Introduction
During the past few decades, a series of studies
have addressed the association of strenuous physical exercise with
increased oxygen uptake and generation of reactive oxygen species
(ROS) in various mammals (1–4). In
general, the body has adequate antioxidant reserves to cope with
the production of ROS under physiological conditions. The
antioxidant system consists of antioxidant vitamins, glutathione
and thiols, and antioxidant enzymes. Each of these antioxidants
plays a unique role within the cell and complements the others
functionally (5). However, when
ROS levels exceed the normal physiological coping range during
strenuous physical exercise, the accumulation of ROS and a
reduction in antioxidant status may result. This scenario increases
oxidative stress and leads to modifications of lipid and protein
structures that consequently compromise the cellular functions in
tissue (6,7). To increase the body’s antioxidant
potential and to decrease levels of oxidative stress, it has been
recommended that individuals increase their intake of dietary
antioxidants. Dietary antioxidants interact with endogenous
antioxidants to form a cooperative antioxidant network (8).
Radix Pseudostellariae, the root of
Pseudostellaria heterophylla (Miq.) Pax. known as ‘Tongshen’
or ‘Taizishen’, has a long history of medicinal use in China. As a
traditional Chinese medicine, it is frequently used to treat
disease, in particular as a lung and spleen tonic (9,10). A
number of studies concerning Radix Pseudostellariae,
including its chemical components and relevant pharmacological
properties, have been performed. Radix Pseudostellariae
polysaccharides (RPPs) have displayed clear anti-infectious,
anti-inflammatory, hypoglycemic, hypolipidemic and immunomodulating
activities (11–14). Studies have also shown that RPPs
exhibit strong antioxidant activities (15), which suggests that they are
beneficial in counteracting exercise-induced oxidative stress.
However, the effects of RPPs on exercise-induced oxidative stress
have not been investigated thus far. Therefore, in the present
study, we investigated the effects of RPP supplementation against
the exercise-induced oxidative stress of forced swimming in male
rats.
Materials and methods
Materials and chemicals
The dried Radix Pseudostellariae (native to
Shandong herbal medicines planting base, China) was purchased from
Tongjitang Herb Shop (Chengdu, China). The authenticity of the
plant was confirmed by Dr MF Li, a botanist at Sichuan University
(Chengdu, China), and a voucher specimen was deposited in the
Herbarium of Sichuan University. The dried Radix
Pseudostellariae was ground with an electric mixer prior to
extraction. The assay kits for blood lactate, hemoglobin, catalase
(CAT), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px)
and malondialdehyde (MDA) were purchased from Jianchen
Bioengineering Institute (Nanjing, China). Other chemicals and
biochemicals were of analytical grade and were purchased from Sigma
Chemical Co. (St. Louis, MO, USA) and Changsheng Pharmaceutical Co.
(Chengdu, China) unless otherwise indicated.
Experiment animals
Healthy male Wistar rats with an average mass of
225–250 g were obtained from Sichuan Research Animal Center
(Chengdu, China). A standard pellet diet and water were provided
ad libitum. The animals were housed in a
temperature-controlled room at 21–23°C and maintained on a 12 h
light : 12 h dark cycle. All animals received humane care according
to the guidelines of the Guidebook for the Care and Use of
Laboratory Animals (16). The
study protocol was approved by the Animal Research Ethics Committee
at Sichuan University (Chengdu, China).
Preparation of RPPs
The preparation of RPPs was carried out according to
the literature (17,18). In brief, the dried Radix
Pseudostellariae was ground into powder. The powder (400 g) was
extracted three times by refluxing with 80% ethanol (1 liter) at
90°C for 2–3 h each time. After filtration, the dregs were
extracted again three times with water (1.5 liter) at 90°C for 2–3
h each time. The extracted solution was condensed to 400 ml and
deproteinated using the Sevag method. The solution was then added
to absolute ethanol until the ethanol concentration was 80% and
kept overnight, followed by filtration. The precipitate was
dissolved with water (100 ml) and then absolute ethanol was added
until the ethanol concentration was 80%, the solution was filtrated
and this method was repeated once again. The precipitate was washed
with 95% ethanol, absolute ethanol and acetone by turns, and then
dried at 50°C.
Experimental protocol
A total of 40 healthy male Wistar rats were
randomized into four equal groups based on body weight following
one week where rats acclimated to the new environment: the control
(C), low-dose RPP supplementation (LRS), medium-dose RPP
supplementation (MRS) and high-dose RPP supplementation (HRS)
groups. The C group received saline solution and the
supplementation groups received different doses of RPPs (100, 200
and 400 mg/kg body weight, respectively). The treatments were
administered orally and daily for 28 days.
Following the final supplementation with RPPs or
saline solution, the rats were allowed to rest for 30 min. The rats
were then removed for the exhaustive swimming exercise. The details
of this apparatus have been reported previously (19). A acrylic plastic pool (90×60×60 cm)
was filled with water to a depth of 40 cm and maintained at 28±1°C.
The rats were forced to swim in the water, and the endurance was
defined as the time active swimming was maintained until the animal
submerged in the water without movement. To diminish stress, all
rats had been accustomed to swimming with repeated short-term
swimming sessions for a week prior to experimentation.
Analysis of biochemical parameters
At the end of the swimming test, the rats were
anesthetized with pentobarbital sodium (5 mg/100 g body weight,
i.p.). Blood was obtained from the orbital sinus for lactate and
hemoglobin level measurements. Hindlimb skeletal muscle was rapidly
removed and homogenized immediately in ice-cold 10% KCl solution
(10 ml/g of tissue) using a teflon/glass homogenizer. The
suspension was centrifuged at 671 × g at 4°C for 10 min and the
clear supernatant was used for CAT, SOD, GSH-Px and MDA level
measurements. All biochemical parameters were determined using
commercial kits following the manufacturer’s recommended
instructions.
Statistical analysis
The data are expressed as the mean ± SD. Statistical
comparisons were compared by one-way analysis of variance (ANOVA).
P<0.05 was considered to indicate a statistically significant
result.
Results and Discussion
Effects of RPP supplementation on the
exhaustive swimming times of rats
Exhaustive swimming was selected as a model of
physical exercise since muscle trauma caused by other types of
physical exercise, including prolonged running on a treadmill,
exercise stimulated by electric shock and plyometric contractions,
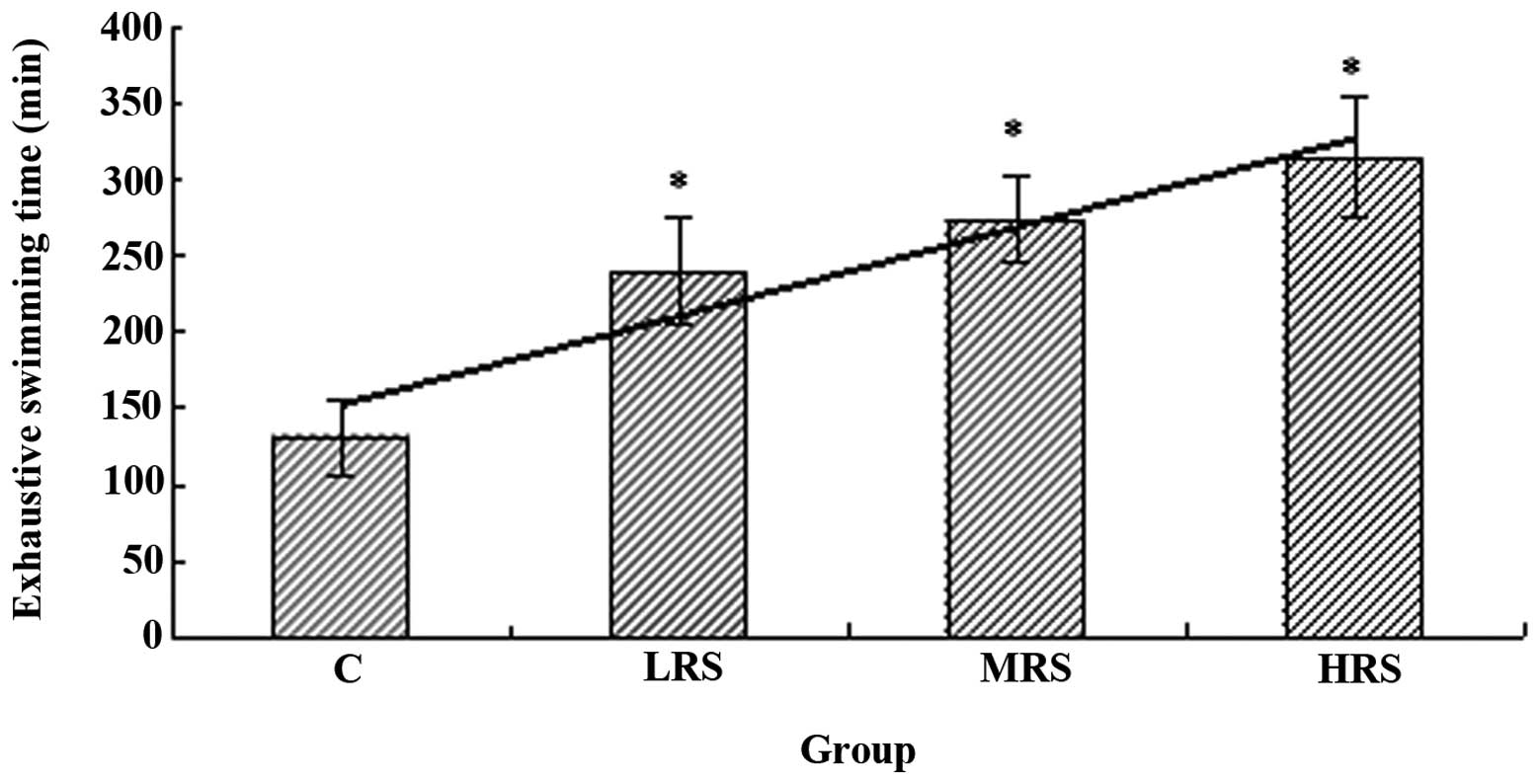
may be avoided (20,21). As shown in Fig. 1, exhaustive swimming times in all
the RPP supplementation groups were significantly longer compared
with that of the C group (P<0.05). These results indicate that
RPP supplementation is able to elevate exercise endurance.
Effects of RPP supplementation on the
blood lactate levels of rats
Lactate serves as an energy source in highly
oxidative tissues. Numerous organs, including the liver and heart,
and tissues such as skeletal muscle, aid the removal of lactate
from the blood, but intense exercise increases lactate production
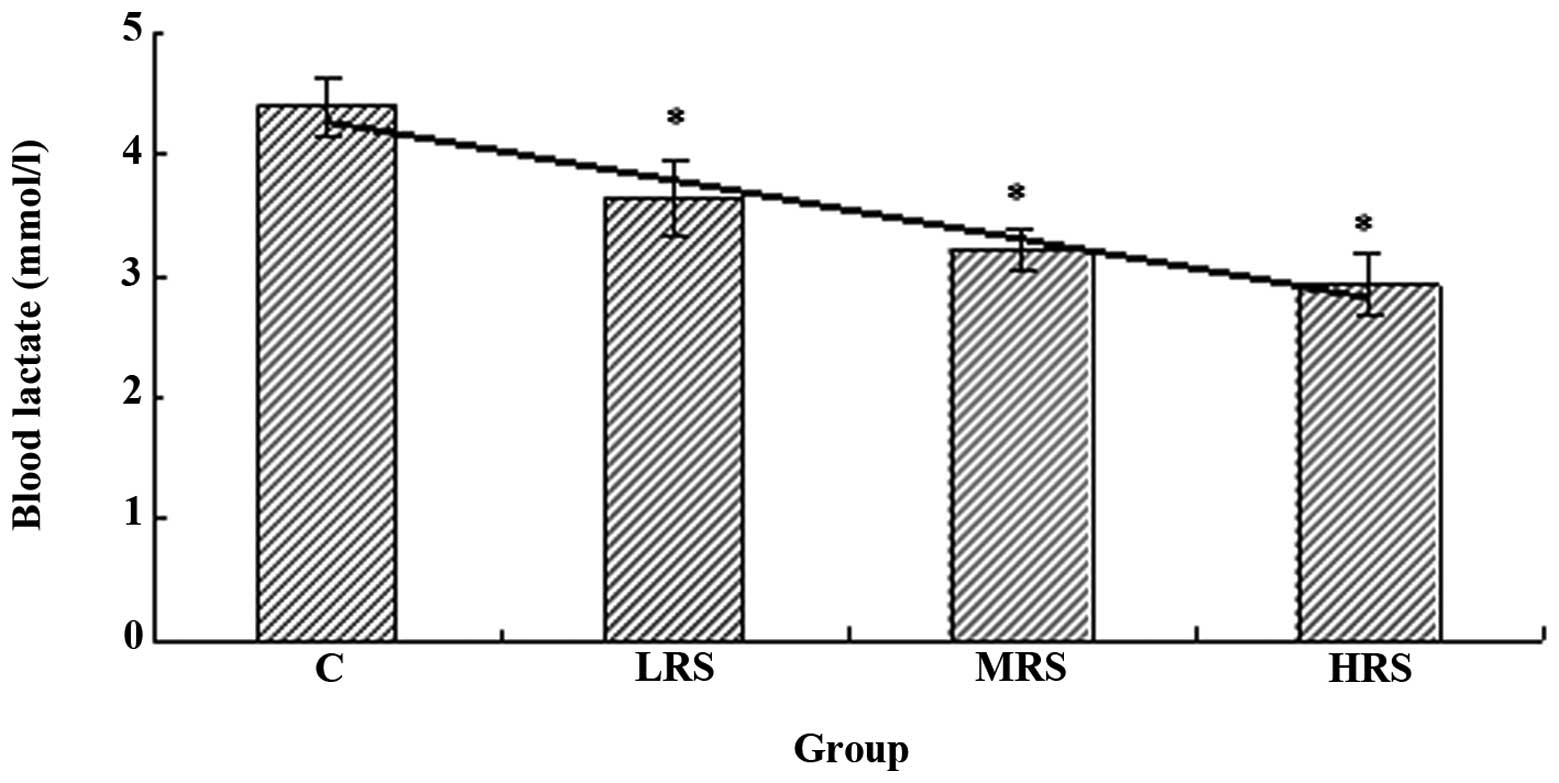
(22). As shown in Fig. 2, the blood lactate level in each of
the RPP supplementation groups was significantly lower compared
with that of the C group (P<0.05). These results indicated that
RPP supplementation effectively attenuates the increase of blood
lactate, which may be responsible for the improvement in exercise
endurance.
Effects of RPP supplementation on the
hemoglobin levels of rats
Hemoglobin is the main component of erythrocytes.
The improvement of cardiopulmonary function and increase of oxygen
supply to tissues caused by an increase in hemoglobin levels are
commonly stated to be major factors that increase endurance
capacity (23). As shown in
Fig. 3, the hemoglobin levels in
the MRS and HRS groups were significantly higher compared with that
of the C group (P<0.05). Although the hemoglobin level in the
LRS group was also increased, no significant difference was
observed (P>0.05). These results indicated that RPP
supplementation may influence the supply of oxygen to tissues by
hemoglobin, and may contribute to the improvement in exercise
endurance.
Effects of RPP supplementation on the MDA
content of rat skeletal muscle
Lipid peroxidation represents oxidative tissue
damage caused by hydrogen peroxide, superoxide anions and hydroxyl
radicals, resulting in structural alteration of the membrane,
release of cell and organelle content and loss of essential fatty
acids with formation of cytosolic aldehyde and peroxide products
(24). MDA is a secondary product
generated during the oxidation of polyunsaturated fatty acids,
which has been frequently measured as an indicator of lipid
peroxidation and oxidative stress in vivo (25). Numerous studies have observed that
strenuous physical exercise induces increases in the MDA
concentration in tissues (24,26,27).
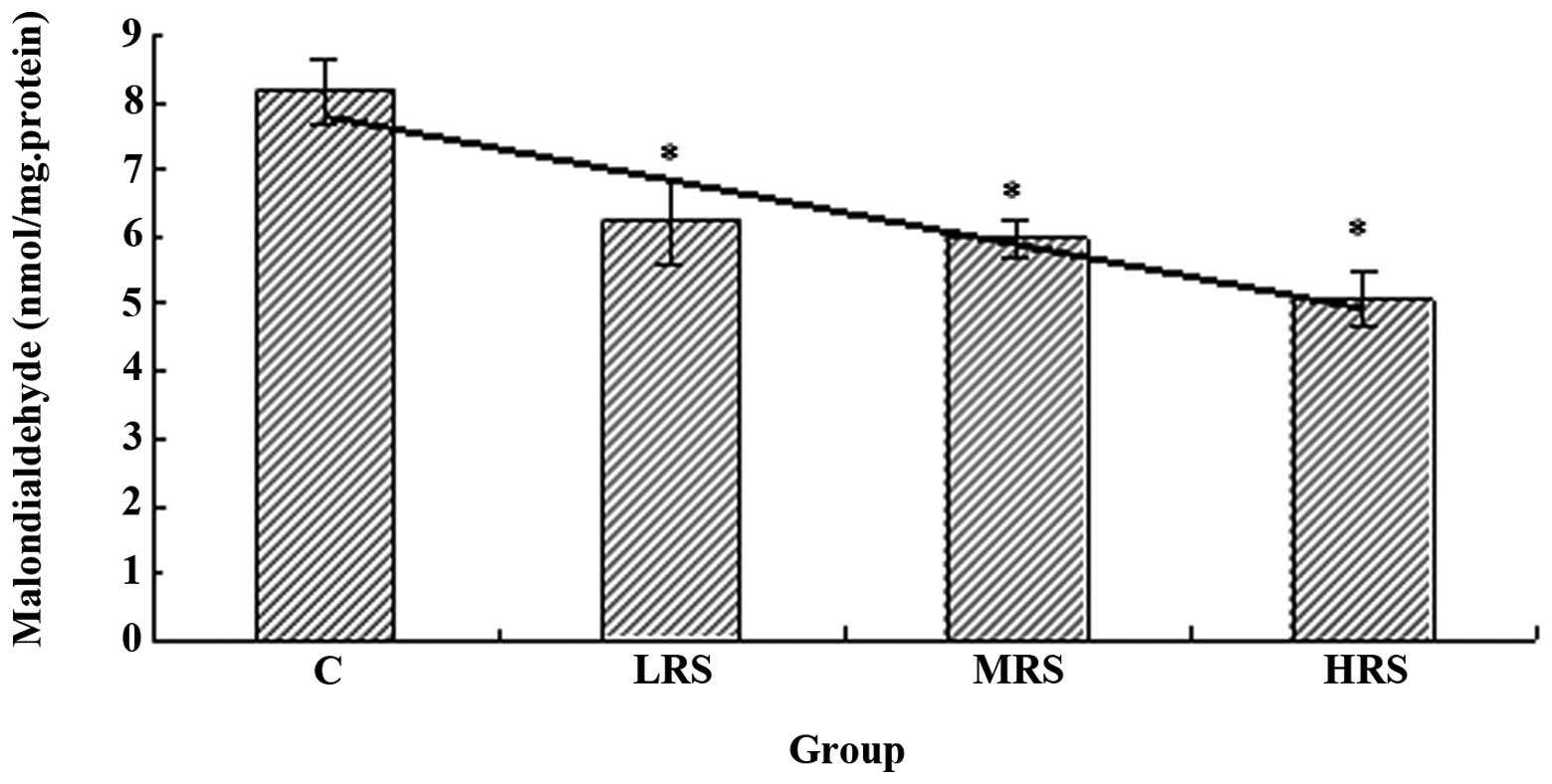
As shown in Fig. 4, the MDA
content in all three RPP supplementation groups was significantly
lower compared with that of the C group (P<0.05). These results
indicate that RPP supplementation effectively reduces lipid
peroxidation.
Effects of RPP supplementation on the
antioxidant enzyme content of rat skeletal muscle
CAT, SOD and GSH-Px are regarded as the first line
of defense by the antioxidant enzyme system against ROS generated
during exhaustive exercise (28).
SOD catalyzes the dismutation of superoxide into oxygen and
hydrogen peroxide. GSH-Px is a selenoenzyme which catalyzes the
reduction of hydroperoxides at the expense of reduced glutathione.
CAT is a primary antioxidant defense component that catalyzes the
decomposition of hydrogen peroxide to water, sharing this function
with GSH-Px (29). As shown in
Table I, the CAT contents in the
MRS and HRS groups were significantly higher compared with that of
the C group (P<0.05). Although the CAT content in the LRS group
was also increased, no significant difference was observed
(P>0.05). The SOD and GSH-Px contents in the RPP supplementation
groups were significantly higher compared with that of the C group
(P<0.05). These results indicate that RPP supplementation
upregulated antioxidant enzymes to protect against oxidative
stress-induced injury during exhaustive exercise.
 | Table IEffects of RPP supplementation on the
antioxidant enzyme content of skeletal muscle in rats. |
Table I
Effects of RPP supplementation on the
antioxidant enzyme content of skeletal muscle in rats.
| Group | CAT (U/mg
protein) | SOD (U/mg
protein) | GSH-Px (U/mg
protein) |
|---|
| C | 3.16±0.32 | 101.31±11.42 | 6.57±1.38 |
| LRS | 3.33±0.41 | 148.53±10.78a | 9.86±1.85a |
| MRS | 4.15±0.39a | 162.87±13.46a | 12.39±1.16a |
| HRS | 4.48±0.46a | 169.52±12.24a | 16.34±1.72a |
From the present findings, we conclude that RPP
supplementation elevates the exercise tolerance and decreases the
blood lactate level of rats following exhaustive swimming exercise.
RPP supplementation augments the levels of hemoglobin and
antioxidant enzymes and effectively decreases the MDA content in
the skeletal muscle, which suggests that RPP supplementation
possesses protective effects against swimming-induced oxidative
stress. Our data relates to rats, therefore future studies using
different subjects, possibly of different sporting backgrounds, are
required to extend these findings.
Acknowledgements
This study was supported by by a
Sichuan University Cross-Disciplinary Research Project grant (No.
SKQY201109)
References
|
1
|
Alessio HM: Exercise-induced oxidative
stress. Med Sci Sports Exerc. 25:218–224. 1993. View Article : Google Scholar
|
|
2
|
Bejma J and Ji LL: Aging and acute
exercise enhance free radical generation in rat skeletal muscle. J
Appl Physiol. 87:465–470. 1999.PubMed/NCBI
|
|
3
|
Akhtar M, Pillai KK and Vohora D: Effect
of thioperamide on modified forced swimming test-induced oxidative
stress in mice. Basic Clin Pharmacol Toxicol. 97:218–221. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pepe H, Balci SS, Revan S, Akalin PP and
Kurtoğlu F: Comparison of oxidative stress and antioxidant capacity
before and after running exercises in both sexes. Gend Med.
6:587–595. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Banerjee AK, Mandal A, Chanda D and
Chakraborti S: Oxidant, antioxidant and physical exercise. Mol Cell
Biochem. 253:307–312. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Viña J, Gomez-Cabrera MC, Lloret A,
Marquez R, Miñana JB, Pallardó FV and Sastre J: Free radicals in
exhaustive physical exercise: mechanism of production, and
protection by anti-oxidants. IUBMB Life. 50:271–277.
2000.PubMed/NCBI
|
|
7
|
Taysi S, Oztasan N, Efe H, Polat MF,
Gumustekin K, Siktar E, Canakci E, Akcay F, Dane S and Gul M:
Endurance training attenuates the oxidative stress due to acute
exhaustive exercise in rat liver. Acta Physiol Hung. 95:337–347.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Powers SK, DeRuisseau KC, Quindry J and
Hamilton KL: Dietary antioxidants and exercise. J Sports Sci.
22:81–94. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gong Z, Dai Y, Ma H, Wang Z and Yu G: The
effect of Radix Pseudostellariae from 8 habitats on
spleen-deficiency and immunologic function. Zhong Yao Cai.
24:281–282. 2001.(In Chinese).
|
|
10
|
Lin H, Chen Q, Zhao J and Zhou P:
Determination of free amino acid content in Radix Pseudostellariae
using near infrared (NIR) spectroscopy and different multivariate
calibrations. J Pharm Biomed Anal. 50:803–808. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wong CK, Leung KN, Fung KP and Choy YM:
The immunostimulating activities of anti-tumor polysaccharides from
Pseudostellaria heterophylla. Immunopharmacology. 28:47–54.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ng TB, Liu F and Wang HX: The antioxidant
effects of aqueous and organic extracts of Panax
quinquefolium, Panax notoginseng, Codonopsis
pilosula, Pseudostellaria heterophylla and Glehnia
littoralis. J Ethnopharmacol. 93:285–288. 2004.PubMed/NCBI
|
|
13
|
Cai J, Li X, Chen X, et al: The immune
effects of crude extract of Pseudostellaria polysaccharide
in mice. Journal of Fujian College of Traditional Chinese Medicine.
15:33–35. 2005.(In Chinese).
|
|
14
|
Li J and Fu Y: Effect of IgY, Coptis
chinensis and Radix Pseudostellariae on gastric mucous
membrane in mice with Helicobacter pylori infection. Chinese
Journal of Clinical Rehabilitation. 10:78–80. 2006.(In
Chinese).
|
|
15
|
Wang WK: Recent advances in studies on
Pseudostellariae Radix. Chinese Journal of Experimental
Traditional Medical Formulae. 17:264–267. 2011.(In Chinese).
|
|
16
|
(NRC) Institute of Laboratory Animal
Resources, Commission on Life Sciences, National Research Council:
Guide for the Care and Use of Laboratory Animals. National
Academies Press; Washington, DC: pp. 11–45. 1996
|
|
17
|
Chen YY, Ding Y, Wang W, Wang RF, Su H and
Du LJ: Determination of polysaccharide in Radix
pseudostellariae extract by size-exclusion high-performance
liquid chromatography. Tsinghua Sci Tech. 12:389–393. 2007.
|
|
18
|
Sheng R, Xu X, Tang Q, Bian D, Li Y, Qian
C, He X, Gao X, Pan R, Wang C, Luo Y, Xia Y and Dai Y:
Polysaccharide of Radix Pseudostellariae improves chronic fatigue
syndrome induced by poly I:C in mice. Evid Based Complement
Alternat Med. 2011:8405162011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee SP, Mar GY and Ng LT: Effects of
tocotrienol-rich fraction on exercise endurance capacity and
oxidative stress in forced swimming rats. Eur J Appl Physiol.
107:587–595. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Misra DS, Maiti R and Ghosh D: Protection
of swimming-induced oxidative stress in some vital organs by the
treatment of composite extract of Withania somnifera,
Ocimum sanctum and Zingiber officinalis in male rat.
Afr J Tradit Complement Altern Med. 6:534–543. 2009.PubMed/NCBI
|
|
21
|
Ou PF and Zhang L: Stimulatory effects of
soybean isoflavones on exercise performance. Int J Phys Sci.
5:2272–2277. 2010.
|
|
22
|
Wei W, Zheng LY, Yu MY, Jiang N, Yang ZR
and Luo X: Anti-fatigue activity of extract form the submerged
fermentation of Ganoderma Lucidum using Radix astragali as
substrate. J Anim Plant Sci. 6:677–684. 2010.
|
|
23
|
Ikeuchi M, Yamaguchi K, Koyama T, Sono Y
and Yazawa K: Effects of fenugreek seeds (Trigonella foenum
greaecum) extract on endurance capacity in mice. J Nutr Sci
Vitaminol (Tokyo). 52:287–292. 2006.
|
|
24
|
Kato J, Ruram AA, Singh SS, Devi SB, Devi
TI and Singh WG: Lipid peroxidation and antioxidant vitamins in
urolithiasis. Indian J Clin Biochem. 22:128–130. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li F, Tang H, Xiao F, Gong J, Peng Y and
Meng X: Protective effect of salidroside from Rhodiolae Radix on
diabetes-induced oxidative stress in mice. Molecules. 16:9912–9924.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kanter MM, Nolte LA and Holloszy JO:
Effects of an antioxidant vitamin mixture on lipid peroxidation at
rest and postexercise. J Appl Physiol. 74:965–969. 1993.PubMed/NCBI
|
|
27
|
Zhang M, Izumi I, Kagamimori S, Sokejima
S, Yamagami T, Liu Z and Qi B: Role of taurine supplementation to
prevent exercise-induced oxidative stress in healthy young men.
Amino Acids. 26:203–207. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang CC, Lin TJ, Lu YF, Chen CC, Huang CY
and Lin WT: Protective effects of L-arginine supplementation
against exhaustive exercise-induced oxidative stress in young rat
tissues. Chin J Physiol. 52:306–315. 2009. View Article : Google Scholar
|
|
29
|
Cotgreave IA, Moldéus P and Orrenius S:
Host biochemical defense mechanisms against prooxidants. Annu Rev
Pharmacol Toxicol. 28:189–212. 1988. View Article : Google Scholar : PubMed/NCBI
|