Introduction
Asthma is one of the most common chronic diseases in
pediatric medicine. It is estimated that the annual morbidity and
mortality rates have been increasing in recent years worldwide.
According to a report by the World Health Organization, >80% of
asthma in children results from an allergic reaction in which the
house dust mite (HDM) is the major pathogen (1). Inhaled corticosteroids (ICSs) and
specific immunotherapy (SIT) are widely used inflammatory
treatments for controlling the symptoms of asthma (2). Although ICSs remain the recommended
agents for asthma control, the use of an ICS alone is not
beneficial due to the risk of side-effects, including oropharyngeal
candidiasis, trachyphonia and cough. Recently, a number of studies
have demonstrated that combined ICS and SIT therapies may alleviate
the symptoms of asthma and nasal allergies, and reduce the required
dose of medications (3,4). It is well known that the
administration of SIT by subcutaneous injection is beneficial to
patients with asthma and its complications. Subcutaneous SIT may
improve the prognosis of asthma and allergic rhinitis and enable
the daily dose of glucocorticoid to be reduced (5,6).
Systemic pre-clinical investigations regarding the long-term
effects of this combination therapy are lacking, and the effects of
combined SIT and ICS administration have only been determined from
patient experience. Moreover, the appropriate dosage, clinical
observations, potential side-effects and outcome of ICS combined
with subcutaneous SQ-standardized specific immunotherapy (SCIT) in
the treatment of children with asthma remain unclear. In the
present study, a systemic three-year evaluation was performed to
compare the efficacy of standardized glucocorticoid management with
or without SCIT in the treatment of children with HDM allergic
asthma.
Subjects and methods
Patients
Ninety asthmatic children (with or without allergic
rhinitis) with a mild to moderate HDM allergy (aged, 5–14 years)
were recruited from January 2009 to December 2009 at Wuxi
Children’s Hospital affiliated to Nanjing Medical University (Wuxi,
China). This was a randomized, double-blind, placebo-controlled
study. The patients were separated into two groups: The treatment
group (n=45; males, 24 and females, 21) and the control group
(n=45; males, 22 and females, 23). Patients in the treatment group
received Alutard SQ (Dermatophagoides pteronyssinus;
ALK-Abelló, Hørsholm, Denmark) SCIT combined with standardized
management (ICS) for 36 months. The patients in the control group
were also treated with a desensitization vaccine. The standardized
management was administered with the vaccine kit (desensitization
vaccine, ALK-Abelló). This study was conducted in accordance with
the Declaration of Helsinki and with approval from the ethics
committee at Wuxi Children’s Hospital affiliated to Nanjing Medical
University. The legal guardians of all patients were informed of
the treatment and written informed consent was obtained from the
participants and (or) their legal guardians.
Inclusion criteria: i) Asthma diagnosis followed the
diagnostic criteria established by the National Pediatric Asthma
Group (7); ii) patients aged
between 5–14 years (including males and females); iii) patients
showed mild to moderate allergic asthma with or without allergic
rhinitis and a forced expiratory volume in 1 sec (FEV1) of ≥70% of
the normal value; iv) patients tested positive in the skin prick
test (SPT) and had a urticaria skin index (SI) of ≥0.5 (++) and/or
tested positive for allergen-specific IgE in the serum; and v)
patients required ICS treatment to control the symptoms of
asthma.
Exclusion criteria: i) Patients displayed a FEV1 of
<70% of the normal value; ii) patients diagnosed with severe
asthma; iii) patients used ICS to control asthma during this study;
iv) patients treated with a daily dose of ICS >800 g
beclomethasone for 15 days or patients routinely administered
prednisone orally; v) patients who were receiving treatment with
other medicines, such as leukotriene modifiers and long-acting β
agonists to control asthma; vi) patients commonly suffering from
respiratory tract infection, acute sinusitis or acute otitis media;
vii) patients treated for HDM or other allergens in the previous
five years; viii) patients previously diagnosed with heart, lung,
liver, kidney or blood diseases; ix) patients receiving treatment
with receptor blockers; and x) patients who had received previous
immunotherapy with immunosuppressants for immunodeficiency.
Treatment
All patients were treated with the standardized
management for HDM. The SCIT treatment was initiated at a dosage of
20 U/ml, and was continued weekly with an increase in the dosage
each week; the dosages were 20, 40, 80, 200, 400, 800, 2,000,
4,000, 8,000, 10,000, 20,000, 40,000, 60,000, 80,000 and 100,000
U/ml, respectively. Following the 15 treatments, patients received
maintenance treatment in weeks 17, 21, 27, 33, 39, 45 and 51 with a
dose of 100,000 U/ml. The SCIT treatment was discontinued following
the final treatment at week 51 according to the symptoms of asthma
in the patients and the clinical experience of agent
administration.
Assessments
To evaluate the efficiency of the combined
immunotherapy, there were 10 check-points during the treatment
period at which certain parameters were monitored. The first
check-point was prior to treatment and the remaining check-points
were following the start of treatment at weeks 1, 15, 27, 39, 51,
75, 99, 123 and 147. Five parameters were monitored, including the
dose of ICS, asthma symptom scores, peak expiratory flow (PEF)
levels, SPT results and serum IgE levels.
Dose of ICS
Patients were treated with ICS according to the
findings of a pediatric asthma control trial (5). The therapeutic protocol was revised
every 1–3 months. When the symptoms of asthma had been controlled
for three months, the dose of ICS was reduced. Complete withdrawal
of the ICS was considered if no asthma symptoms had been observed
in the patient for six months. The glucocorticoids inhaled were
budesonide (AstraZeneca, North Ryde, NSW, Australia) and
fluticasone propionate (Glaxo Wellcome, Brentford, UK).
Asthma symptom scores
The symptoms of asthma were scored at daytime and
night-time as follows: Daytime score: 0, no symptoms; 1, mild
symptoms appear intermittently; 2, moderate symptoms frequently
appear; 3, enduring symptoms affecting routine activity. Night-time
score: 0, no symptoms; 1, discomfort when waking up once or waking
up early; 2, discomfort when waking up more than once; 3,
discomfort when waking up at night frequently but able to fall
asleep; 4, sleeplessness.
PEF
The evaluation of PEF was performed using a
spirometer (AS-407; Minato Medical Science Co., Ltd., Osaka,
Japan). To measure the PEF levels of a patient, the indicator was
adjusted to ‘0’ and the instument was steadied. The patient was
required to breathe deeply and then blow strongly into the
instrument for minimal time. This evaluation was performed three
times and the highest PEF level was recorded.
SPT
For the SPT, the standard prick antigen ALK
histamine dihydrochloride (ALK, Copenhagen, Denmark) was used as a
positive control and saline was defined as a negative control
(8). The diameter of the wheal and
red spot (S) was calculated using the following formula: S = (d +
D)/2, where d is the smallest transverse diameter and D is the
largest transverse diameter. D and d crossed at right angles. A
positive result was achieved if the wheal diameter (S) was larger
than that of the negative control by 3 mm. The SI of the patients
was calculated using the following formula: SI = diameter of the
allergen-induced wheal/diameter of the histamine-induced wheal. The
SI was graded as follows: Normal, ‘0’ = negative; grade I, ‘+’ =
SI<0.5; grade II, ‘++’=0.5≤SI<1.0; grade III,
‘+++’=1.0≤SI<2.0; grade IV, ‘++++’=2.0≤SI.
Serum IgE analysis
The serum IgE levels of HDMs in the two groups were
measured using a specific house mite test kit (Dr. Fooke-Achterrath
Laboratorien GmbH, Neuss, German) and the UniCAP immune detection
system (Pharmacia and Upjohn, Stockholm, Sweden). The reference
value of the test result was 0–0.35 kUA/l for a normal
result.
Statistical analysis
All data are expressed as the mean ± standard
deviation and were analyzed with SPSS software, version 11.5 (SPSS,
Inc., Chicago, IL, USA). Comparisons within groups and among the
groups were analyzed by nonparametric tests for multiple samples
and t- and q-tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
General evaluation
In the treatment group, there were 45 patients (24
males and 21 females) and the average age was 10.1±2.2 years. In
the control group there were 45 patients (22 males, and 23 females)
and the average age was 9.8±1.5 years. There were no statistical
differences between the two groups regarding the baseline
parameters, such as age, gender, duration of asthma, dose of ICS,
SPT results and levels of PEF and serum IgE (Table I). In the treatment group, five
patients were withdrawn (three males and two females) within the 12
months following the first injection. In three of these cases,
local indurations remained following injection. One patient
experienced pharyngeal discomfort and coughing and another
displayed a systemic allergic reaction. Twenty-four months
following the first injection, a further two patients were
withdrawn from the study (one of each gender); one demonstrated
urticaria and the other experienced coughing 15–30 min after
injection. By 36 months following the initial injection, two more
male patients were withdrawn; one left the study and the other
experienced a tight chest 30 min after injection that was not
alleviated by dose reduction. In the control group, four patients
(three males and one female) were withdrawn within the first 12
months. All four patients experienced no improvement of asthma
symptoms. At 24 months, two further patients were withdrawn (one of
each gender) for personal reasons. At 36 months, an additional male
patient left the study.
 | Table IGeneral patient data. |
Table I
General patient data.
| Characteristic | Treatment group | Control group | Z- or t-test | P-value |
|---|
| Gender |
| Male | 24 | 22 | | |
| Female | 21 | 23 | Z=−1.026 | 0.305 |
| Age (years) | 10.1±2.2 | 9.8±1.5 | t=0.542 | 0.590 |
| Course (years) | 3.5±1.4 | 3.4±0.9 | t=0.217 | 0.829 |
| Asthma score |
| Day | 2.8±0.7 | 2.8±0.5 | t=0.094 | 0.925 |
| Night | 1.8±0.4 | 1.9±0.4 | t=−1.139 | 0.259 |
| ICS (μg) | 196.7±65.6 | 206.7±45.0 | t=−1.775 | 0.081 |
| Serum IgE
(kUA/l) | 91.4±29.1 | 90.9±19.2 | t=0.074 | 0.941 |
| PEF value (%) | 63.3±5.4 | 62.3±5.1 | t=0.074 | 0.941 |
| SPT | 1.2±0.5 | 1.3±0.5 | t=−0.629 | 0.532 |
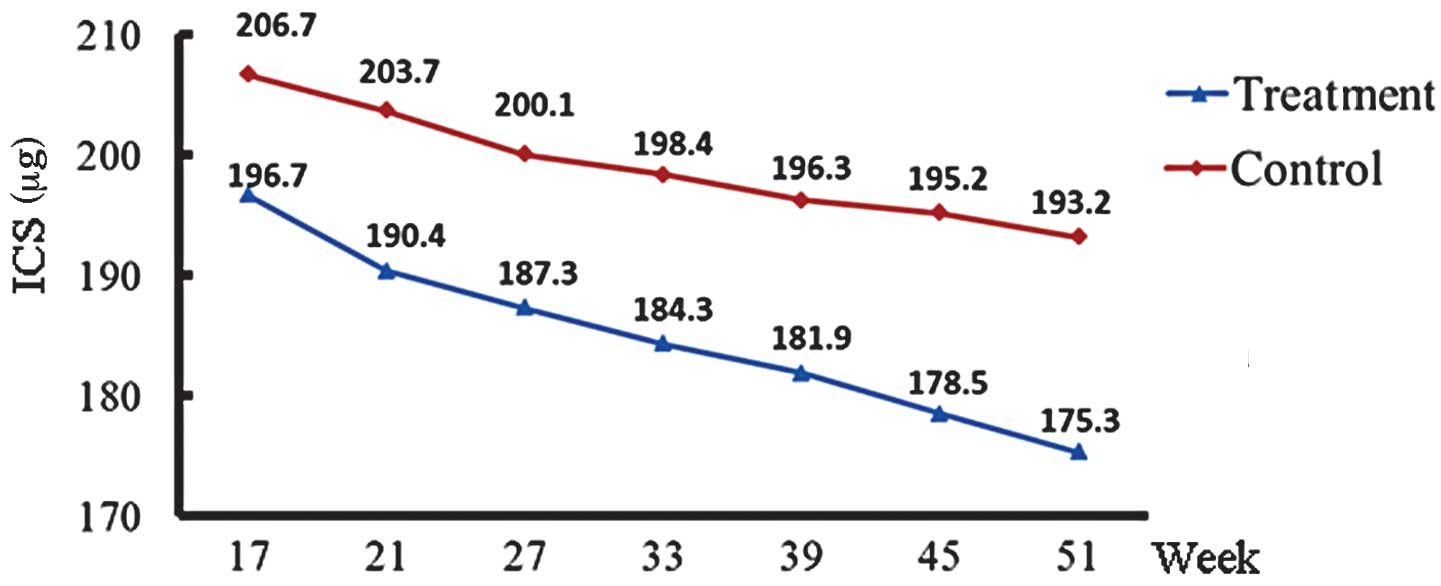
Dose of ICS
The ICS doses in the treatment and control groups
decreased gradually with time. The ICS doses in the treatment and
control groups in the first year are shown in Fig. 1. The dose of ICS in the treatment
group was significantly lower than that in the control group in the
second (P=0.015) and third years (P=0.027; Table II). At the end of the third year,
13 cases in the treatment group and nine cases in the control group
had ceased ICS treatment. The ICS discontinuation rate in the
treatment group (28.9%) was significantly higher than that in the
control group (20.0%) (Z=−2.327, P=0.020).
 | Table IIICS doses in the treatment and control
groups. |
Table II
ICS doses in the treatment and control
groups.
| Variable | Baseline (μg) | Year 1 (μg) | Year 2 (μg) | Year 3 (μg) |
|---|
| Treatment group | 196.7±65.6 | 170.8±64.4 | 115.0±54.1 | 71.3±53.8 |
| Control group | 206.7±45.0 | 190.4±46.8 | 147.9±47.0 | 101.3±48.5 |
| t | −0.689 | −1.346 | −2.516 | −2.269 |
| P-value | 0.494 | 0.183 | 0.015 | 0.027 |
Evaluation of asthma symptoms
Daytime and night-time asthma symptom scores were
recorded. The scores of the treatment and control groups declined
from baseline during the course of treatment. Consistent with the
ICS dose, the asthma symptom scores of the treatment group were
significantly lower each year compared with those of the control
group (P<0.05; Table
III).
 | Table IIIAsthma symptom scores of the treatment
and control groups. |
Table III
Asthma symptom scores of the treatment
and control groups.
| Baseline | Year 1 | Year 2 | Year 3 |
|---|
|
|
|
|
|
|---|
| Variable | Day | Night | Day | Night | Day | Night | Day | Night |
|---|
| Treatment group | 2.8±0.7 | 1.8±0.4 | 2.0±0.7 | 1.1±0.4 | 1.1±0.7 | 0.8±0.3 | 0.7±0.5 | 0.4±0.3 |
| Control group | 2.8±0.5 | 1.9±0.4 | 2.5±0.6 | 1.5±0.3 | 1.6±0.6 | 1.2±0.3 | 1.0±0.5 | 0.7±0.3 |
| t-value | 0.094 | 1.139 | 1.945 | 1.805 | 2.064 | 2.027 | 2.206 | 2.365 |
| P-value | 0.925 | 0.259 | 0.013 | 0.024 | 0.012 | 0.011 | 0.009 | 0.007 |
PEF evaluation
The accumulation of standardized allergen extracts
of HDM during treatment resulted in a significantly increased PEF
value compared with that prior to injection (P<0.05). The
increase in PEF was more marked in the second and third years than
that of the first year (Table
IV).
 | Table IVPEF results of the treatment and
control groups (l/min). |
Table IV
PEF results of the treatment and
control groups (l/min).
| Variable | Baseline | Year 1 | Year 2 | Year 3 |
|---|
| Treatment group | 63.3±5.4 | 72.5±6.3 | 87.4±9.2 | 91.3±5.8 |
| Control group | 62.3±5.1 | 69.4±4.8 | 73.5±5.1 | 81.6±4.5 |
| t-value | 0.941 | 1.346 | 2.324 | 2.769 |
| P-value | 0.074 | 0.063 | 0.018 | 0.007 |
SPT evaluation
The SPT results remained essentially unchanged at
the annual re-assessments. No differences between the two groups
were identified.
Serum IgE levels
The serum IgE levels were significantly reduced
compared with baseline levels at the end of the third year in the
treatment group (P<0.01), but not in the control group
(P=0.241). For this phenotype, the serum IgE level was
significantly downregulated by combined therapy. This effect was
only observed following at least three years of treatment; no
significant differences were identified in in the first (P=0.897)
and second (P=0.665) years. No significant differences were
observed within the control group (P=0.241; Table V).
 | Table VSerum IgE levels in the treatment and
control groups (kUA/l). |
Table V
Serum IgE levels in the treatment and
control groups (kUA/l).
| Variable | Baseline | Year 1 | Year 2 | Year 3 |
|---|
| Treatment group | 91.4±29.1 | 85.3±18.2 | 80.4±14.2 | 77.6±26.4 |
| Control group | 92.6±24.5 | 92.1±18.8 | 90.3±25.6 | 90.8±20.5 |
| t-value | 1.846 | 0.818 | 2.582 | 3.147 |
| P-value | 0.092 | 0.073 | 0.024 | 0.003 |
Adverse reactions
Adverse reactions following injection were monitored
and it was identified that 203 out of the 1,735 injections were
associated with an adverse reaction. One of the 203 injections was
a systemic adverse reaction and the remainders were local adverse
reactions.
Discussion
The primary pathogenesis of asthma is an immune
reaction in which HDM is the most common pathogen. ICSs,
anti-allergic agents and support treatments are well-known clinical
asthma therapies. However, the long-term use of ICSs is not
beneficial due to the risk of side-effects. SCIT may improve
allergic diseases, including asthma (9,10).
The present study aimed to systemically evaluate the effects of SIT
in children. This study was an open clinical observation following
the intention-to-treat principle, in which parallel controls and
self-controls were established (9).
This study demonstrated that ICS treatment improved
asthma symptom scores, serum IgE levels and PEF values with or
without immunotherapy. However, a comparison of the treatment and
control groups showed certain differences. The daytime and
night-time asthma symptom scores in the treatment group were
significantly lower than those of the control group. In addition,
the PEF values and serum IgE levels were lower in the treatment
group compared with those of the control group, indicating that
specific immunotherapy was effective in the treatment of children
with asthma. The dose of ICS also decreased each year in the two
groups. However, the reduction was most evident in the treatment
group in the second and third years. Following the third year of
treatment, the rate of ICS discontinuation in the treatment group
was significantly higher than that in the control group. The serum
IgE levels in the treatment group were lower than those in the
control group. These results suggest that long-term SCIT may
alleviate asthma symptoms and reduce the required dose of ICS.
Previous studies have demonstrated that subcutaneous injection
immunotherapy is effective in the treatment of allergic rhinitis
and asthma, which may improve the symptom scores by >40%
(11–15). A previous study indicated that SCIT
treatment may alleviate the clinical symptoms of allergic rhinitis
as early as 6 weeks following the initiation of treatment (16). The present three-year retrospective
study on the effects of SIT in the treatment of HDM-allergic
asthmatic children showed that SCIT may improve lung function and
the clinical symptoms of asthma. It may also reduce the number of
asthmatic attacks and enable the ICS dose to be reduced.
In the present study, the total number of injections
was 1,735. Among them, 203 injections resulted in adverse
reactions. One case displayed a systemic adverse reaction and the
remaining cases showed local adverse reactions. Similar to previous
studies (17,18), the local adverse reactions in the
present study were manifested as local induration, induced cough
and urticaria. The rate of adverse reactions was 11.7%. The
incidence rate of adverse reactions in asthma-SIT was previously
observed to be 5–33% (19). Those
adverse reactions occurred in the dose-increasing and maintenance
periods, which is also consistent with a previous study (20).
At present, the recommended course of treatment with
SIT is 3–5 years and the effects may last for a long time even once
treatment has finished (21).
However, there is no standard course of treatment for asthma and
there is a 0–55% relapse frequency rate. Different courses of
treatment and the diversity of allergens may affect the length of
clinical remission following drug withdrawal (9). The efficacy of SCIT treatment may
also vary according to the severity of the disease and the purity
of the extracts. Therefore, the most effective SCIT treatment
should be an individualized treatment and its standards should be
determined by clinical studies.
In conclusion, this study demonstrated that SCIT is
effective and safe for the treatment of children with allergic
asthma, alleviates asthma symptoms and reduces the required ICS
dose.
References
|
1
|
Carrard A and Pichler C: House dust mite
allergy. Ther Umsch. 69:249–252. 2012.(In German).
|
|
2
|
Barnes PJ: New drugs for asthma. Semin
Respir Crit Care Med. 33:685–694. 2012. View Article : Google Scholar
|
|
3
|
Wang CM and Chuang JJ: Effect of mite
allergen immunotherapy on the altered phenotype of dendritic cells
in allergic asthmatic children. Ann Allergy Asthma Immunol.
110:107–112. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maazi H, Shirinbak S, Willart M, et al:
Contribution of regulatory T cells to alleviation of experimental
allergic asthma after specific immunotherapy. Clin Exp Allergy.
42:1519–1528. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eifan AO, Akkoc T, Yildiz A, Keles S,
Ozdemir C, Bahceciler NN and Barlan IB: Clinical efficacy and
immunological mechanisms of sublingual and subcutaneous
immunotherapy in asthmatic/rhinitis children sensitized to house
dust mite: an open randomized controlled trial. Clin Exp Allergy.
40:922–932. 2010. View Article : Google Scholar
|
|
6
|
Blumberga G, Groes L and Dahl R:
SQ-standardized house dust mite immunotherapy as an
immunomodulatory treatment in patients with asthma. Allergy.
66:178–185. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
The Breathing Group of Pediatric Academy
affiliated to Chinese Medical Association and the Editorial Board
of Chinese Journal of Pediatrics affiliated to Chinese Medical
Association. The routine for prevention and treatment of bronchial
asthma in children (for trial). Zhonghua Er Ke Za Zhi. 42:100–106.
2004.(In Chinese).
|
|
8
|
Yang BZ, Chen HB, Hou JM, et al: Clinical
analysis on 520 children with bronchial asthma of allergens by skin
prick test. Journal of North Sichuan Medical College. 26:331–333.
2011.(In Chinese).
|
|
9
|
Akdis CA and Akdis M: Mechanisms of
allergen-specific immunotherapy. J Allergy Clin Immunol. 127:18–27.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Han J, Huang Y and Wang MK: Influence of
specific immunotherapy on Dermatophagoides
pteronyssinus-specific IgG4 and pulmonary function in children
suffering from allergic asthma. Journal of Third Military Medical
University. 33:618–620. 2011.(In Chinese).
|
|
11
|
Cox L, Calderón M and Pfaar O:
Subcutaneous allergen immunotherapy for allergic disease: examining
efficacy, safety and cost-effectiveness of current and novel
formulations. Immunotherapy. 4:601–616. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gonzales M, Fratianni C, Mamillapali C and
Khardori R: Immunotherapy in miscellaneous medical disorders Graves
ophthalmopathy, asthma, and regional painful syndrome. Med Clin
North Am. 96:635–654. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Trebuchon F, David M and Demoly P: Medical
management and sublingual immunotherapy practices in patients with
house dust mite-induced respiratory allergy: a retrospective,
observational study. Int J Immunopathol Pharmacol. 25:193–206.
2012.
|
|
14
|
Hedlin G and van Hage M: The role of
immunotherapy in the management of childhood asthma. Ther Adv
Respir Dis. 6:137–146. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
La Rosa M, Lionetti E, Leonardi S, et al:
Specific immunotherapy in children: the evidence. Int J
Immunopathol Pharmacol. 24:69–78. 2011.PubMed/NCBI
|
|
16
|
Stelmach I, Sobocińska A, Majak P, Smejda
K, Jerzyńska J and Stelmach W: Comparison of the long-term efficacy
of 3- and 5-year house dust mite allergen immunotherapy. Ann
Allergy Asthma Immunol. 109:274–278. 2012.PubMed/NCBI
|
|
17
|
Eifan AO, Shamji MH and Durham SR:
Long-term clinical and immunological effects of allergen
immunotherapy. Curr Opin Allergy Clin Immunol. 11:586–593. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Klimek L, Mewes T, Wolf H, Hansen I,
Schnitker J and Mann WJ: The effects of short-term immunotherapy
using molecular standardized grass and rye allergens compared with
symptomatic drug treatment on rhinoconjunctivitis symptoms, skin
sensitivity, and specific nasal reactivity. Otolaryngol Head Neck
Surg. 133:538–543. 2005. View Article : Google Scholar
|
|
19
|
Creticos PS: The consideration of
immunotherapy in the treatment of allergic asthma. J Allergy Clin
Immunol. 105:S559–S574. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Frew AJ: 25. Immunotherapy of allergic
disease. J Allergy Clin Immunol. 111(Suppl 2): S712–S719. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mellerup MT, Hahn GW, Poulsen LK and
Malling H: Safety of allergen-specific immunotherapy. Relation
between dosage regimen, allergen extract, disease and systemic
side-effects during induction treatment. Clin Exp Allergy.
30:1423–1429. 2000. View Article : Google Scholar
|