Introduction
Gallbladder cancer (GBC) is a common and lethal
cancer with a poor prognosis due to its insensitivity to
radioactive and chemical therapies. The 5-year survival rate of GBC
is <5% (1,2). Early tumor resection is the only
effective and potentially curative treatment. However, a number of
patients present with GBC in the later stages when surgical
intervention is no longer effective. Therefore, the identification
of a biomarker with diagnostic and prognostic values is crucial for
the treatment of GBC.
P-glycoprotein (P-gp) is encoded by the multidrug
resistance 1 (MDR1) gene and is a membrane glycoprotein. It acts as
an energy-dependent drug efflux pump and is important in
pharmacokinetics. Overexpression of P-gp is a major mechanism of
drug resistance (3), thus, P-gp is
used as a biomarker of drug resistance during the systemic
treatment of various malignancies (4). In previous years, studies have
identified that the expression of P-gp correlates with tumor
progression and the prognosis of breast, colon and lung cancers
(5–8)
Glutathione S-transferase π (GST-π) is a
subclass of GSTs, a polymorphic supergene family of detoxification
enzymes involved in the metabolism of numerous potential
carcinogens (9). GST-π is mainly
distributed in the placenta, lung, kidney, liver and red blood
cells at a low levels of expression (10). In addition, GST-π is the most
important phase II drug-metabolizing enzyme and is involved in the
metabolism and detoxification of environmental carcinogens and
chemotherapeutics. Previously, it was reported that the expression
levels of GST-π correlate with the prognosis of malignances,
including glioblastoma and breast, prostate and colorectal cancer
(9,11–13).
However, the clinical significance of P-gp and GST-π
in the progression and prognosis of GBC remain unknown. Therefore,
the present study aimed to investigate the expression levels and
prognostic values of P-gp and GST-π in GBC.
Materials and methods
Samples
In total, 42 tissue samples of GBC were obtained
from patients at the First Hospital of Xi’an Jiao Tong University
(Xi’an, China) between January 2000 and July 2005. Following
excision, samples were paraffin-embedded. The diagnosis of GBC was
established by histopathological analysis and surgery was performed
according to the stage defined by the Nevin classification
(14). In addition, 10 tissue
samples obtained from patients with chronic cholecystitis were used
as the controls.
Demographic and clinical data, including age,
gender, the presence of gallstones and disease history, were
obtained. The patient cohort included 26 females and 16 males and
the mean age at surgical resection was 60.81±1.30 years. All
patients did not present with complications, such as hypertension,
diabetes and chronicle hepatitis. Histological types of GBC were
classified according to previous studies (15,16),
which included adenocarcinoma (not otherwise specified), papillary
adenocarcinoma, adenosquamous, mucinous, adenocarcinomas and
undifferentiated carcinoma.
This study was approved by the Ethical Committee of
Xi’an JiaoTong University. All patients received oral and written
information regarding the study protocol and signed an informed
consent prior to inclusion in the study.
Immunohistochemistry
Paraffin-embedded tumor tissues were sliced into
5-μm thick sections and mounted on glass. Slides were
deparaffinized and rehydrated in 10 min through a graded alcohol
series to deionized water in 1% Antigen Unmasking Solution (Vector
Laboratories, Burlingame, CA, USA) and microwaved to enhance
antigen retrieval. Tissue samples were sequentially incubated with
anti-mouse immunoglobulin coupled to horseradish peroxidase (HRP).
Slides were incubated with the specific primary anti-GST-π
(ab47709; Abcam, Cambridge, UK) and anti-P-gp (P7965; Sigma, St.
Louis, MO, USA) monoclonal antibodies with an HRP-conjugated
secondary antibody (A1293; Sigma), and then stained with
3,3-diaminobenzidine and counterstained with hematoxylin and eosin.
In addition, 10 tissue samples from patients with chronic
cholecystitis obtained by cholecystectomy were used as the control
group. Two pathologists independently observed and interpreted the
results of the immunohistochemical staining.
Assessment of staining
Staining of P-gp and GST-π was evaluated according
to the percentage of positive cells under an optical microscope
(Leica Microsystems, GmbH, Wetzlar, Germany; magnification, ×20).
Staining intensity was classified as the following: Negative (−),
no immunopositive staining or <10% of positive cells observed;
weak to moderate (+), 10–30% positive cells; and high (++), >30%
positive cells.
Patient follow-up
Patients were advised to undergo 2-year regular
follow-ups following GBC diagnosis. Follow-up data from 36 patients
were obtained and the remaining data were lost.
Statistical analysis
Statistical analysis was performed using SPSS
software (version 11.5; SPSS, Inc., Chicago, IL, USA). Differences
of expression rate among groups were analyzed by Pearson’s
χ2 test. The Fisher’s exact test was used to assess the
differences between the positive rates when the number of total
cases was <40. All statistical tests were two-sided. To
elucidate the risk factors for prognosis (2-year survival rate),
multivariate analysis was performed using the logistic regression
model. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression levels of P-gp and GST-π in
GBC
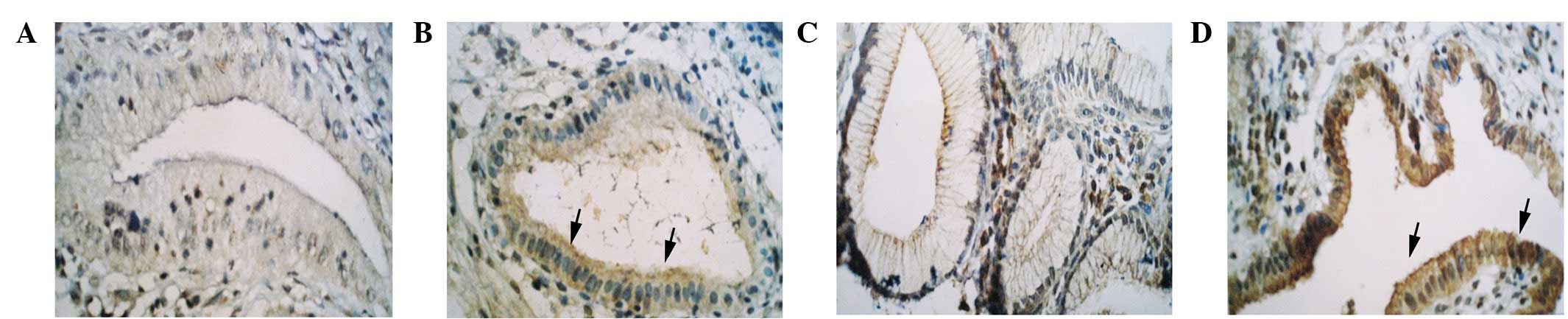
P-gp and GST-π were mainly expressed in the
cytoplasm or membrane of GBC cells (Fig. 1). The positive expression rate of
P-gp and GST-π in the GBC tissues were 76.2 and 64.3%,
respectively, which was significantly higher than that in the
chronic cholecystitis tissues (30 and 20%, respectively) (P=0.014
and P=0.035, respectively) (Fig.
2A). The expression levels were not correlated with gender,
age, pathology, presence of gallstones and histological grading
(Table I).
 | Table IDemographic and clinical data and
expression of P-gp and GST-π of gallbladder carcinoma patients. |
Table I
Demographic and clinical data and
expression of P-gp and GST-π of gallbladder carcinoma patients.
| Parameter | Cases, n | P-gp staining | Positive rate, % | P-value | GST-π staining | Positive rate, % | P-value |
|---|
|
|
|---|
| − | + | ++ | − | + | ++ |
|---|
| Gender |
| Male | 16 | 3 | 11 | 2 | 81 | - | 6 | 8 | 2 | 63 | - |
| Female | 26 | 7 | 11 | 8 | 73 | 0.22 | 9 | 12 | 5 | 65 | 0.86 |
| Age, years |
| ≥60 | 26 | 5 | 12 | 9 | 81 | - | 7 | 16 | 3 | 73 | - |
| <60 | 16 | 5 | 10 | 1 | 69 | 0.11 | 8 | 4 | 4 | 50 | 0.10 |
| Presence of
gallstones |
| Yes | 20 | 5 | 8 | 7 | 75 | - | 7 | 9 | 4 | 65 | - |
| No | 22 | 5 | 14 | 3 | 78 | 0.21 | 8 | 11 | 3 | 64 | 0.85 |
| Pathology |
| Adenocarcinoma
(NOS) | 28 | 5 | 17 | 6 | 82 | - | 9 | 14 | 5 | 68 | - |
| Papillary
adenocarcinoma | 5 | 2 | 2 | 1 | 60 | - | 3 | 2 | - | 40 | - |
| Adenosquamous | 3 | 1 | 2 | - | 67 | - | 2 | 1 | - | 33 | - |
| Mucinous
adenocarcinoma | 2 | - | 1 | 1 | 100 | - | - | 1 | 1 | 100 | - |
| Undifferentiated
carcinoma | 4 | 2 | - | 2 | 50 | 0.48 | 1 | 2 | 1 | 75 | 0.41 |
| Histological
grade |
| I | 9 | 4 | 4 | 1 | 56 | - | 4 | 5 | - | 56 | - |
| II | 19 | 3 | 12 | 4 | 84 | - | 6 | 10 | 3 | 68 | - |
| III | 14 | 3 | 6 | 5 | 79 | 0.35 | 5 | 5 | 4 | 64 | 0.46 |
Expression levels of P-gp and GST-π
correlate with the Nevin stage
The expression levels of P-gp and GST-π in the early
Nevin stages of GBC (I, II and III) were lower (33.3 and 16.7%,
respectively) than that in the later stages (IV and V) (83.3 and
72%, respectively) (P=0.021 and 0.016, respectively) (Fig. 2B). As Nevin staging is classified
by tumor metastasis, multivariate analysis was performed using the
logistic regression model and found that P-gp staining is an
independent risk factor for metastasis of GBC (R2=3.09;
P=0.044) (Table II).
 | Table IILogistic regression analysis of
metastasis of gallbladder carcinoma. |
Table II
Logistic regression analysis of
metastasis of gallbladder carcinoma.
| Variable | Regression
coefficent | Standard error | P-value |
|---|
| P-gp | 3.09 | 1.53 | 0.044 |
| Tumor grade | 0.48 | 1.00 | 0.631 |
| Age | 0.03 | 0.08 | 0.705 |
| Gender | 2.61 | 1.69 | 0.123 |
| Pathological
type | 0.55 | 1.59 | 0.727 |
| Gallstones | 2.41 | 1.71 | 0.158 |
P-gp and GST-π expression positively
correlates with the prognosis of GBC
According to follow-up data of 36 cases, the
expression levels of P-gp and GST-π significantly correlated with
the 2-year survival rate. The 2-year survival rate in P-gp-negative
patients (55.6%) was higher than that in the P-gp-positive patients
(11.1%) (P=0.013). Similarly, 2-year survival rate in the
GST-π-negative patients (45.5%) was also higher than that in the
GST-π-positive patients (12.0%) (P=0.036). In addition,
coexpression of P-gp and GST-π demonstrated the lowest 2-year
survival rate (4.3%) (P=0.001) (Fig.
2C). P-gp was also found to be an independent prognostic marker
of the 2-year survival rate by logistic regression analysis
(R2=−2.76, P=0.061) (Table III).
 | Table IIILogistic regression analysis of
2-year survival of gallbladder carcinoma patients. |
Table III
Logistic regression analysis of
2-year survival of gallbladder carcinoma patients.
| Variable | Regression
coefficent | Standard error | P value |
|---|
| P-gp | −2.76 | 1.47 | 0.061 |
| Tumor stage | 0.84 | 2.13 | 0.693 |
| Tumor grade | −1.28 | 0.90 | 0.153 |
| Age | 0.01 | 0.06 | 0.959 |
| Gender | −0.46 | 1.15 | 0.687 |
| Pathological
type | 0.16 | 1.19 | 0.896 |
| Gallstones | 0.05 | 1.18 | 0.969 |
Correlation between P-gp and GST-π
A significant positive correlation was also found
between the expression levels of P-gp and GST-π in tumor tissues
(R2=0.20; P=0.003) and the coexpression rate was 59.5%
(Fig. 2D).
Discussion
In the present study, expression levels of P-gp and
GST-π in malignant lesions were found to be higher than that of
benign lesions, indicating multiple drug resistance of GBC. In
addition, majority of positive cells were located on the mucosal
surface of the gallbladder, this phenomena was consistent with the
role of P-gp as a multidrug transporter and support the mechanism
of GBC. Similar results have also previously been identified
suggesting that P-gp is substantially expressed on the biliary
surface of hepatocytes and small biliary ductules (17).
Although P-gp and GST-π correlate with drug
resistance, their mechanisms and drug-resistant spectrum are
different. GST-π is regulated in vivo by reactive oxygen
species and its induction represents part of an adaptive response
mechanism to chemical stress caused by electrophiles (18). The drug-resistance spectrum of
GST-π is cisplatin. However, the drug-resistance spectrum of P-gp
is vincristine and doxorubicin (19,20).
Therefore, the expression of P-gp and GST-π should be taken into
consideration when designing clinical trials.
It has been previously suggested that aromatic
compounds can induce GST-π expression (21,22).
In addition, it is well known that GBC is closely associated with
gallstone and chronic cholecystitis, which may generate aromatic
compounds due to the long-term stasis of bile and bacterial
infection (23). Thus, we
hypothesized that aromatic compounds are not only comprised of
chemical factors for the carcinogenesis of GBC, but are also
important for GST-π expression.
The main prognostic factors of GBC are the clinical
or pathological stages (24) and
the Nevin staging system for GBC is widely used (25). The Nevin stage of GBC is mainly
defined according to metastasis and invasion. In the present study,
P-gp and GST-π showed lower levels in non-metastatic tumors (Nevin
stage, I, II and III) than in metastatic tumors (Nevin stage, IV
and V), suggesting that P-gp and GST-π may be used as indicators
for invasion and metastasis. In the present study, patients with
positive P-gp or GST-π expression showed a shorter 2-year survival
rate compared with patients with a negative expression.
Furthermore, patients with coexpression of P-gp and GST-π were
associated with the worst prognosis. These results demonstrate that
P-gp and GST-π may be used as prognostic markers for GBC and were
consistent with previous studies on liver, colon, breast and
ovarian cancer (26–28).
A close correlation between GST-π and P-gp
expression was also identified in the current study, with
coexpression observed in 59.5% of patients with GBC. Similar
studies have shown that the rate of coexpression was 93% in
patients with leukemia and 80% in patients with lung cancer
(29,30). It has been demonstrated there are
abnormal expression of genes, such as c-erbB-2, neu, P53, ras,
INT2, HSTF1, bcl-2, c-fos and c-jun in various types of cancer,
including GBC, and these genes not only correlate with drug
resistance but also frequently regulate and co-amplify P-gp and
GST-π genes (28–34). Therefore, the higher expression
levels of P-gp and GST-π in patients with GBC may be a reflection
of the abnormal expression of oncogenes and cancer suppressor
genes.
In conclusion, results of the present study suggest
that P-gp is a prognostic marker for GBC. In the future, the
detection of P-gp and GST-π in patients with GBC may contribute to
chemotherapeutic and surgical decisions.
Acknowledgements
The authors thank Mei-Rong Han, He-Ping Tian, Yi-Jun
Yang and Yue Han for their assistance in the immunostaining
experiments and clinical data collection.
References
|
1
|
Mekeel KL and Hemming AW: Surgical
management of gallbladder carcinoma: a review. J Gastrointest Surg.
11:1188–1193. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miller G and Jarnagin WR: Gallbladder
carcinoma. Eur J Surg Oncol. 34:306–312. 2008. View Article : Google Scholar
|
|
3
|
Khdair A, Handa H, Mao G and Panyam J:
Nanoparticle-mediated combination chemotherapy and photodynamic
therapy overcomes tumor drug resistance in vitro. Eur J Pharm
Biopharm. 71:214–222. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schrader AJ, Seger M, Konrad L, et al:
Clinical impact of MDR1-expression in testicular germ cell cancer.
Exp Oncol. 29:212–216. 2007.PubMed/NCBI
|
|
5
|
Bark H, Xu HD, Kim SH, Yun J and Choi CH:
P-glycoprotein down-regulates expression of breast cancer
resistance protein in a drug-free state. FEBS Lett. 582:2595–2600.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Berger W, Setinek U, Hollaus P, et al:
Multidrug resistance markers P-glycoprotein, multidrug resistance
protein 1, and lung resistance protein in non-small cell lung
cancer: prognostic implications. J Cancer Res Clin Oncol.
131:355–363. 2005. View Article : Google Scholar
|
|
7
|
Bottke D, Koychev D, Busse A, et al:
Fractionated irradiation can induce functionally relevant multidrug
resistance gene and protein expression in human tumor cell lines.
Radiat Res. 170:41–48. 2008. View
Article : Google Scholar
|
|
8
|
Hanson JA, Gillespie JW, Grover A, et al:
Gene promoter methylation in prostate tumor-associated stromal
cells. J Natl Cancer Inst. 98:255–261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pandey SN, Jain M, Nigam P, Choudhuri G
and Mittal B: Genetic polymorphisms in GSTM1, GSTT1, GSTP1, GSTM3
and the susceptibility to gallbladder cancer in North India.
Biomarkers. 11:250–261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tiirikainen MI, Elonen E, Syrjälä MT,
Jansson SE and Krusius T: Flow cytometric analysis of
glutathione-S-transferase-pi in acute leukemia. Leukemia.
8:978–984. 1994.PubMed/NCBI
|
|
11
|
Somlo G, Chu P, Frankel P, et al:
Molecular profiling including epidermal growth factor receptor and
p21 expression in high-risk breast cancer patients as indicators of
outcome. Ann Oncol. 19:1853–1859. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Perry AS, Loftus B, Moroose R, et al: In
silico mining identifies IGFBP3 as a novel target of methylation in
prostate cancer. Br J Cancer. 96:1587–1594. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sutoh I, Kohno H, Nakashima Y, et al:
Concurrent expressions of metallothionein, glutathione
S-transferase-pi, and P-glycoprotein in colorectal cancers. Dis
Colon Rectum. 43:221–232. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nevin JE, Moran TJ, Kay S and King R:
Carcinoma of the gallbladder: staging, treatment, and prognosis.
Cancer. 37:141–148. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Henson DE, Albores-Saavedra J and Corle D:
Carcinoma of the gallbladder. Histologic types, stage of disease,
grade, and survival rates. Cancer. 70:1493–1497. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Levy AD, Murakata LA and Rohrmann CA Jr:
Gallbladder Carcinoma: radiologic-pathologic correlation.
Radiographics. 21:295–314. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gottesman MM and Pastan I: The multidrug
transporter, a double-edged sword. J Biol Chem. 263:12163–12166.
1988.PubMed/NCBI
|
|
18
|
Rodriguez C, Commes T, Robert J and Rossi
JF: Expression of P-glycoprotein and anionic glutathione
S-transferase genes in non-Hodgkin’s lymphoma. Leuk Res.
17:149–154. 1993.PubMed/NCBI
|
|
19
|
Kufe DW, Pollock RE, Weichselbaum RR, et
al: Holland-Frei Cancer Medicine. 6th edition. Hamilton (ON): BC
Decker; pp. 711–726. 2003
|
|
20
|
Stewart DJ: Mechanisms of resistance to
cisplatin and carboplatin. Crit Rev Oncol Hematol. 63:12–31. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen YK and Lin LM: Placental glutathione
S-transferase isoenzyme expression in polycyclic aromatic
hydrocarbon-induced hemster buccal pouch mucosa. Oral Oncol.
34:180–185. 1998. View Article : Google Scholar
|
|
22
|
Tsuchida S and Sato K: Glutathione
transferses and cancer. Crit Rev Biochem Mol Biol. 27:337–384.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takabayashi A, Watkins JB, Soloway RD,
Rios-Dalenz J and Henson DE: Glycolithocolic acid is greatly
increased in stones from patients with carcinoma of the
gallbladder. Gastroenterology. 79:1058–1063. 1980.
|
|
24
|
Henson DE, Albores-Saavedra J and Corle D:
Carcinoma of the gallbladder. Histologic types, stage of disease,
grade, and survival rates. Cancer. 70:1493–1497. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lazcano-Ponce EC, Miquel JF, Muñoz N, et
al: Epidemiology and molecular pathology of gallbladder cancer. CA
Cancer J Clin. 51:349–364. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Seo S, Hatano E, Higashi T, et al:
Fluorine-18 fluorodeoxyglucose positron emission tomography
predicts tumor differentiation, P-glycoprotein expression, and
outcome after resection in hepatocellular carcinoma. Clin Cancer
Res. 13:427–433. 2007. View Article : Google Scholar
|
|
27
|
Sinicrope FA, Hart J, Brasitus TA,
Michelassi F, Lee JJ and Safa AR: Relationship of P-glycoprotein
and carcinoenbryonic antigen expression in human colon carcinoma to
local invision, DNA ploidy, and disease relapse. Cancer.
74:2908–2907. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Saint-Ruf C, Malfoy B, Scholl S, Zafrani B
and Dutrillaux B: GST gene is frequently complified with INT2 and
HSTF1 proto-oncogene in human breast cancers. Oncogene. 16:403–406.
1991.PubMed/NCBI
|
|
29
|
Slebos RJ, Kibbelaar RE, Dalesio O, et al:
K-ras oncogene activation as a prognostic marker in adenocarcinomar
of the lung. N Eng J Med. 323:561–565. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tsuda H, Hirohashi S, Shimosato Y, et al:
Correlation between long-term survival in breast cancer patients
and amplification of two putive oncogene-coamplification units:
hst-1/int-2 and c-erbB-2/ear-1. Cancer Res. 49:3104–3108.
1989.PubMed/NCBI
|
|
31
|
Charpin C, Vielh P, Duffaud F, et al:
Quantitative immunocytochemical assays of P-glycoprotein in breast
carcinoma: correlation to messanger RNA experession and to
immunohistochemical prognostic indicators. J Natl Cancer Inst.
86:1539–1545. 1994. View Article : Google Scholar
|
|
32
|
Ralhan R, Swain RK, Agarwal S, et al:
P-glycoprotein is positively correlated with p53 in human oral
pre-malignant and malignant lesions and is associated with poor
prognosis. Int J Cancer. 84:80–85. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Malik IA: Gallbladder cancer: current
status. Expert Opin Pharmacother. 5:1271–1277. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Brotherick I, Shenton BK, Egan M, et al:
Examination of multidrug resistance in cell lines and primary
breast tumours by flow cytometry. Eur J Cancer. 32A:2334–2341.
1996. View Article : Google Scholar : PubMed/NCBI
|