cells treated
with Ang II. Pre-treatment with the angiotensin receptor blocker
losartan was also applied to reveal the potential reversibility of
AT-1 mediated effects.
Materials and methods
Cell cultures
THP-1 is a myelomonocytic cell line. THP-1 cells
were cultured as previously described (7). In brief, RPMI-1640 medium
supplemented with 10% decomplemented FBS and 2 mM glutamine, 25 mM
HEPES, penicillin (50 U/ml) and streptomycin (50 U/ml) was used.
Cells were cultured at a density of 500,000/ml, at 37°C, in a
humidified 50 ml/l CO2 atmosphere. The chemokine
receptor phenotype of the monocyte subpopulation was assessed by
re-evaluating the mean fluorescence intensity (Geo Mean) and rate
of chemokine receptor-positive cells in the monocyte gates of flow
cytometer density plots.
Cells were treated with Ang II (Sigma-Aldrich, St.
Louis, MO, USA) or lipopolysaccharide (LPS) in the presence or
absence of Ang II type 1 receptor blocker (ARB) losartan or
telmisartan. Three time points of 0, 24 and 48 h and concentrations
of Ang II ranging from 0.2 to 20 μM were initially
evaluated. Losartan was evaluated in concentrations ranging from 10
to 1,000 μM. Optimal results were obtained for 100 μM
of losartan. Bacterial LPS was used in a standard concentration of
10 ng/ml.
Flow cytometry
The expression of chemokine receptors CXCR1 and
CXCR2 was evaluated by flow cytometry using anti-CXCR1 fluorescein
isothiocyanate-conjugated and anti-CXCR2 phycoerythrin-conjugated
antibodies (BD Bioscience, Franklin Lakes, NJ, USA). Experiments
were performed at least in triplicate and the mean fluorescence
intensity ± standard deviation (SD) was reported. In all cases, the
intra-assay coefficient of variation (CV) was <5% while the
inter-assay CV was <10%.
ELISA
Cells were seeded at a density of 500,000/ml. In the
pre-set time points culture media were collected and centrifuged at
200 × g for 8 min to remove particles. The supernatants were frozen
at −20°C until used for ELISA. The concentration of IL-8 was
measured using an ELISA kit (R&D Systems, Minneapolis, MN, USA)
according to the manufacturer’s instructions. Experiments were
performed at least in triplicate and the mean concentrations
(ng/ml) ± SD were reported.
Statistical analysis
The paired sample t-test was applied to evaluate the
differences between the means since this better eliminated bias
attributed to the different baseline expressions of IL-8 among
different experiments. P<0.05 was considered to indicate a
statistically significant difference. Experiments were performed at
least in triplicate (or as indicated by degrees of freedom at the
reported results) and the mean concentrations ± SD or mean
fluorescence intensity ± SD were reported.
Results
The impact of the ARB losartan on IL-8 production
and the CXCR1/CXCR2 phenotype of Ang II- and LPS-treated THP-1
monocytes is summarized in Table
I.
 | Table I.Effects of ARB losartan on the
CXCR1/CXCR2 phenotype and Interleukin-8 (IL-8) production of LPS or
Ang II treated THP-1 monocytes. |
Table I.
Effects of ARB losartan on the
CXCR1/CXCR2 phenotype and Interleukin-8 (IL-8) production of LPS or
Ang II treated THP-1 monocytes.
Interleukin 8 production
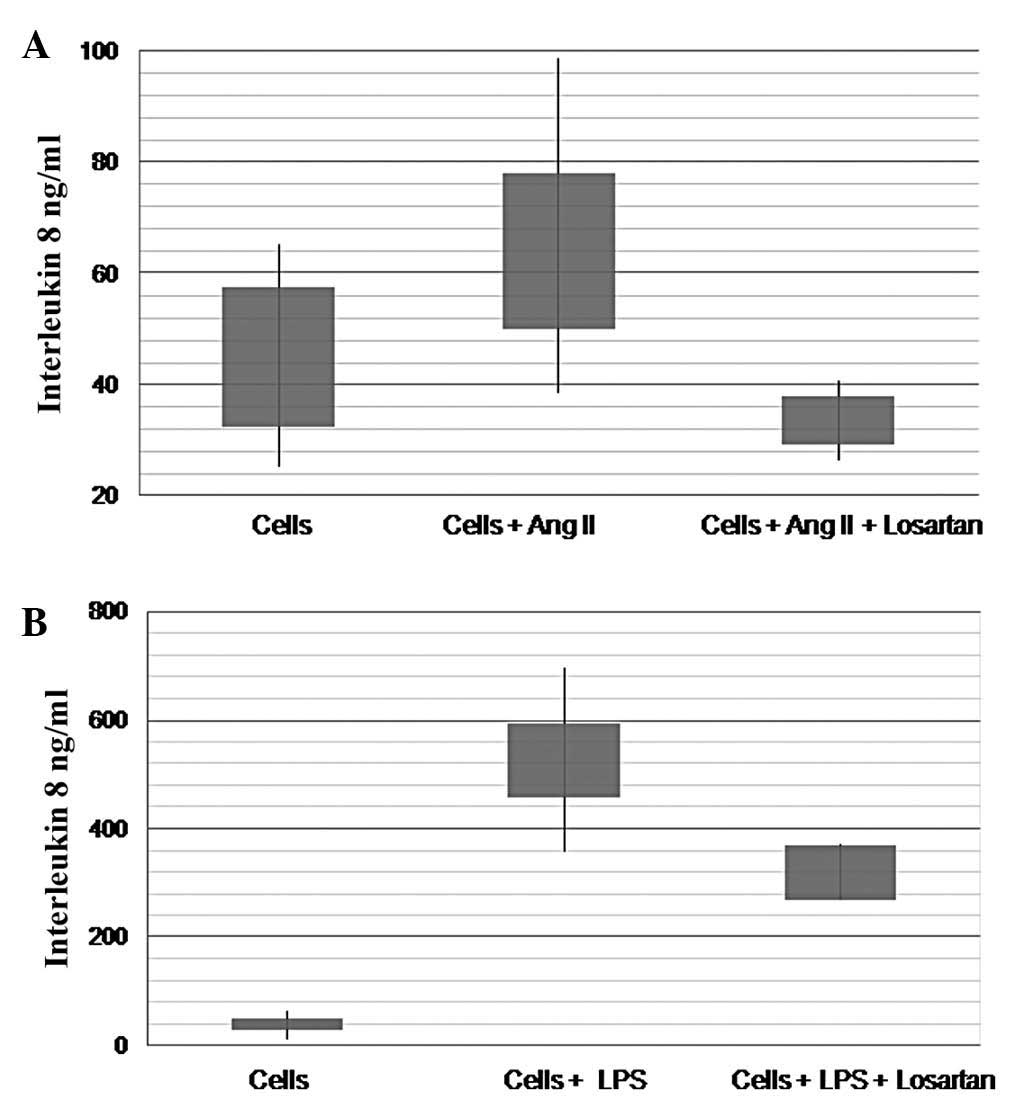
Ang II produced a significant increase of IL-8
production by THP-1 monocytes. A maximum effect was achieved by 10
μM of Ang II (45.2±12.5 vs. 68.8±18.9 ng/ml, df=3, t=−6.96,
P=0.006) (Fig. 1B). LPS produced a
similar but more pronounced effect (40.3±9.5 vs. 527±68.1 ng/ml,
df=2, t=−14.2, P=0.005) (Fig. 1A).
Similar results were obtained for the two substances in time points
ranging from 12 to 48 h after treatment.
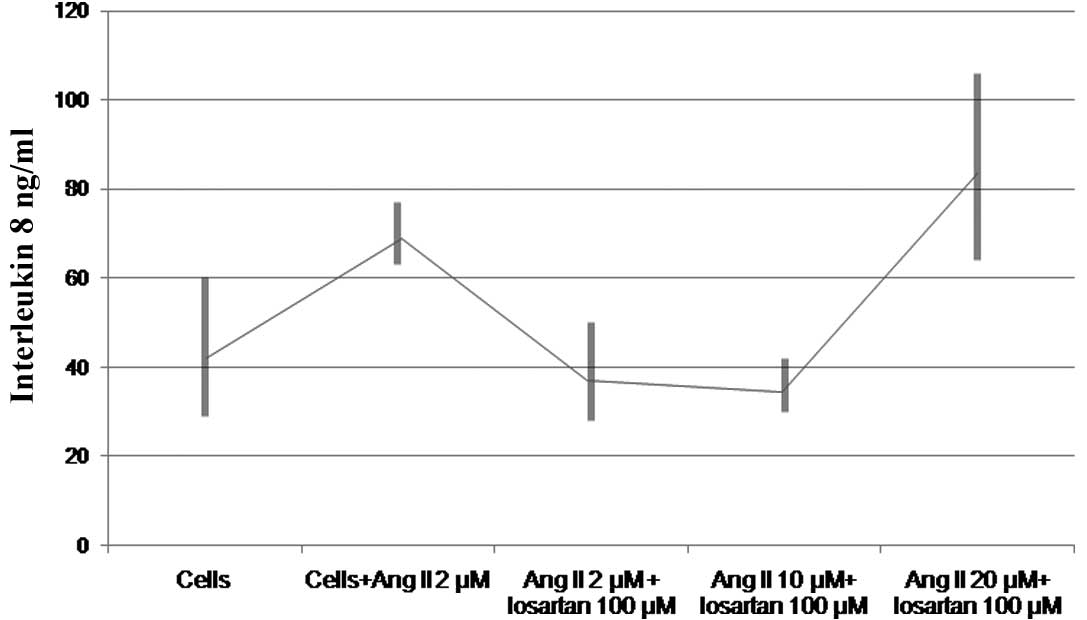
Losartan significantly inhibited the effect of Ang
II on the production of IL-8 by THP-1 monocytes (Fig. 1B). Losartan (100 μM)
successfully reversed the effect of 10 μM or less of Ang II
(76.7.8±12.6 vs. 36.0±4 ng/ml, df=2, t=8.2, P=0.015) (Fig. 2). The phenomenon was reproduced
utilizing either a 2-h pretreatment with losartan or simultaneous
incubation with Ang II and losartan (data not shown). Losartan
significantly reduced the increase of IL-8 production induced by 10
ng/ml of LPS (527±68 vs. 320±20 ng/ml, df=2, t=7.3, P=0.018)
(Fig. 1A). Additionally, losartan
significantly reduced the baseline production of IL-8 in naïve
(non-Ang II- or LPS-treated) THP-1 monocytes (58.3±28.4 vs.
28.4±5.9 ng/ml, df=4, t=5.1, P=0.006) (Fig. 3).
CXCR1/CXCR2 phenotype
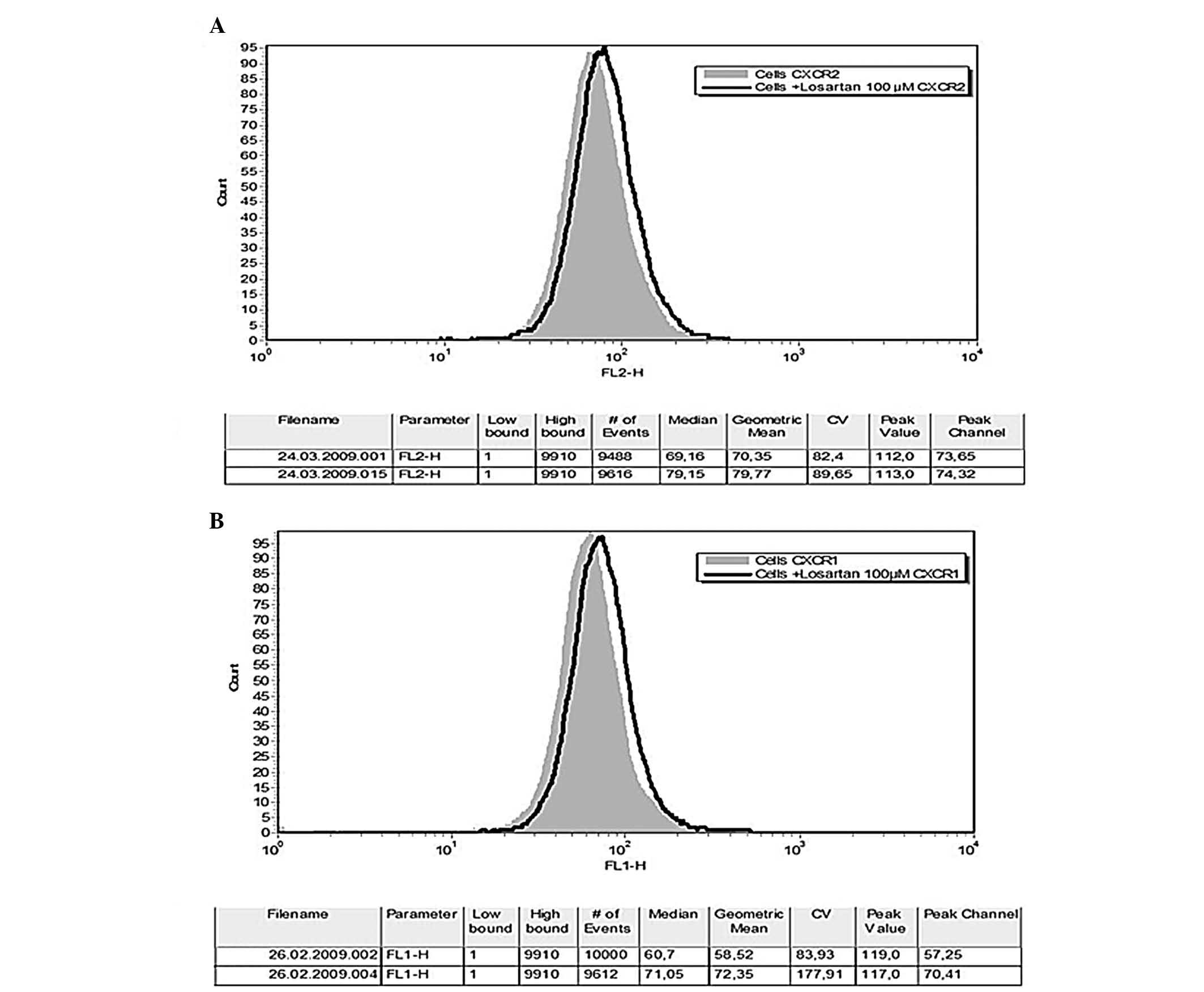
Neither Ang II nor LPS affected the CXCR1/CXCR2
fluorescence intensity of THP-1 monocytes. Losartan significantly
altered the CXCR1/CXCR2 phenotype of naïve or LPS or Ang II
pre-treated THP-1 monocytes. Losartan (100 μM) resulted in a
small but constantly detected and statistically significant
increase of the fluorescence intensity of CXCR1- and CXCR2-positive
THP-1 cells (59.1±9.4 vs. 73.2±11, df=8, t=−8.4, P<0.0001 and
67.2±26.7 vs. 74±29, df=8 t=−4.19, P=0.003, respectively) (Figs. 4 and 5). In order to explore the possibility of
a drug- instead of a class-effect, cells were also incubated with
the ARB telmisartan before CXCR1 and CXCR2 fluorescence intensity
was assessed. As with losartan, telmisartan increased the
fluorescence intensity of CXCR1-positive cells (74±27.5 vs.
105±30.8, df=2, t=−9.6, P=0.01). However, no change was detected
regarding the CXCR2 receptor (82.3±25.4 vs. 89.1±23, df=2, t=−2.1
P=0.17).
No effect was observed by the ACE captopril and
lisinopril on IL-8 production by LPS- or Ang II-treated THP-1
cells.
Discussion
There is sufficient amount of evidence in the
scientific literature supporting the pre-inflammatory and
pre-atherogenic properties of Ang II. In fact, most of the
beneficial pleiotropic effects of the RAS blockade are attributed
to the inhibition of Ang II-induced vascular damage (8,9). A
considerable amount of evidence in this field has been derived from
in vitro models that barely resemble actual biochemical
pathways, but are able to identify an isolated cellular reaction to
a particular stimulus at the biochemical and molecular level
(10). In the present study, THP-1
monocytes were utilized for the study of Ang II effects on the
activation of the IL8/CXCR1/2 pathway. The THP-1 cell line is a
well-established model in the study of monocyte behavior since it
shares many common characteristics with the normal human monocytes,
including morphology, as well as the expression of plasma membrane
receptors and cytokines (11). In
the latter cell model, we demonstrated that Ang II significantly
upregulated IL-8. ARB losartan attenuated this effect suggesting
the existence of an AT-1-mediated pathway. We observed that
losartan has the potential to attenuate LPS-induced IL-8
overexpression, a finding that supports the broader spectrum of
losartan’s anti-inflammatory properties. In accordance with our
observation, Chen et al(12) in similar settings, reported that
Ang II elevated the levels of monocyte chemoattractant protein
(MCP)-1, IL-8 and tumor necrosis factor-α and upregulated the CCR2
and CXCR2 mRNA expression of THP-1 monocytes. The authors further
reported that pretreatment with losartan eliminated the effects
mediated by Ang II. Similarly, Schmeisser et al(13) reported that the Ang II-induced
upregulation of IL-8 and MCP-1 protein and RNA in monocytes was
inhibited by the AT1R-blocker losartan. Ramiprilat was also found
to suppress the Ang II-induced upregulation of IL-8 and MCP-1 in a
dose-dependent manner. Thus, it appears that there is agreement on
the effects of Ang II on IL-8 production by monocytic cells.
However, our study further demonstrated that losartan treatment can
reduce the baseline levels of IL-8 production and increase
CXCR1/CXCR2 expression of cultured THP-1 cells. The latter
observation opposes previously reported results supporting that Ang
II upregulates IL-8 and its receptors and that losartan inhibits
both effects (12,13). In this study, an opposite effect of
losartan on IL-8 and CXCR1/2 receptors was observed. This is not
the first time that such a phenomenon is observed. Reverse
regulation of CXCR1 and/or CXCR2 in response to IL-8 alteration was
previously reported in both in vivo and in vitro
systems (14,15). Moreover, we previously observed and
reported a similar effect of losartan on CX3CR1 expression of THP-1
monocytes, although the impact on the ligand was not assessed
(7). The biochemical pathway
leading to the losartan-induced upregulation of CXCR1/2 is obscure.
Browning et al(16)
provided direct evidence that autocrine IL-8 production occurs in
monocytes stimulated with IL-8 and that this cell response is
regulated at the receptor level. The authors assumed that the
preferential usage of CXCR1 in autocrine IL-8 production occurs in
certain types of cells, such as multinucleate cells. Samanta et
al(17) reported on data
suggesting that the IL-8 receptor expression is markedly regulated
by IL-8. Since IL-8 regulates both its own and CXCR1/2 expression
through CXCR1 activation, this opposite effect of losartan on IL-8
ligand and receptor expression could be attributed to the
activation of auto-regulation pathways. Although no data are
currently available to support this hypothesis, we observed a more
pronounced losartan-induced increase in CXCR1 fluorescence
intensity (CXCR1 is reported to be most actively involved in IL-8
auto-regulation). By contrast, this theory is opposed by the fact
that a 10-fold increase of IL-8 induced by LPS did not affect the
CXCR1/CXCR2 phenotype of THP-1 monocytes.
In conclusion, the in vitro model of this
study demonstrated that Ang II increased IL-8 production by THP-1
monocytes through an AT-1-mediated pathway. ARB losartan attenuated
both the Ang II- and LPS-induced overexpression of IL-8 and
produced a small but statistically significant downregulation of
baseline IL-8 production by THP-1 monocytes. Losartan also produced
a small but statistically significant increase in the fluorescence
intensity of CXCR1- and CXCR2-positive THP-1 cells. The biochemical
basis of the latter observation deserves further investigation.
Extrapolating this in vitro observation to in vivo
pathways, we suggest Ang II-induced IL-8 production by monocytes as
another pre-atherogenic potential of Ang II that can be effectively
blocked by the AT1 receptor blockade.
References
|
1.
|
Apostolakis S, Vogiatzi K, Amanatidou V
and Spandidos DA: Interleukin 8 and cardiovascular disease.
Cardiovasc Res. 84:353–360. 2009. View Article : Google Scholar
|
|
2.
|
Ryoo SW, Kim DU, Won M, Chung KS, Jang YJ,
Oh GT, Park SK, Maeng PJ, Yoo HS and Hoe KL: Native LDL induces
interleukin-8 expression via H2O2, p38
kinase, and activator protein-1 in human aortic smooth muscle
cells. Cardiovasc Res. 62:185–193. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Dje N’Guessan P, Riediger F, Vardarova K,
Scharf S, Eitel J, Opitz B, Slevogt H, Weichert W, Hocke AC,
Schmeck B, Suttorp N and Hippenstiel S: Statins control oxidized
LDL-mediated histone modifications and gene expression in cultured
human endothelial cells. Arterioscler Thromb Vasc Biol. 29:380–386.
2009.PubMed/NCBI
|
|
4.
|
Dandona P, Dhindsa S, Ghanim H and
Chaudhuri A: Angiotensin II and inflammation: the effect of
angiotensin-converting enzyme inhibition and angiotensin II
receptor blockade. J Hum Hypertens. 21:20–27. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Apostolakis S, Vlata Z, Vogiatzi K,
Krambovitis E and Spandidos DA: Angiotensin II up-regulates CX3CR1
expression in THP-1 monocytes: impact on vascular inflammation and
atherogenesis. J Thromb Thrombolysis. 29:443–448. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Ferrario CM and Strawn WB: Role of the
renin-angiotensin aldosterone system and proinflammatory mediators
in cardiovascular disease. Am J Cardiol. 98:121–128. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Apostolakis S, Krambovitis E, Vlata Z,
Kochiadakis GE, Baritaki S and Spandidos DA: CX3CR1 receptor is
up-regulated in monocytes of coronary artery diseased patients:
impact of pre-inflammatory stimuli and renin-angiotensin system
modulators. Thromb Res. 121:387–395. 2007. View Article : Google Scholar
|
|
8.
|
Koh KK, Ahn JY, Han SH, Kim DS, Jin DK,
Kim HS, Shin MS, Ahn TH, Choi IS and Shin EK: Pleiotropic effects
of angiotensin II receptor blocker in hypertensive patients. J Am
Coll Cardiol. 42:905–910. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Sierra C and de la Sierra A:
Antihypertensive, cardiovascular, and pleiotropic effects of
angiotensin-receptor blockers. Curr Opin Nephrol Hypertens.
14:435–441. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Devlin RB, Frampton ML and Ghio AJ: In
vitro studies: what is their role in toxicology? Exp Toxicol
Pathol. 57(Suppl 1): 183–188. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Tsuchiya S, Yamabe M, Yamaguchi Y,
Kobayashi Y, Konno T and Tada K: Establishment and characterization
of a human acute monocytic leukemia cell line (THP-1). Int J
Cancer. 26:171–176. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Chen M, Li Y, Yang T, Wang Y, Bai Y and
Xie X: ADMA induces monocyte adhesion via activation of chemokine
receptors in cultured THP-1 cells. Cytokine. 43:149–159. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Schmeisser A, Soehnlein O, Illmer T,
Lorenz HM, Eskafi S, Roerick O, Gabler C, Strasser R, Daniel WG and
Garlichs CD: ACE inhibition lowers angiotensin II-induced chemokine
expression by reduction of NF-kappaB activity and AT1 receptor
expression. Biochem Biophys Res Commun. 325:532–540. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Chishti AD, Dark JH, Kesteven P, Powell H,
Snowden C, Shenton BK, Kirby JA and Baudouin SV: Expression of
chemokine receptors CXCR1 and CXCR2 during cardiopulmonary bypass.
J Thorac Cardiovasc Surg. 122:1162–1166. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Gessler P, Pfenninger J, Pfammatter JP,
Carrel T, Baenziger O and Dahinden C: Plasma levels of
interleukin-8 and expression of interleukin-8 receptors on
circulating neutrophils and monocytes after cardiopulmonary bypass
in children. J Thorac Cardiovasc Surg. 126:718–725. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Browning DD, Diehl WC, Hsu MH,
Schraufstatter IU and Ye RD: Autocrine regulation of interleukin-8
production in human monocytes. Am J Physiol Lung Cell Mol Physiol.
279:L1129–L1136. 2000.PubMed/NCBI
|
|
17.
|
Samanta AK, Oppenheim JJ and Matsushima K:
Interleukin 8 (monocyte-derived neutrophil chemotactic factor)
dynamically regulates its own receptor expression on human
neutrophils. J Biol Chem. 265:183–189. 1990.
|