Introduction
The number of patients with large-area soft tissue
defects accompanied by serious injury involving bone exposure in
the lower leg have increased due to rapid increases in road
accidents and work-related injuries. In large-area skin soft tissue
defects with poor blood supply and bone exposure, it is not
feasible to conduct skin flap transplantation to close the wound
and maintain the limb; therefore, amputation is often selected.
Even if limb salvage treatment is selected, the traditional
dressing therapy easily causes complications, including wound
infection and non-healing, bone fracture and non-union,
osteonecrosis, osteomyelitis and fistula formation. As a result,
limb function is poor and the treatment efficacy is unsatisfactory.
A novel vacuum sealing drainage (VSD) (1) technique has become the standard
treatment method for treating various types of wound surfaces and
wounds that are difficult to heal (2). Compared with other traditional
drainage modes, it has clear advantages. VSD is an efficient
drainage system and its efficiency embodies its comprehensive
drainage and thorough drainage under high vacuum. It promptly and
thoroughly leads seepage, pus and necrotic tissues from the
drainage area out of the body to cause ‘zero accumulation’ in the
drainage area. It also significantly speeds up infected lacuna
closure and infected wound surface healing to effectively prevent
surgical field effusion. Additionally, VSD is particularly suitable
for the treatment of complex wounds. Experiments in animals suggest
that the negative pressure environment promotes subcutaneous and
intradermal blood flow in the wound, reduces bacterial reproduction
and promotes granulation growth (3). Halvorson et al(4), by studying pediatric cases, confirmed
that VSD is a safe and effective method with a low infection rate
for treating open fractures. VSD combined with debridement and
antibiotics increases the wound healing rate, shortens the
hospitalization time and enhances patients’ comfort and
satisfaction (5). To resolve the
limb salvage problem, we used free dermatoplasty combined with VSD
to treat 36 patients with large-area soft tissue defects
accompanied by bone exposure in the lower leg from June 2006 to
June 2011 and obtained a satisfactory efficacy.
Subjects and methods
General data
In this study, there were 36 patients, including 29
males and 7 females, aged 22–66 years with a mean age of 36.8
years. Among them, there were 14 cases whose left limb was injured,
19 cases whose right limb was injured and three cases with two
injured limbs. Soft tissue defect areas ranged from 25×12 to 35×30
cm and bone exposure areas ranged from 6×4 to 10×6 cm. When
evaluated by the open fracture Gustilo classification, 14 cases
were of Gustilo type IIIA and 22 cases were of type IIIB. Regarding
the cause of injury, 29 cases were due to traffic injury, five
cases were due to crushing by heavy objects and two cases were due
to wringer injury. The time interval from trauma to surgery was
∼4–10 h. In addition, emergency debridement was conducted in all
cases. This study was conducted in accordance with the Declaration
of Helsinki. and with approval from the Ethics Committee of the
Second Hospital of Tangshan. Written informed consent was obtained
from all participants.
Treatment method
Emergency debridement was conducted under tourniquet
control following epidural anesthesia or continuous epidural
anesthesia. Wounds were repeatedly and thoroughly washed with a
high pressure pulse flushing pump and sterilized with conventional
disinfection solution. Debridement and hemostasis were strictly and
thoroughly conducted to remove soft tissues without the loss of
blood circulation. The initially denuded skins of the unclear
necrosis boundary were maintained briefly. Once the necrosis
boundary was clear, they were thoroughly removed. After stripping
the skin and cleaning the subcutaneous fat, the fat was punctured
with a knife to create meshed holes to enable the seepage of
secreta, pus and necrotic tissues to drain away. Following
emergency debridement, adjacent available muscle flaps were
transferred to cover the outer areas of the exposed bone and reduce
the bone exposure range. Several holes were drilled through the
bare bone cortex surface into the contralateral cortex to enable
blood capillaries and fresh granulation tissues to grow from the
channels to cover the exposed bone. In this study, all cases were
treated by VSD. After 1–4 rounds of treatment, granulation tissues
on the wound surface grew well and free dermatoplasty was conducted
to close the wound. Following the free dermatoplasty, sustained
low-pressure VSD was conducted. In the donor skin area, sustained
VSD was conducted for 12 cases. The wound surface was firstly
covered with Vaseline gauze and then covered with VSD dressing. For
situations involving open fractures, external fixing frame fixation
or simple fixation, including Kirschner wire and screw fixation,
was used.
When applying a sterile medical sponge (VSD
dressing) to the wound surface, it is appropriate to cover only the
wound surface. A transparent adhesive membrane (semipermeable
membrane) was then used to seal the wound and the VSD dressing. The
semipermeable membrane extended >3 cm from the edge of wound to
seal the surrounding normal skin. Thus, the open wound was closed.
A silicone tube was connected between the VSD dressing and a VSD
special treatment instrument or a negative pressure drainage
device, and negative pressure treatment was conducted in a closed
condition. A study has confirmed that a negative pressure
maintained at 125 mmHg increases microcirculation while a negative
pressure >400 mmHg causes retroaction (6). According to the particular situation
of each wound, either intermittent or sustained constant negative
pressure treatment was selected. When the wound surface was
relatively small, the amounts of seepage and secretions were less
and the wound surface was cleaner and an intermittent constant
negative pressure was selected only when it was required to rapidly
promote the growth of granulation tissues. When the wound surface
was larger and the amounts of seepage and secreta were greater, a
sustained constant negative pressure treatment was selected in
order to promptly and effectively lead waste out of the body.
Generally, the VSD dressing and semipermeable membrane were
replaced once every ∼7 days. When there was a substantial amount of
waste, including pus, secreta and necrotic tissues, drainage tubes
in the VSD dressing or VSD dressing micropores became obstructed,
which affected the filtering and suction of the VSD dressing. In
these conditions, the VSD dressing and semipermeable membrane were
replaced once every 3–4 days. During treatment, it was necessary to
maintain a sealed environment and an effective negative pressure
status, as well as unobstructed pipelines.
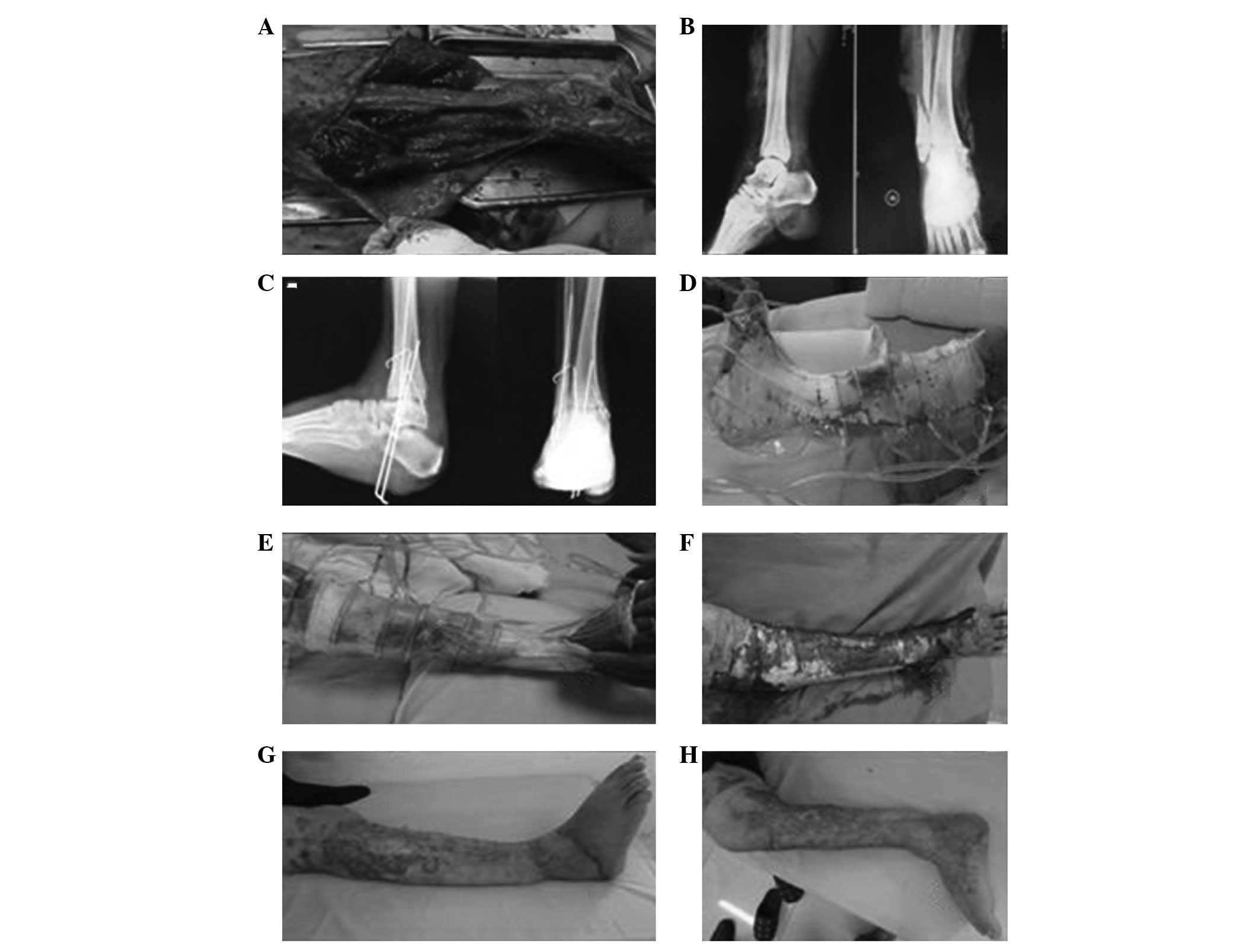
Typical case 1
Case 1 was a female patient aged 60 years with
traumatic hemorrhagic shock, left lower leg and left foot traumas
(Gustilo type IIIB) and right ankle trauma (Gustilo type IIIB).
After VSD had been conducted on the right foot for 3 weeks, free
dermatoplasty was conducted on the fresh granulation tissues on the
wound surface and sustained low-pressure VSD treatment was
conducted in the skin transplantation area. After 1 week, the skin
graft had completely survived and the wound was healed. In
addition, after VSD treatment had been conducted on the left lower
leg and foot for 3 weeks, local muscle flap transfer was conducted
to cover the exposed tibia. After 1 week, the muscle flaps had
survived and free dermatoplasty was conducted. In the skin
tranplantation area, sustained low-pressure VSD treatment was
conducted. After 1 week, the skin graft had completely survived and
the wound had healed well (Fig.
1).
Typical case 2
Case 2 was a male patient aged 34 years, with an
open fracture dislocation of the right lower extremity (Gustilo
type IIIB). After two VSD treatments, local muscle flap transfer
was conducted to cover the exposed tibia. After 1 week, the muscle
flaps had survived and free dermatoplasty was conducted. In the
skin tranplantation area, sustained low-pressure VSD treatment was
conducted. After 1 week, the skin graft had completely survived and
the wound had healed well (Fig.
2).
Results
Treatment results
Among the 36 patients, VSD was conducted 1–4 times
according to the status of the wounds and the degree of bone
exposure (larger bone exposure meant a greater frequency of VSD).
Among them, seven cases of soft tissue injuries were mild and the
bone exposure area was 6×4 cm. In the 6–14 days following the first
treatment with VSD, the wound surfaces were completely covered by
granulation tissues. In 12 cases of bone exposure, the area ranged
from 7×4 to 57×5 cm. At days 13–24, following two VSD treatments,
the wound surfaces were completely covered by granulation tissues.
In nine cases of bone exposure, the area ranged from 8×4 to 8×5 cm.
At days 25–27, following three VSD treatments, the wound surfaces
were completely covered by granulation tissues. In eight cases of
bone exposure, the area ranged from 9×5 to 10×5 cm. At days 26–39,
following four VSD treatments, the wound surfaces were completely
covered by granulation tissues. Following emergency debridement,
the exposed bone was covered with adjacent muscle flaps. Following
VSD treatment, abundant, fresh and actively bleeding granulation
tissues with good elasticity grew on the wound surface. In stage
II, the exposed bone was covered with adjacent muscle flaps in 12
cases and the exposed bone was covered with rapidly growing
granulation tissues. The time taken for the exposed bone to be
completely covered in the 36 patients ranged from 6 to 29 days and
the mean was 18.2 days. Once free dermatoplasty combined with
sustained low-pressure VSD was conducted, 36 cases of skin flap
grafts survived. Among them, stamp skin transplantation was
conducted for five cases for 6–8 days due to the larger free skin
transplantation area and the limited donor skin area. Once the VSD
dressing had been removed, scattered wound areas remained in gaps
of the skin graft. Dressings were actively administered and the
wound surfaces healed. Fracture healing durations ranged from 3 to
6 months and the mean duration was 4 months. No osteonecrosis and
no osteomyelitis occurred. In addition, three cases suffered from
achilles tendon contracture of equinus deformity and diorthosis was
conducted.
Postoperative follow-up
The cases in this study were followed up for 1–5
years (mean duration, 2.5 years). The textures of the free skin
grafts on the wound surfaces were good and recovery of lower leg
and ankle functions were satisfactory. Lower leg and ankle
functions were scored according to the Iowa evaluation rating
system criteria (7): excellent,
eight cases; good, 21 cases; qualified, five cases and poor, two
cases. The rate of excellent or good functional results was
80.56%.
Discussion
VSD reduces wound infection and promotes wound
healing. Following the debridement of large-area soft tissue
defects accompanied by bone exposure in the lower leg, VSD is
conducted to transform the open wound into a closed wound and
prevent external bacteria from invading the wound surface.
Therefore, it eliminates the conditions that favor bacterial
culture, inhibits bacterial growth and reproduction, blocks
infection spread and toxin absorption, decreases bacterial
infection and reproduction levels in the tissue, decreases the
wound infection rate, helps the rapid growth of granulation tissues
on the wound surface and promotes wound healing. Experimental and
clinical studies have confirmed that the negative pressure-assisted
wound surface closing technique effectively promotes wound surface
healing by multiple mechanisms (8). The closed environment and continuous
negative pressure suction of VSD cause the wound surface to form a
sustained hypoxic or relatively anoxic subacid environment and thus
inhibits the growth of pathogenic microorganisms on the wound
surface. Additionally, VSD causes a drop in the oxygen tension
around the wound surface to stimulate initiation of the repair
signal. Therefore, the release of fibrinolytic activator and other
enzymes is promoted to form an environment of quickening
fibrinolysis. Subsequently, fibrinolysis occurs on the wound
surface and autolytic debridement is conducted (9), which contributes to wound surface
healing. VSD promotes cellular proliferation and migration
(10). Labler et
al(8) confirmed that following
VSD treatment, the levels of interleukin-8 and vascular endothelial
growth factor expression on the wound surface were significantly
increased, which promoted vascularization of the wound surface.
Morykwas et al(11)
reported that VSD treatment accelerates the blood circulation on
the wound surface and that the intermittent peak in the soft tissue
blood supply flow was 4-fold higher than the normal baseline blood
flow. The treatment of this group of cases also confirmed that VSD
enables the soft tissue to obtain a good blood supply and rapidly
relieves swelling of injured tissue, reduces local edema, improves
local circulation and oxygen level, speeds up the growth of fresh
granulation tissues and promotes wound healing. The treatment of
complex wounds often requires multiple debridements and the wounds
are finally closed by skin flaps or skin grafts. VSD helps to
shorten the treatment time and simplifies the treatment method
(12).
Large-area soft tissue defects accompanied by bone
exposure in the lower leg are difficult to treat. Due to the large
area of the skin soft tissue defects, poor blood supply and bone
exposure, it is not feasible to use skin flap transplantation to
close the wound surface. Additionally, the defects are often
accompanied by surrounding infection and osteonecrosis, as well as
osteomyelitis. Therefore, it is difficult to maintain the limb and
amputation is the only available option. Even if limb salvage
treatment is selected, with traditional dressing therapy the
drainage of seepage, pus and necrotic tissues is poor, which causes
the wound surface to become a bacterial culture medium. Bacterial
growth and reproduction are likely to cause complications,
including wound infection and non-healing, bone fracture and
non-union, osteonecrosis, osteomyelitis and fistula formation. As a
result, limb function is poor and treatment efficacy is
unsatisfactory. Free dermatoplasty combined with VSD is an
effective treatment method. Li et al(13) used VSD to treat large-area soft
tissue defects in child lower leg and identified that VSD
effectively prevents exposed deep soft tissue infection, and
granulation tissues grew well around exposed bones and tendons. The
early surgical treatment principle of large-area soft tissue
defects accompanied by bone exposure in the lower leg resolves soft
tissue coverage and healing problems on the wound surface. Prior to
surgery, it is necessary to evaluate the status of the soft tissue
injury to guide the treatment and judge the prognosis. For all
cases in this study, several holes were drilled through the bare
bone cortex surface into the contralateral cortex. Following VSD
treatment, fresh granulation tissues grew from the channels and an
adjacent muscle flap transfer was conducted to cover the bone
surface. The time taken for the bone to be completely covered
ranged from 6 to 29 days and the mean was 18.2 days. Therefore, a
good soft tissue base was provided for free skin graft reparation
of the wound surface. For all cases in this study, free
dermatoplasty of transferred adjacent muscle flaps combined with
VSD was used for treatment. It avoids amputation and complex
surgery, solves the problem of the traditional dressing method by
allowing thorough drainage, promotes the rapid growth of
granulation tissues and speeds up wound healing. Mouës et
al(14) compared the
efficacies of local negative pressure therapy and traditional
closing treatment of the wound surface of serious injuries in a
prospective randomized study, and identified that in the negative
pressure therapy group, the healing of the wound surface occurred
earlier and the complication occurrence rate was lower. In the
current study, three cases suffered from achilles tendon
contracture of equinus deformity (diorthosis was conducted) and in
seven cases, the functional evaluations were relatively poor
(qualified, five cases; poor, two cases) due to severe injuries, a
number of soft tissue defects around the lower leg, lack of
muscular strength and flexibility and bone paste scar formation.
However, the patients themselves felt that the result was better
than wearing a prosthesis. As plantar skin soft tissue injuries are
milder, there are normally no severe injuries; therefore, patients
are able to apply a load and walk.
The current study confirms that the application of
VSD combined with free dermatoplasty of transferred muscle flaps in
large-area soft tissue defects accompanied by bone exposure in the
lower leg avoids amputation and complex surgery, greatly shortens
the treatment time, speeds up wound healing, significantly reduces
complications, markedly decreases the infection rate and clearly
increases the treatment success rate. It is a simple, fast and
effective treatment method. Hou et al(15) suggested that VSD treatment of a
tibial Gustilo type IIIB fracture may reduce the skin graft or free
skin flap area. The retrospective study conducted by Babiak et
al(16) suggested that VSD
shortened the treatment time.
For large-area soft tissue defects accompanied by
bone exposure in the lower leg, it is inappropriate to conduct
adjacent flap transfer and contralateral cross leg flap transfer to
close the wound surface and more appropriate to conduct skin flap
transplantation to close the wound, due to the large area of the
soft tissue defect, the severity of the soft tissue injury and poor
vascular conditions. A good soft tissue base bed and healthy fresh
granulation tissues are required for free skin graft reparation of
the wound surface. For exposed bone, adjacent muscle flap transfer
is conducted to cover the outer areas of the exposed bone and
reduce the bone exposure range. In the current study, once the soft
tissue defect areas had been treated by VSD, abundant, fresh and
active-bleeding granulation tissues with good elasticity grew on
the wound surface, which provided a good soft tissue bed for free
skin graft reparation of the wound surface. Large-area free skin
flaps are preferred and skin flaps are connected by suturing. Stamp
skin grafts or punctuate skin grafts are not used if possible in
order to reduce scarring and the repeated damage generated by
granulation tissue proliferation in graft gaps. Large-area free
skin grafts in the lower leg are inappropriately compressed by
packing. One reason lies in the large area of the wound surface as
it is impossible to apply a compression bandage uniformly. Another
reason lies the pressure not being evenly controlled, which is
likely to affect the venous return of the limb. Webster et
al(17) confirmed that VSD
reduces the transplantation failure rate. Although the daily cost
of VSD is higher, VSD reduces dressing requirements and promotes
wound healing and thus reduces hospitalization time (18). For all cases in this study,
sustained VSD treatment was conducted following free dermatoplasty.
Clinical studies confirm that VSD causes a mechanical reaction to
negative pressure but does not readily move skin flaps.
Furthermore, it removes surplus fluid, reduces infection, speeds up
wound vascularization and promotes the rapid growth and healing of
free skin grafts.
The following matters require attention during VSD:
i) it is important to conduct thorough debridement of wounds to
remove all inactive tissues, since necrotic tissues act as
bacterial reproduction foci and resolvase and bacteriotoxin are
factors that hinder wound healing. ii) The wound range must be
reduced as far as possible. Available adjacent muscle flaps are
transferred to cover the outer areas of the exposed bone to reduce
the bone exposure range. Exposed tendon and bone surfaces are
covered with a layer of medical foam sponge to avoid cavities on
the wound surfaces. iii) VSD treatment should comply with surgical
procedures. It is necessary to maintain a sealed, effective and
negative pressure status and maintain unobstructed pipelines.
Retrograde flushing should not be conducted. iv) The silicone tube
inserted for intermittent flushing during surgery should be
maintained in a closed status during the on-flushing period. v)
During treatment, peripheral blood circulation of the toes and the
patient’s vital signs should be observed to prevent negative
nitrogen balance. As the removed exudant contains large amounts of
proteins, it is necessary to supplement adequate nutrients. At the
beginning of VSD treatment, individual patients may present wound
pain and it is feasible to apply analgesics or reduce the negative
pressure. With an increase in tolerance, the pressure may be
adjusted gradually to the required negative pressure status. If
wound secretions increase, they are removed by sustained high
negative pressure. vii) Active hemorrhaging in the drainage area is
a contraindication of VSD treatment.
VSD dressing is a non-absorbable material and the
material itself does not provide nutritional elements for the
exposed tendons and bones on wound surfaces. The development of
biological products into VSD dressings providing nutritional
elements for exposed tendons and bones on wound surfaces, for fresh
granulation tissues rapidly growing on the surfaces of exposed
tendons and bones and for preventing dry necrosis of exposed
tendons and bones, it is likely to be a new milepost in the
development of VSD and should be further investigated.
Acknowledgements
This study was supported by the
Medical Science Research Project of Hebei Province (20090633).
References
|
1.
|
Argenta LC and Morykwas MJ:
Vacuum-assisted closure: a new method for wound control and
treatment: clinical experience. Ann Plast Surg. 38:563–577. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Polykandriotis E, Kneser U, Kopp J and
Horch RE: Modified gloving technique for vacuum therapy in the
hand. Zentralbl Chir. 131(Suppl 1): S36–S39. 2006.(In German).
|
|
3.
|
Miller Q, Bird E, Bird K, Meschter C and
Moulton MJ: Effect of subatmospheric pressure on acute wound
healing. Curr Surg. 61:205–208. 2004. View Article : Google Scholar
|
|
4.
|
Halvorson J, Jinnah R, Kulp B and Frino J:
Use of vacuum-assisted closure in pediatric open fractures with a
focus on the rate of infection. Orthopedics. 34:e256–e260.
2011.PubMed/NCBI
|
|
5.
|
Chiummariello S, Guarro G, Pica A and
Alfano C: Evaluation of negative pressure vacuum-assisted system in
acute and chronic wounds closure. Our experience G Chir.
33:358–362. 2012.PubMed/NCBI
|
|
6.
|
Yildiz CE, Cöhçen S, Mert M and Çetin G:
Successful management of an unwanted complication; VAC therapy.
Anadolu Kardiyol Derg. 12:615–616. 2012.PubMed/NCBI
|
|
7.
|
Merchant TC and Dietz FR: Long-term
follow-up after fracture of the tibial and fibular shafts. J Bone
Joint Surg Am. 71:599–606. 1989.PubMed/NCBI
|
|
8.
|
Labler L, Rancan M, Mica L, Härter L,
Mihic-Probst D and Keel M: Vacuum-assisted closure therapy
increases local inter-leukin and vascular endothelial growth factor
levels in trauma wounds. J Trauma. 66:749–757. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Moran SG, Windham ST, Cross JM, Melton SM
and Rue LW III: Vacuum-assisted complex wound closure with elastic
vessel loop augmentation: a novel technique. J Wound Care.
12:212–213. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
McNulty AK, Schmidt M, Feeley T and
Kieswetter K: Effects of negative pressure wound therapy on
fibroblast viability, chemotactic signaling, and proliferation in a
provisional wound (fibrin) matrix. Wound Repair Regen. 15:838–846.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Morykwas MJ, Simpson J, Punger K, Argenta
A, Kremers L and Argenta J: Vacuum-assisted closure: state of basic
research and physiologic foundation. Plast Reconstr Surg. 117(Suppl
7): 121S–126S. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Negosanti L, Sgarzani R, Nejad P, et al:
VAC therapy for wound management in patients with contraindications
to surgical treatment. Dermatol Ther. 25:277–280. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Li RG, Yu B, Wang G, Chen B, et al:
Sequential therapy of vacuum sealing drainage and free-flap
transplantation for children with extensive soft-tissue defects
below the knee in the extremities. Injury. 43:822–828. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Mouës CM, van den Bemd GJ, Heule F and
Hovius SE: Comparing conventional gauze therapy to vacuum-assisted
closure wound therapy: a prospective randomised trial. J Plast
Reconstr Aesthet Surg. 60:672–681. 2007.
|
|
15.
|
Hou Z, Irgit K, Strohecker KA, Matzko ME,
et al: Delayed flap reconstruction with vacuum-assisted closure
management of the open IIIB tibial fracture. J Trauma.
71:1705–1708. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Babiak I, Zakiewicz W and Luterek M:
Application of negative-pressure wound therapy in complex therapy
of open tibia fractures IIIB and IIIC with massive soft tissue
loss. Chir Narzadow Ruchu Ortop Pol. 76:154–60. 2011.(In
Polish).
|
|
17.
|
Webster J, Scuffham P, Sherriff KL,
Stankiewicz M and Chaboyer WP: Negative pressure wound therapy for
skin grafts and surgical wounds healing by primary intention.
Cochrane Database Syst Rev. 4:CD0092612012.
|
|
18.
|
Dhir K, Reino AJ and Lipana J: Vacuum
assisted closure therapy in the management of head and neck wounds.
Laryngoscope. 119:54–61. 2009. View Article : Google Scholar : PubMed/NCBI
|