Introduction
Radix Bupleuri (RB), isolated from the dried
roots of Bupleurum chinense DC or Bupleurum
scorzonerifolium Willd, has been used as a health product and
natural remedy for centuries in traditional Chinese medicine, based
on its hepato-protective, antipyretic, analgesic, immunomodulatory
and anti-inflammatory effects (1,2). As
major bioactive compounds isolated from RB, saikosaponins have
numerous biological activities, including immunoregulatory,
anti-inflammatory, anti-bacterial and anti-viral activity (3,4). One
study demonstrated that saikosaponin A (SSA) exhibits
anti-inflammatory activity (5).
However, the potential molecular mechanism of SSA in terms of the
anti-inflammatory signaling pathways has not been fully
determined.
Inflammation is a beneficial host response to
foreign challenge or tissue injury, helping facilitate the
restoration of tissue structure. However, prolonged inflammation is
not beneficial as it contributes to the pathology of a number of
diseases (6,7). Therefore, anti-inflammatory agents
have potential therapeutic effects for various inflammation-related
diseases. It is well established that activated immunocytes are
involved in the inflammation process, particularly macrophages,
which play a crucial role in the specific and non-specific immune
responses during inflammation (8).
Lipopolysaccharide (LPS) induces the release of inflammatory
mediators in macrophages, leading to the production of inducible
nitric oxide synthase (iNOS), tumor necrosis factor (TNF)-α,
interleukin (IL)-1β and IL-6 (9,10).
Cytokines play essential roles in the inflammatory
response, mainly due to their crucial effects on the
differentiation, maturation and activation of cells (11). However, excessive production of
cytokines harms organisms (6). It
has been reported that patients suffering from inflammatory
diseases present abnormalities in pro- and anti-inflammatory
cytokines (12). Inflammatory
cytokine release in response to LPS is mediated by the activation
of nuclear factor κ-light-chain enhancer of activated B cells
(NF-κB) and mitogen-activated protein kinase (MAPK) (13,14).
NF-κB is a family of transcription factors and regulates the
expression of a number of immune-related cytotoxic factors,
including iNOS and cyclooxygenase-2 (COX-2), and pro-inflammatory
cytokines, including TNF-α, IL-1β, IL-6 and IL-8 (15,16).
The MAPK family also induces the production of immune-related
cytotoxic factors and pro-inflammatory cytokines (17,18).
Therefore, NF-κB and MAPKs are well-recognized as targets of
anti-inflammatory agents.
In the present study, we examined the effects of SSA
on the production of various inflammatory cytokines in
LPS-stimulated mouse RAW 264.7 macrophages. We also investigated
its anti-inflammatory mechanism, focusing on inflammatory signaling
pathways. To our knowledge, this is the first report demonstrating
that SSA inhibits the production of immune-related cytotoxic
factors and inflammatory cytokines induced by LPS by inhibiting the
NF-κB and MAPK signaling pathways.
Materials and methods
Reagents
SSA was purchased from Sichuan Victory Biotechnology
Co., Ltd. (Sichuan, China), with 98% purity detected by high
performance liquid chromatography (HPLC). LPS (Escherichia
coli 026:B6), dimethyl sulfoxide (DMSO) and
3-[4,5-dimethylthiazol- 2-yl]-2,5-diphenyltetrazolium bromide (MTT)
were purchased from Sigma (St. Louis, MO, USA). TNF-α, IL-1β, IL-6
and IL-10 enzyme-linked immunosorbent assay (ELISA) kits were
purchased from R&D Systems (Minneapolis, MN, USA). Dulbecco’s
modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were
purchased from HyClone Laboratories of Thermo Scientific (Logan,
UT, USA).
The antibodies, including iNOS, COX-2, NF-κB (p65)
and β-actin were obtained from Cayman Chemical Co. (Ann Arbor, MI,
USA). Antibodies for phospho-extracellular signal-regulated kinases
(ERK)1/2, ERK, phospho-p38, p38, phospho-Jun N-terminal kinase
(JNK), JNK, IκBα and p65 were obtained from Cell Signaling
Technology (Danvers, MA, USA).
Cell culture and sample treatment
The mouse macrophage cell line RAW 264.7 was
obtained from the Center of Cellular Resources, Central South
University, Changsha, China. Cells were cultured in DMEM
supplemented with 10% heat-inactivated FBS, 3 mM glutamine, 100
U/ml penicillin and 100 μg/ml streptomycin at 37°C under a
humidified atmosphere of 5% CO2. In all experiments,
cells were left to acclimate for 24 h before treatment. SSA was
added 1 h prior to LPS (1 mg/l) treatment. The study was approved
by the ethics committee of Central South University, Changsha,
China.
MTT assay for cell viability
Cytotoxicity induced by SSA was analyzed by MTT
assay. RAW 264.7 cells were plated at a density of 1×104
cells/ml onto 96-well plates containing 100 μl DMEM and
incubated overnight. After acclimating for 24 h, the cells were
treated with 100 μl SSA at various concentrations (3.125,
6.25, 12.5, 25, 50 and 100 μM) for 1 h, followed by
stimulation with 50 μl LPS (1 mg/l) for 18 h. Subsequently,
20 μl MTT (5 mg/ml, 20 μl/well) in FBS-free medium
was added to each well and further incubated for 4 h. Cell-free
supernatants were then removed and cells were resolved with 150
μl DMSO per well, followed by optical density measurement at
490 nm with a ELX800-UV absorbance microplate reader (BioTek
Instruments Inc., Winooski, VT, USA).
Cytokine determination
To determine the effects of SSA on cytokine release
in LPS-stimulated cells, the production of TNF-α, IL-1β, IL-6 and
IL-10 was measured by ELISA. RAW 264.7 cells were grown in a 6-well
plate at a density of 3×105 cells/well for 24 h. The
cells were pretreated with various concentrations of SSA compounds
for 2 h and further challenged with LPS for an additional 18 h at
37°C with 5% CO2. The supernatants were then collected
and centrifuged at 1,000 x g, 4°C for 10 min. The levels of TNF-α,
IL-1β, IL-6 and IL-10 in the supernatants were determined using
ELISA kits, according to the manufacturer’s instructions.
Real-time fluorescent quantitative
polymerase chain reaction (PCR)
RAW 264.7 cells (4×105 cells/ml),
cultured in 6-well plates for 24 h, were pretreated with various
concentrations (3.125, 6.25 and 12.5 μM) of SSA for 2 h
before treatment with 1 μg/ml LPS for 3 h in a 37°C, 5%
CO2 incubator. Following two washes with ice-cold
phosphate-buffered saline (PBS), the cells were harvested and total
cellular RNA was isolated using the TRIzol reagent, according to
the manufacturer’s instructions (Invitrogen Life Technologies,
Carlsbad, CA, USA). For the real-time PCR, 1 μg total RNA
was reverse-transcribed to synthesize cDNA using a first-strand
cDNA synthesis kit (Takara, Dalian, China). Quantitative real-time
PCR was performed on a Bio-Rad CFX 96 real-time PCR detection
system in a 30 ml reaction volume containing iQ™ SYBR-Green
Supermix (Bio-Rad, Hercules, CA, USA), 100 nM primers and 1 ml
appropriately diluted cDNA template. The parameters of the PCR
reaction were as follows: 94°C for 3 min for one cycle, then 94°C
for 30 sec, 55–59°C for 30 sec, 72°C for 45 sec for 30 cycles and
72°C for 5 min for one cycle. The relative gene expression was
calculated by the comparative Ct method (2−ΔΔCt), using
glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as the house
keeping gene. The primer sequences for analysis of TNF-α, IL-1β,
IL-6 and GAPDH mRNA are presented in Table I.
 | Table IPrimers used for real-time PCR. |
Table I
Primers used for real-time PCR.
| Gene | Primer | Sequence (5′-3′) |
|---|
| iNOS | Sense |
CAAGCTGAACTTGAGCGAGGA |
| Antisense |
TTTACTCAGTGCCAGAAGCTGGA |
| COX-2 | Sense |
CTGGAACATGGACTCACTCAGTTTG |
| Antisense |
AGGCCTTTGCCACTGCTTGT |
| TNF-α | Sense |
CCGCTCGTTGCCAATAGTGATG |
| Antisense |
CATGCCGTTGGCCAGGAGGG |
| IL-1β | Sense |
GCACTACAGGCTCCGAGATGAA |
| Antisense |
GTCGTTGCTTGGTTCTCCTTGT |
| IL-6 | Sense |
CTTGGGACTGATGCTGGTGACA |
| Antisense |
GCCTCCGACTTGTGAAGTGGTA |
| IL-10 | Sense |
CGATGTTCTGTTCTGGTT |
| Antisense |
AAGACGCTTGACTTGAAG |
| GAPDH | Sense |
AGTGGCAAAGTGGAGATT |
| Antisense |
GTGGAGTCATACTGGAACA |
Western blot analysis
Western blot analysis was performed to evaluate the
effect of the test compound on iNOS, COX-2, NF-κB (p65) and
inhibitory NF-κB inhibitor α (IκBα) in the cytosol and nucleus, as
well as the expressions of P38 MAPK, c-JNK and ERK. The RAW 264.7
cells were cultivated in a 6-well plate for 24 h and then received
appropriate treatment with SSA in the absence or presence of LPS
for 2 h. After treatment for 18 h with LPS, the cells were
harvested and the total protein, cytosol protein and nuclear
protein were extracted using a Nuclear-Cytosol Extraction Kit (Cell
Signaling Technology). β-actin was used as the control. The protein
was separated on polyacrylamide gels and then transferred onto a
polyvinylidene fluoride (PVDF) membrane. The membranes were blocked
and incubated with different antibodies, followed by incubation
with the horseradish peroxidase (HRP)-linked secondary antibody.
The signals were detected using an enhanced chemiluminescence (ECL)
reagent (Bio-Rad). The images were quantified by Bio-Rad Quantity
One software. The quantities of the target bands were normalized by
β-actin.
Statistical analysis
Data, expressed as means ± standard deviation, were
analyzed by one-way analysis of variance (ANOVA). Significant
differences were determined with Tukey’s multiple range tests. All
tests were performed using SPSS 13.0 software (SPSS Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Cytotoxicity of SSA on RAW 264.7
cells
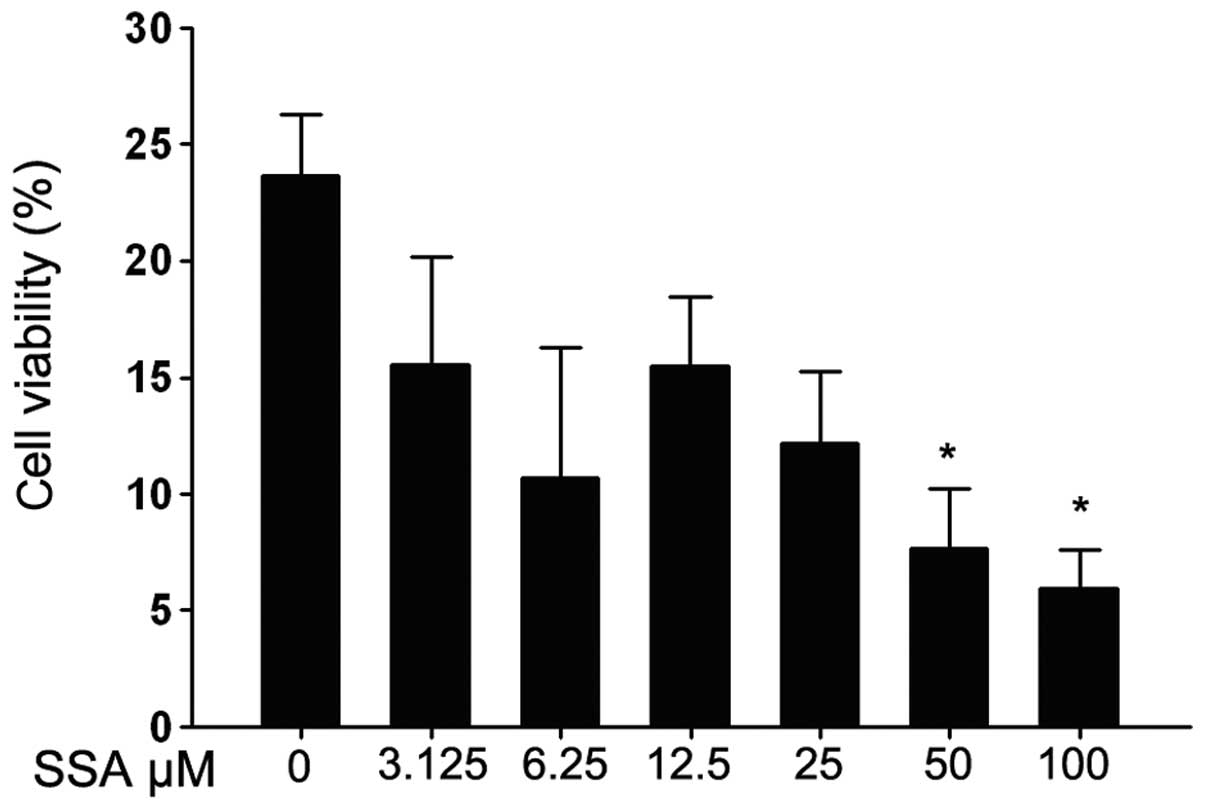
Prior to evaluating the anti-inflammatory activity
of SSA, the cytotoxic effect of SSA on RAW 264.7 cells was tested
using the MTT assay. As shown in Fig.
1, cell viability was significantly reduced with 12.5–100
μM SSA, while 3.125 and 6.25 μM SSA had no effect on
LPS-stimulated RAW 264.7 cells.
SSA inhibits the release of LPS-induced
pro-inflammatory cytokines in RAW 264.7 cells
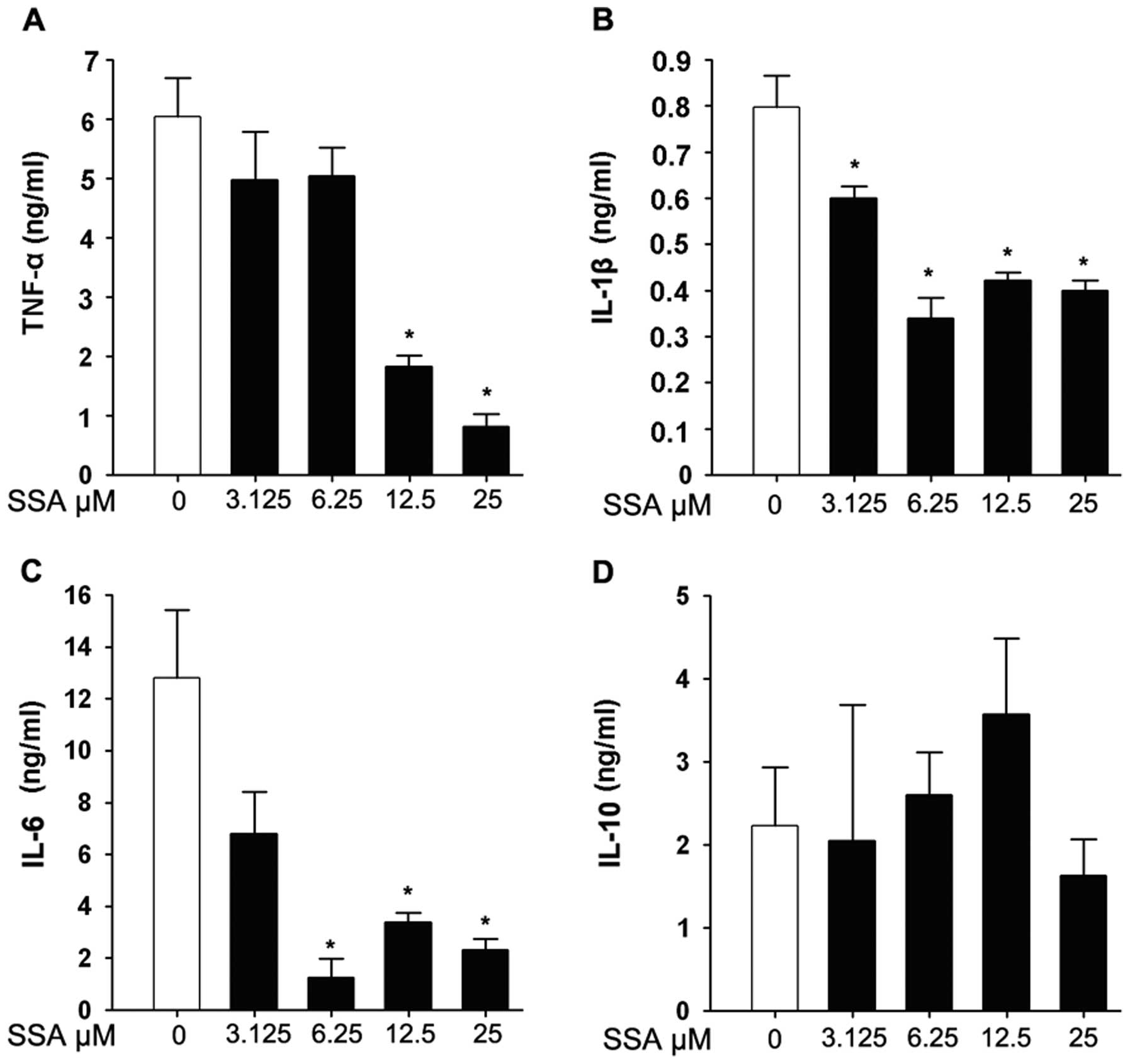
TNF-α, IL-1β, IL-6 and IL-10 concentrations in the
culture supernatants of RAW 264.7 cells were evaluated by ELISA. As
shown in Fig. 2A, TNF-α was
significantly inhibited by pretreatment with SSA in a
dose-dependent manner. A similar tendency was also observed in IL-6
and IL-1β production at various concentrations of SSA (Fig. 2B and C). However, SSA pretreatment
had no significant effect on IL-10 compared to the control group in
this assay (Fig. 2D).
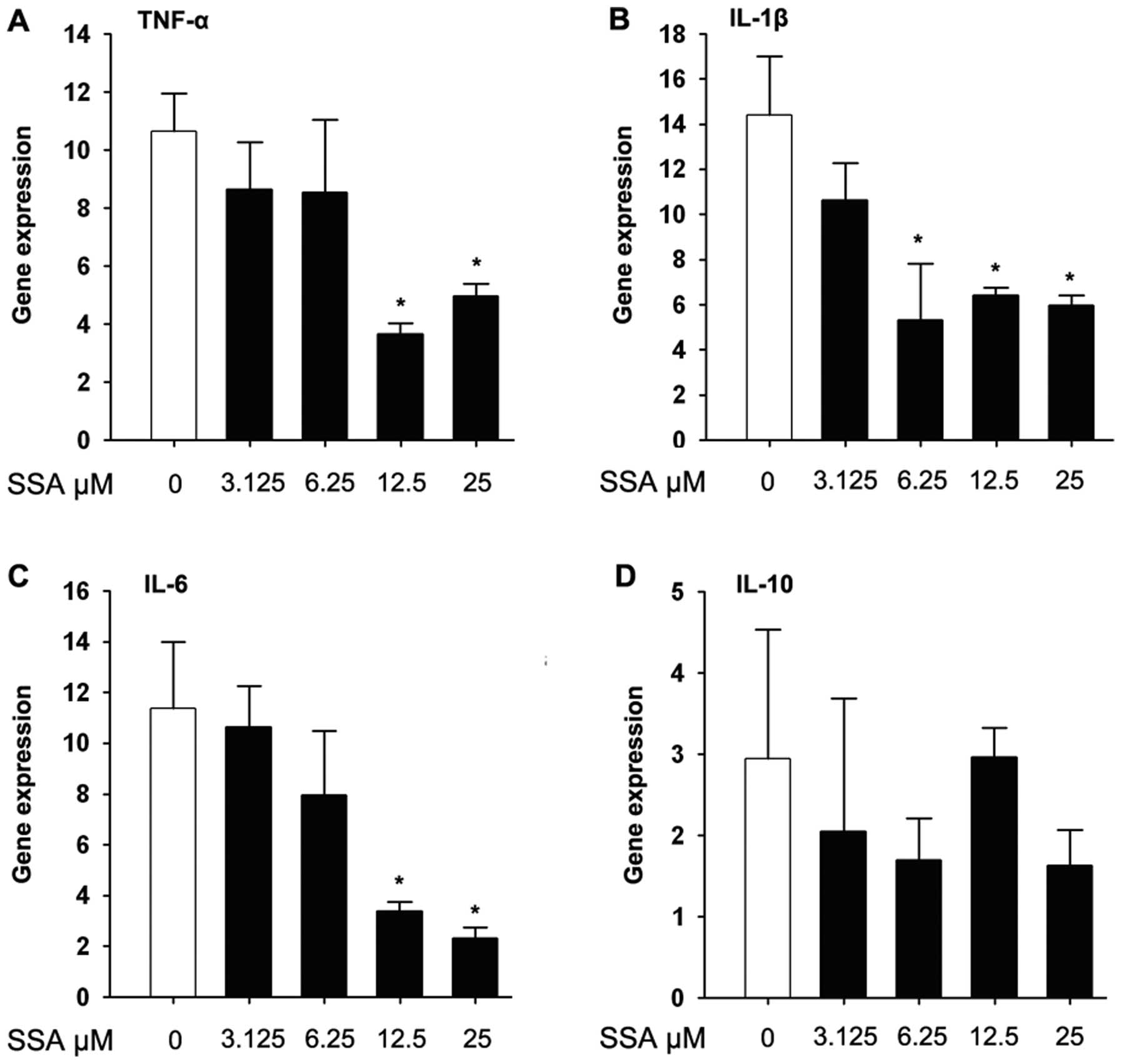
SSA inhibits the mRNA level of TNF-α,
IL-1β, IL-6 and IL-10 in LPS-stimulated RAW 264.7 cells
Real-time PCR was employed to quantitate TNF-α,
IL-6, IL-1β and IL-10 gene expression from cDNA samples. For the
mRNA expression of pro-inflammatory cytokines, SSA pretreatment for
1 h significantly inhibited the expression of TNF-α, IL-1β and IL-6
compared to the control group, and upregulated the expression of
IL-10 (Fig. 3).
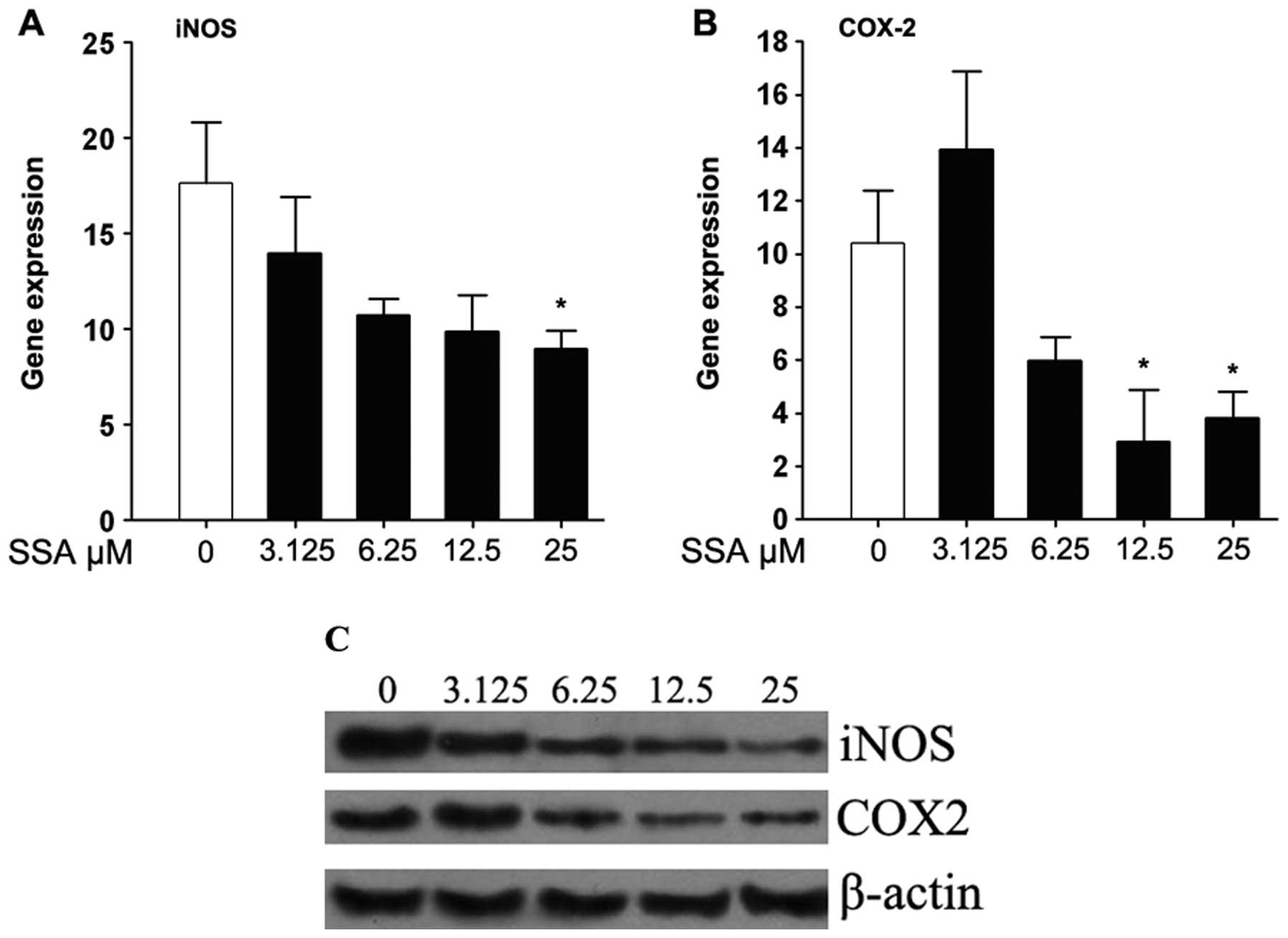
SSA suppresses the expression of iNOS and
COX-2 in LPS-stimulated RAW 264.7 cells
Real-time PCR and western blotting were performed to
determine the inhibitory effect of SSA on the mRNA and protein
levels of iNOS and COX-2, respectively. As shown in Fig. 4A and B, the mRNA expressions of
iNOS and COX-2 were reduced in a dose-dependent manner by SSA in
LPS-stimulated RAW 264.7 cells. Also, SSA strongly downregulated
iNOS and COX-2 protein expression (Fig. 4C).
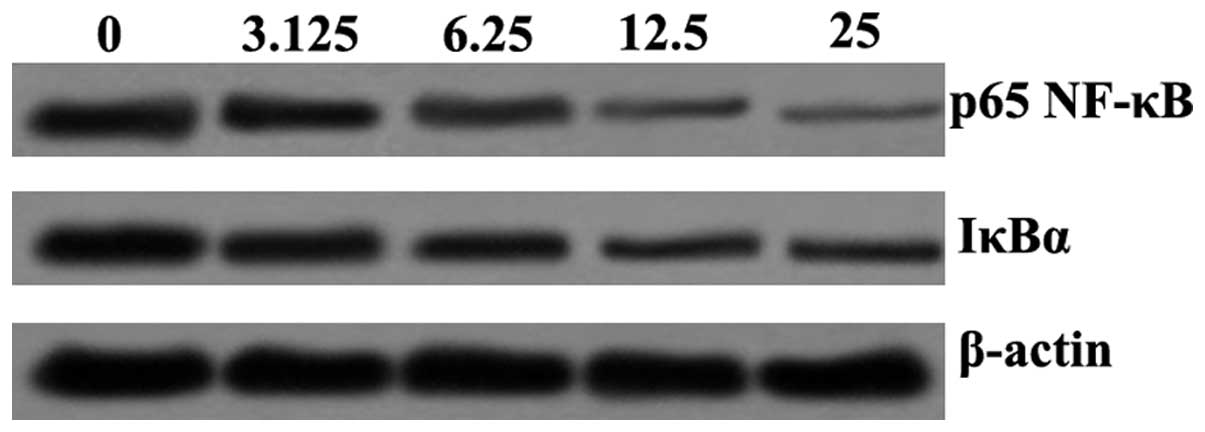
SSA suppresses the LPS-induced activation
of NF-κB signaling in RAW 264.7 cells
Western blotting was performed to determine the
effect of SSA on LPS-induced NF-κB activation. The results revealed
that p65 NF-κB and IκBα protein expression were downregulated by
SSA. The p65 NF-κB and IκBα protein expression demonstrated a
dose-dependent effect on suppression induced by SSA (Fig. 5).
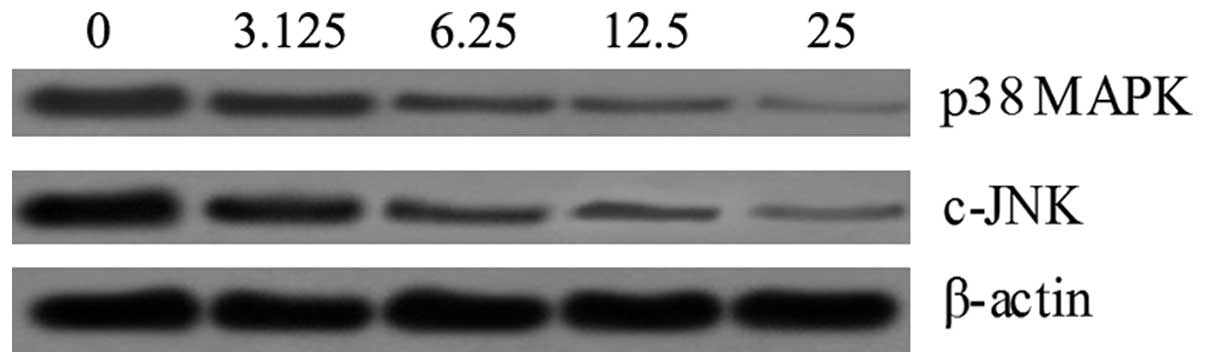
SSA suppresses the LPS-induced activation
of MAPK signaling in RAW 264.7 cells
In order to understand the mechanism by which SSA
inhibits LPS-induced production of inflammatory cytokines, we
detected the possible connection between SSA and the MAPK pathway.
Following SSA treatment, the phosphorylation of p38 MAPK and c-JNK
had markedly decreased compared to the control in a dose-dependent
manner (Fig. 6).
Discussion
Macrophages play a crucial role in the specific and
non-specific immune responses during the inflammatory process by
producing a large amount of inflammatory mediators, including
immune-related cytotoxic factors and inflammatory cytokines.
Despite the beneficial effect during infection, excessive
production of inflammatory mediators may cause edema, cellular
metabolic stress and tissue necrosis (12). As a result, agents regulating
inflammatory cytokines may have therapeutic effects. The present
study demonstrated that LPS effectively induces the activation of
macrophages, which is consistent with previous reports (19,20).
By activating several signals and transcription factors, including
MAPKs and NF-κB, LPS induces the activation of inflammatory
cytokines in macrophages, leading to the production of TNF-α, IL-6,
IL-1β and IL-10 (9,10). In the present study, we
demonstrated that SSA markedly inhibits immune-related cytotoxic
factors, including iNOS and COX-2, and pro-inflammatory cytokines,
including TNF-α, IL-1β and IL-6. It also increased the protein and
mRNA levels of the anti-inflammatory cytokine, IL-10, in
LPS-stimulated RAW 264.7 macrophages. These data demonstrate the
anti-inflammatory activity of SSA in macrophages stimulated by
LPS.
To further clarify the molecular mechanism of the
inhibitory effect of SSA on inflammatory mediators, we investigated
the effects of SSA on the activation of two signaling pathways,
NF-κB and MAPKs, in LPS-stimulated macrophages. LPS has been shown
to induce the NF-κB signaling pathway in macrophages (21). NF-κB, a family of transcription
factors, is universally expressed in various types of cells and
regulates the transcription of a number of key inflammatory
mediators, including COX-2, TNF-α, IL-1β, IL-6 and IL-10 (22). Therefore, the NF-κB signaling
pathway acts as a core regulator of inflammation. Under normal
conditions, NF-κB associates with IκBs, which sequester NF-κB in
the cytoplasm. The activation of NF-κB begins with the
phosphorylation of IκBα. Then, the phosphorylation of IκBα allows
itself to be ubiquitinated and eventually degraded by the 26S
proteasome (23). Once IκBα is
degraded, the nuclear localization signal of NF-κB is not masked
and NF-κB is able to translocate to the nucleus and promote the
transcription of target genes (24). As demonstrated in the present
study, in the control group, the phosphorylation levels of IκBα and
p65 NF-κB were high following exposure to LPS; however, following
administration of SSA, the phosphorylation of p65 NF-κB and IκBα
were markedly decreased in a dose-dependent manner. These data
indicate that SSA blocks the NF-κB signaling pathway by inhibiting
the phosphorylation of IκBα, preventing NF-κB translocation to the
nucleus.
The other major extracellular signaling pathway
induced by inflammatory mediators is the MAPK pathway. In the MAPK
family, p38 MAPK, c-JNK and ERKs are the most important components
(18). LPS has been shown to
induce the MAPK signaling pathway in macrophages (25), which is consistent with our data.
In the present study, we identified that phosphorylation of p38
MAPK and c-JNK was high in LPS-stimulated macrophages; however,
following administration of SSA, the phosphorylation of p38 MAPK
and c-JNK significantly reduced in a dose-dependent manner,
suggesting that the activation of the MAPK signaling pathway is
inhibited by SSA. Since it is well established that MAPKs regulate
various inflammatory mediators, including TNF, IL-1, IL-2, IL-6
COX-2 and iNOS (26–28), we consider that the
anti-inflammatory activity of SSA is associated with its inhibitory
effect on the MAPK signaling pathway.
In conclusion, this study demonstrated that SSA has
an inhibitory effect on pro-inflammatory cytokines, as well as a
facilitative effect on anti-inflammatory cytokines in
LPS-stimulated macrophages. The mechanism of these actions involves
the regulation of MAPK and NF-κB signals.
References
|
1.
|
Ashour ML and Wink M: Genus
Bupleurum: a review of its phytochemistry, pharmacology and
modes of action. J Pharm Pharmacol. 63:305–321. 2011. View Article : Google Scholar
|
|
2.
|
Kong XY, Hao Y, Wu TX and Xie YM: Adverse
drug reactions or adverse events of Chaihu Injection: a systematic
review. Zhong Xi Yi Jie He Xue Bao. 8:1124–1132. 2010.(In
Chinese).
|
|
3.
|
Wu GC, Wu H, Fan LY and Pan HF:
Saikosaponins: a potential treatment option for systemic lupus
erythematosus. Ir J Med Sci. 180:259–261. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Sui C, Zhang J, Wei J, et al:
Transcriptome analysis of Bupleurum chinense focusing on
genes involved in the biosynthesis of saikosaponins. BMC Genomics.
12:5392011.PubMed/NCBI
|
|
5.
|
Lu CN, Yuan ZG, Zhang XL, et al:
Saikosaponin a and its epimer saikosaponin d exhibit
anti-inflammatory activity by suppressing activation of NF-kappaB
signaling pathway. Int Immunopharmacol. 14:121–126. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Philippou A, Maridaki M, Theos A and
Koutsilieris M: Cytokines in muscle damage. Adv Clin Chem.
58:49–87. 2012. View Article : Google Scholar
|
|
7.
|
Lee IT and Yang CM: Role of NADPH
oxidase/ROS in pro-inflammatory mediators-induced airway and
pulmonary diseases. Biochem Pharmacol. 84:581–590. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Romeo GR, Lee J and Shoelson SE: Metabolic
syndrome, insulin resistance and roles of inflammation - mechanisms
and therapeutic targets. Arterioscler Thromb Vasc Biol.
32:1771–1776. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Wang Y, Yu C, Pan Y, et al: A novel
compound C12 inhibits inflammatory cytokine production and protects
from inflammatory injury in vivo. PLoS One. 6:e243772011.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Borges MC, Vinolo MA, Crisma AR, et al:
High-fat diet blunts activation of the nuclear factor-kappaB
signaling pathway in lipopolysaccharide-stimulated peritoneal
macrophages of Wistar rats. Nutrition. Oct 19–2012.(Epub ahead of
print).
|
|
11.
|
Paulsen G, Mikkelsen UR, Raastad T and
Peake JM: Leucocytes, cytokines and satellite cells: what role do
they play in muscle damage and regeneration following eccentric
exercise? Exerc Immunol Rev. 18:42–97. 2012.PubMed/NCBI
|
|
12.
|
Ren G, Zhao X, Zhang L, et al:
Inflammatory cytokine-induced intercellular adhesion molecule-1 and
vascular cell adhesion molecule-1 in mesenchymal stem cells are
critical for immunosuppression. J Immunol. 184:2321–2328. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Wu YH, Chuang SY, Hong WC, Lai YJ, Chang
GJ and Pang JH: Berberine reduces leukocyte adhesion to
LPS-stimulated endothelial cells and VCAM-1 expression both in vivo
and in vitro. Int J Immunopathol Pharmacol. 25:741–750.
2012.PubMed/NCBI
|
|
14.
|
Shao J, Liu T, Xie QR, et al: Adjudin
attenuates lipopolysaccharide (LPS)- and ischemia-induced
microglial activation. J Neuroimmunol. 254:83–90. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Sohn KH, Jo MJ, Cho WJ, et al:
Bojesodok-eum, a herbal prescription, ameliorates acute
inflammation in association with the inhibition of
NF-kappaB-mediated nitric oxide and proinflammatory cytokine
production. Evid Based Complement Alternat Med. 2012 Oct
8–2012.(Epub ahead of print).
|
|
16.
|
Cortez M, Carmo LS, Rogero MM, Borelli P
and Fock RA: A high-fat diet increases IL-1, IL-6 and TNF-alpha
production by increasing NF-kappaB and attenuating PPAR-gamma
expression in bone marrow mesenchymal stem cells. Inflammation. Oct
19–2012.(Epub ahead of print).
|
|
17.
|
Xie GC and Duan ZJ: Signal transduction of
innate immunity to virus infection. Bing Du Xue Bao. 28:303–310.
2012.(In Chinese).
|
|
18.
|
Kyriakis JM and Avruch J: Mammalian MAPK
signal transduction pathways activated by stress and inflammation:
a 10-year update. Physiol Rev. 92:689–737. 2012.PubMed/NCBI
|
|
19.
|
Gyorfy Z, Duda E and Vizler C:
Interactions between LPS moieties and macrophage pattern
recognition receptors. Vet Immunol Immunopathol. Sep 26–2012.(Epub
ahead of print).
|
|
20.
|
Liu Z, Li W, Wang F, et al: Enhancement of
lipopolysaccharide-induced nitric oxide and interleukin-6
production by PEGylated gold nanoparticles in RAW264.7 cells.
Nanoscale. Oct 16–2012.(Epub ahead of print).
|
|
21.
|
Tacchi L, Casadei E, Bickerdike R,
Secombes CJ and Martin SA: MULAN related gene (MRG): A potential
novel ubiquitin ligase activator of NF-kB involved in immune
response in Atlantic salmon (Salmo salar). Dev Comp Immunol.
38:545–553. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
DiDonato JA, Mercurio F and Karin M:
NF-kappaB and the link between inflammation and cancer. Immunol
Rev. 246:379–400. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Iwai K: Diverse ubiquitin signaling in
NF-kappaB activation. Trends Cell Biol. 22:355–364. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Dyson HJ and Komives EA: Role of disorder
in IkappaB-NFkappaB interaction. IUBMB Life. 64:499–505. 2012.
View Article : Google Scholar
|
|
25.
|
Tang X and Zhu Y: TLR4 signaling promotes
immune escape of human colon cancer cells by inducing
immunosuppressive cytokines and apoptosis resistance. Oncol Res.
20:15–24. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Wang Z, Jiang W, Zhang Z, Qian M and Du B:
Nitidine chloride inhibits LPS-induced inflammatory cytokines
production via MAPK and NF-kappaB pathway in RAW 264.7 cells. J
Ethnopharmacol. 144:145–150. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Cheng W, Chen L, Yang S, et al: Puerarin
suppresses proliferation of endometriotic stromal cells partly via
the MAPK signaling pathway induced by 17ss-estradiol-BSA. PLoS One.
7:e455292012. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Ihara H, Yamamoto H, Ida T, et al:
Inhibition of nitric oxide production and inducible nitric oxide
synthase expression by a polymethoxyflavone from young fruits of
Citrus unshiu in rat primary astrocytes. Biosci Biotechnol Biochem.
76:1843–1848. 2012. View Article : Google Scholar : PubMed/NCBI
|