Introduction
Depression is a commonly occurring, debilitating and
life-threatening psychiatric disorder. Various antidepressants,
including tricyclic antidepressants, monoamine oxidase inhibitors
and noradrenaline (NA) reuptake inhibitors, are widely available in
the pharmaceutical market. However, the majority of these
antidepressants have undesirable side-effects (1-3).
Consequently, new antidepressants are sought. The root of
Paeonia lactiflora Pall. (Ranunculaceae), commonly
known as peony, is a commonly used in herbal medicines in China,
Korea and Japan. It is a component herb of numerous traditional
formulae, including Jiaweisinisan and Dang Gui Shao Yao San,
prescribed for the treatment of depression-like disorders (4,5). The
antidepressant-like effect of the total glycoside fraction of peony
has also been observed in mice exposed to chronic unpredictable
stress (6). Paeoniflorin, the main
and active component of peony, has been widely studied as an
antioxidant, anticonvulsant, antithrombotic agent, cognition
enhancer or learning impairment-attenuating agent and
neuroprotecting agent (7-13). However, there is no information
available regarding the antidepressant activity of paeoni-florin
following intraperitoneal injection in mice (14). In the present study, we assessed
the antidepressant-like effects of paeoniflorin and its mechanisms
by means of behavioural and pharmacological procedures.
Materials and methods
Animals
Male Institute of Cancer Research (ICR) mice (18–22
g) were obtained from the Laboratory Animal Unit of Zhejiang
Chinese Medicine University (Zhejiang, China). The animals were
housed five per cage and acclimatized to a colony room with
controlled ambient temperature (24±1°C), humidity (50±10%) and a 12
h light/dark cycle. They were fed a standard diet and water ad
libitum and were left to acclimate for 4 days before use in
experiments. The use of experimental mice was approved by the
Animal Experimentation Ethics Committee of the Zhejiang Chinese
Medicine University and conducted in accordance with the National
Institutes of Health (NIH) Principles of Laboratory Animal Care
(publication No. 80-23, revised 1996).
Drugs
Paeoniflorin (purity >98%) was purchased from
Guizhou Dida Technology Co., Ltd. (Zhejiang, China). Imipramine
hydrochloride was purchased from Sigma-Aldrich (St. Louis, MO, USA)
as a positive control. Reserpine was purchased from Bangmin
(Guangzhou, China) and 5-hydroxytryptamine (5-HT), NA, dopamine
(DA) and 5-hydroxyindoleacetic acid (5-HIAA) were purchased from
Sigma-Aldrich. For intraperitoneal injection, paeoniflorin,
imipramine and reserpine were separately dissolved in 0.9% normal
saline and diluted to the desired concentration on the day of
testing. In this study, various doses of paeoniflorin (10, 20 and
40 mg/kg) and imipramine (10 mg/kg) were injected intraperitoneally
(i.p.) 60 min before testing.
Tail suspension test
The tail suspension test was based on the method of
Steru et al (15). Animals
were suspended 50 cm above the floor by means of an adhesive tape,
placed ∼1 cm from the tip of the tail. The duration of immobility
was recorded during the last 4 min of the 6-min testing period.
Mice were considered immobile only when they hung passively and
completely motionless.
Forced swimming test
The forced swimming test was similar to those
described previously (16,17). The mice were individually placed
into glass cylinders (height, 25 cm; diameter, 10 cm) containing 10
cm of water (22±1°C). The duration of immobility was defined as the
time the mouse spent without struggling, floating motionless or
making only small movements necessary to keep its head above water
for the 6-min period. The water was exchanged following each
trial.
Locomotor activity
Locomotor activity was studied using an open-field
test which was performed on mice using a slightly modified method
(18,19). Briefly, the locomotor activity of
the mice was measured using a box (30×30×15 cm) with the floor
divided into 25 squares illuminated with light from the ceiling.
Mice were placed in the central square and the total number of
squares entered was recorded for 2 min. The open field arena was
cleaned following each trial.
Tests of reserpine-induced ptosis,
akinesia and hypothermia
The tests of reserpine-induced ptosis, akinesia and
hypothermia were in accordance with those of Bourin et al
(20). The mice were treated with
reserpine (2.5 mg/kg, i.p.) 10 min after the administration of
paeoniflorin. Three parameters of akinesia, the degree of palpebral
ptosis and the rectal temperature were recorded at 1 h, 1 h and 4
h, respectively, after the administration of reserpine. The degree
of palpebral ptosis was evaluated according to the following rating
scale: 0, eyes open; 1, eyes one-quarter closed; 2, eyes half
closed; 3, eyes three-quarters closed and 4, eyes completely
closed. To measure akinesia, mice were placed in the center of a
circle (diameter, 7.5 cm). The total time the mice remained within
the circle during a 1-min period was counted.
Monoamine neurotransmitter assay
Levels of 5-HT, NA, DA and 5-HIAA in the hippocampus
were measured by high-performance liquid chromatography (HPLC)
coupled with an electrochemical detector as described previously
(4). Briefly, each frozen tissue
sample was homogenized in 0.4 M perchloric acid (solution A). The
homogenate was stored on ice for 1 h and then centrifuged at 12,000
× g (4°C) for 20 min. The pellet was discarded. A 160-μl
aliquot of the supernatant was added to 80 μl solution B
[(containing 0.2 M potassium citrate, 0.3 M dipotassium hydrogen
phosphate and 0.2 M ethylenediamine tetraacetic acid (EDTA)]. The
mixture was stored on ice for 1 h and then centrifuged at 12,000 ×
g (4°C) for 20 min. Then, 20 μl of the resultant supernatant
was directly injected into an ESA liquid chromatography system
equipped with a reversed-phase C18 column (150×4.6 mm, 5 μm)
and an electrochemical detector (ESA CoulArray, Chelmsford, MA,
USA). The detector was set at 450 mV. The mobile phase consisted of
125 mM citric acid-sodium citrate (pH 4.3), 0.1 mM EDTA, 1.2 mM
sodium octanesulfonate and 16% methanol. The flow rate was 1.0
ml/min. NA, 5-HT, DA and 5-HIAA were identified and quantified by
comparing their retention times and peak areas to those of standard
solutions. The contents of 5-HT, NA, DA and 5-HIAA were expressed
as ng/g in wet weight tissue.
Statistical analysis
The results were expressed as the mean ± standard
error of the mean. All data were analyzed statistically using
one-way analysis of variance, followed by Dunnett’s test. P<0.05
was considered to indicate a statistically significant
difference.
Results
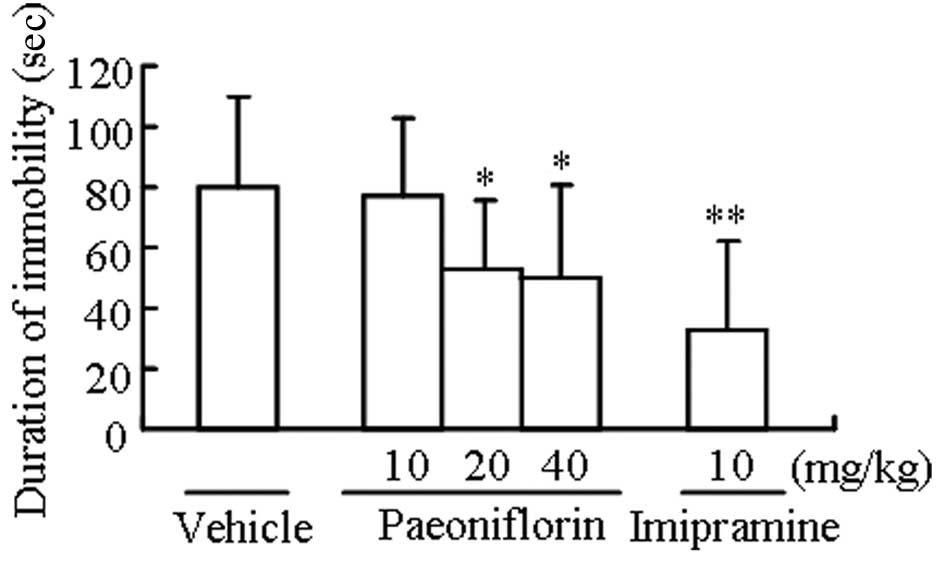
Paeoniflorin decreases the duration of
immobility in the tail suspension test
Paeniflorin at doses of 20 and 40 mg/kg
significantly decreased the duration of immobility in the tail
suspension test compared with the control group (P<0.05).
Imipramine (10 mg/kg) treatment also had a significant effect on
immobility (P<0.01; Fig.
1).
Paeoniflorin decreases the duration of
immobility in the forced swimming test
Paeoniflorin (40 mg/kg) significantly inhibited
immobility in the forced swimming test (P<0.05). Imipramine (10
mg/kg) treatment also decreased the duration of immobility
(P<0.01; Fig. 2).
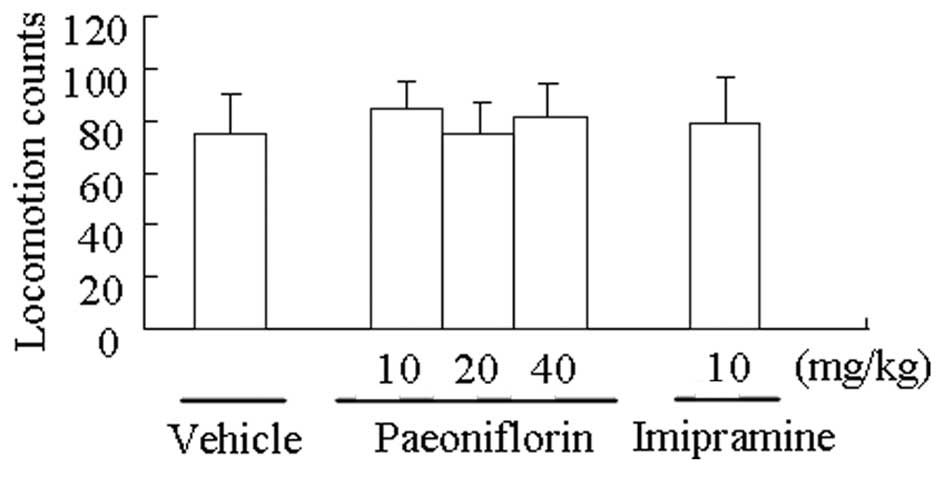
Paeoniflorin has no effect on locomotor
activity
The effect of paeoniflorin on the locomotor activity
of mice is shown in Fig. 3.
Neither paeoniflorin nor imipramine affected locomotor activity at
the doses that significantly reduced the immobility response in the
tail suspension and forced swimming tests.
Paeoniflorin attenuates reserpine-induced
ptosis, akinesia and hypothermia
The effects of paeoniflorin on reserpine-induced
ptosis, akinesia and hypothermia are shown in Table I. The paeoniflorin treatment at
doses of 20 and 40 mg/kg significantly decreased the scores of
reserpine-induced ptosis (P<0.01). The paeoniflorin treatment at
40 mg/kg significantly reduced the time of reserpine-induced
akinesia (P<0.05) and significantly antagonized
reserpine-induced hypothermia compared with vehicle treatment
(P<0.05). Imipramine at a dose of 10 mg/kg significantly
antagonized the hypothermia, ptosis and akinesia induced by
reserpine (P<0.05, P<0.01 and P<0.05, respectively).
 | Table IAntagonism of paeoniflorin on the
reserpine-induced ptosis, akinesia and hypothermia in mice. |
Table I
Antagonism of paeoniflorin on the
reserpine-induced ptosis, akinesia and hypothermia in mice.
| Treatment | Rectal temperature
(°C) | Scores of ptosis | Akinesia (sec) |
|---|
| Vehicle | 32.00±0.0 | 3.5±0.7 | 60.0±0 |
| Paeoniflorin (10
mg/kg) | 32.05±0.2 | 3.5±0.5 | 54.8±17 |
| Paeoniflorin (20
mg/kg) | 32.03±0.1 | 2.1±1.3b | 43.0±27 |
| Paeoniflorin (40
mg/kg) | 32.07±0.1a | 2.0±1.3b | 32.4±29a |
| Imipramine (10
mg/kg) | 34.94±0.6a | 0.7±0.8b | 32.3±23a |
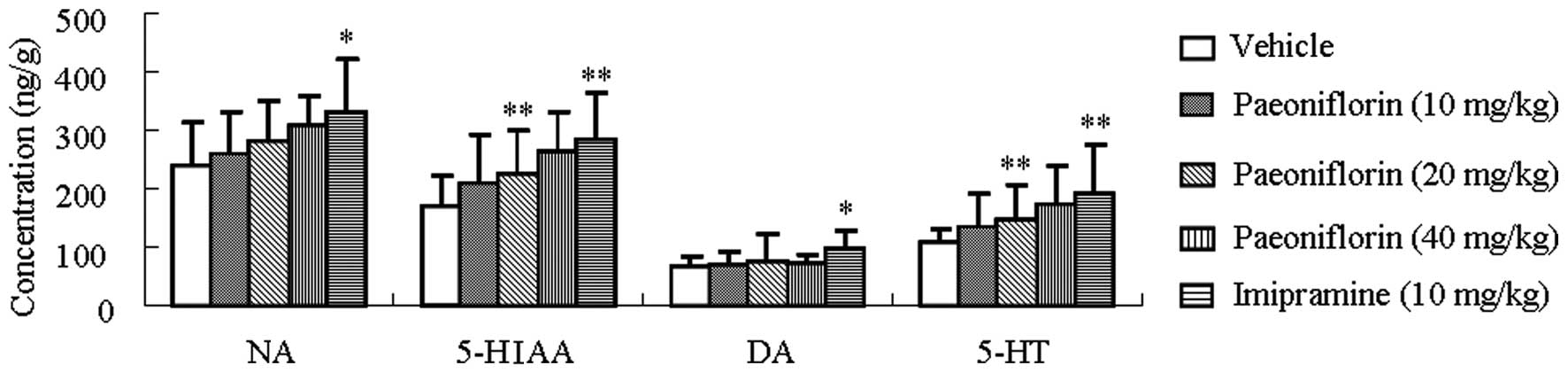
Paeoniflorin increases the concentrations
of monoamine neurotransmitters and a major metabolite in the
hippocampus
The effect of paeoniflorin on hippocampal monoamine
neurotransmitters and a major metabolite of 5-HT in mice is
presented in Fig. 4. Paeoniflorin
(40 mg/kg) treatment significantly increased the concentration of
5-HIAA and 5-HT in the hippocampus (P<0.01) compared with
vehicle treatment, while imipramine treatment significantly
affected hippocampal NA, 5-HIAA, DA and 5-HT concentrations
(P<0.05, P<0.01, P<0.05 and P<0.01, respectively).
Discussion
The tail suspension and forced swimming tests are
widely used for the screening of antidepressant activity (16,18).
The forced swimming and tail suspension-induced state of immobility
in animals is similar to human depression and is amenable to
reversal by antidepressant drugs (16,18).
These animal models are based on the despair or helpless behaviour
to an inescapable and confined space in animals. The present
results demonstrated that paeoniflorin induces significant
antidepressant-like effects in these models. The decrease in
immobility time was dose-dependent in two models.
In these behavioural tests, false-positive results
are occasionally obtained for agents that stimulate locomotor
activity (21). Therefore, we
determined whether paeoniflorin has excitatory or inhibitory
actions on the central nervous system. In our study, paeoniflorin
had no effect on the spontaneous locomotor activity of mice,
indicating that paeoniflorin had no excitatory or inhibitory action
on the central nervous system in effective doses, which eliminates
the probability of false-positive results in the tail suspension
and forced swimming tests. This finding suggests that the reduction
of immobility time elicited by paeoniflorin treatment in the tail
suspension and forced swimming tests is likely to be due to a
psychomotor-stimulant effect and not a psychomotor-stimulant
action.
Reserpine is an antihypertensive drug that depletes
neuronal storage granules of biogenic amines in the brains of
rodents and produces a clinically significant depression-like state
(22). Mice become hypothermic,
akinetic and diarrhetic, with eyelid drooping, in response to
reserpine. Reserpine irreversibly inhibits the vesicular uptake of
monoamine neurotransmitters, including NA, DA and 5-HT (21,23).
The symptoms are reversed by major classes of antidepressant drugs.
The results obtained in the present study demonstrate that
paeoniflorin dose-dependently antagonizes the ptosis, akinesia and
hypothermia induced by reserpine in mice, which indicates that
paeoniflorin has an antidepressant-like effect and may have an
effect on monoamine neurotransmitters. We then explored the
antidepressant mechanism of paeoniflorin by determining the levels
of monomine neurotransmitters in the hippocampus by
HPLC-electrochemical detection (ECD). The results revealed that
paeoniflorin increased the levels of monoamine neurotransmitters in
the mouse hippocampus. This observation suggests that the
antidepressant-like effects of paeoniflorin may be caused by the
preservation of monoamine neurotransmitters.
In a conclusion, upregulation of serotonergic
systems may be an important mechanism in the antidepressant-like
effects of paeoniflorin in mice.
Acknowledgements
This study was supported by the
Zhejiang Provincial Natural Science Foundation of China
(Y2101288).
References
|
1
|
Shen JW and Liang ZJ: Advances in research
on biological etiology of depression and the antidepressants.
Pharmaceut Care Res. 7:94–99. 2007.
|
|
2
|
Mandrioli R, Mercolini L, Saracino MA and
Raggi MA: Selective serotonin reuptake inhibitors (SSRIs):
therapeutic drug monitoring and pharmacological interactions. Curr
Med Chem. 19:1846–1863. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Park SW, Kim YK, Lee JG, Kim SH, Kim JM,
et al: Antidepressant-like effects of the traditional Chinese
medicine kami-shoyo-san in rats. Psychiatry Clin Neurosci.
61:401–406. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang Z, Mao QQ, Zhong XM, Li ZY, Qiu FM
and Ip SP: Mechanistic study on the antidepressant-like effect of
danggui-shaoyao-san, a chinese herbal formula. Evid Based
Complement Alternat Med. 2012:1735652012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xie ZL and Wang XH: Clinical study of
Jiawei Sini Decoction in the treatment of 38 dysthymic patients.
Chin J Inf TCM. 12:8–9. 2005.
|
|
6
|
Mao QQ, Ip SP, Ko KM, Tsai SH, Xian YF and
Che CT: Effects of peony glycosides on mice exposed to chronic
unpredictable stress: further evidence for antidepressant-like
activity. J Ethnopharmacol. 124:316–320. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ye J, Duan H, Yang X, Yan W and Zheng X:
Anti-thrombosis effect of paeoniflorin: evaluated in a
photochemical reaction thrombosis model in vivo. Planta Med.
67:766–767. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xiao L, Wang YZ, Liu J, Luo XT, Ye Y and
Zhu XZ: Effects of paeoniflorin on the cerebral infarction,
behavioral and cognitive impairments at the chronic stage of
transient middle cerebral artery occlusion in rats. Life Sci.
78:413–420. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhong SZ, Ge QH, Li Q, Qu R and Ma SP:
Peoniflorin attenuates Abeta(1-42)-mediated neurotoxicity by
regulating calcium homeostasis and ameliorating oxidative stress in
hippocampus of rats. J Neurol Sci. 280:71–78. 2009. View Article : Google Scholar
|
|
10
|
Mao QQ, Huang Z, Ip SP and Che CT:
Antidepressant-like effect of ethanol extract from Paeonia
lactiflora in mice. Phytother Res. 22:1496–1499. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mao QQ, Ip SP, Ko KM, Tsai SH, Zhao M and
Che CT: Peony glycosides protect against corticosterone-induced
neurotoxicity in PC12 cells. Cell Mol Neurobiol. 29:643–647. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mao QQ, Xian YF, Ip SP, Tsai SH and Che
CT: Protective effects of peony glycosides against
corticosterone-induced cell death in PC12 cells through antioxidant
action. J Ethnopharmacol. 133:1121–1125. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mao QQ, Zhong XM, Qiu FM, Li ZY and Huang
Z: Protective effects of paeoniflorin against
corticosterone-induced neurotoxicity in PC12 cells. Phytother Res.
26:969–973. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mao QQ, Ip SP, Xian YF, Hu Z and Che CT:
Anti-depressant-like effect of peony: a mini-review. Pharm Biol.
50:72–77. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Steru L, Chermat R, Thierry B and Simon P:
The tail suspension test: a new method for screening
antidepressants in mice. Psychopharmacology (Berl). 85:367–370.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yin C, Gou L, Liu Y, Yin X, Zhang L, Jia G
and Zhuang X: Antidepressant-like effects of L-theanine in the
forced swim and tail suspension tests in mice. Phytother Res.
25:1636–1639. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sánchez-Mateo CC, Bonkanka CX, Prado B and
Rabanal RM: Antidepressant activity of some Hypericum
reflexum L. fil. extracts in the forced swimming test in mice.
J Ethnopharmacol. 112:115–121. 2007.
|
|
18
|
Porsolt RD, Bertin A and Jalfre M:
Behavioral despair in mice: a primary screening test for
antidepressants. Arch Int Pharmacodyn Ther. 229:327–336.
1977.PubMed/NCBI
|
|
19
|
Nesterova YV, Povetieva TN, Suslov NI,
Semenov AA and Pushkarskiy SV: Antidepressant activity of diterpene
alkaloids of Aconitum baicalense Turcz. Bull Exp Biol Med.
151:425–428. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bourin M, Poncelet M, Chermat R and Simon
P: The value of the reserpine test in psychopharmacology.
Arzneimittelforschung. 33:1173–1176. 1983.PubMed/NCBI
|
|
21
|
Bourin M, Fiocco AJ and Clenet F: How
valuable are animal models in defining antidepressant activity? Hum
Psychopharmacol. 16:9–21. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mao QQ and Huang Z: Antidepressant drugs
and animal models of depression. Foreign Med Sci. 32:216–220.
2005.
|
|
23
|
Dhingra D and Sharma A:
Antidepressant-like activity of Glycyrrhiza glabra L. in
mouse models of immobility tests. Prog Neuropsychopharmacol Biol
Psychiatry. 30:449–454. 2006.
|