Introduction
Hepatocellular carcinoma (HCC) is one of the most
malignant tumors in the tropics and the Far East, including China.
It is the fourth most common cause of cancer and accounts for 53%
of liver cancer mortalities worldwide (1). HCC is also a hypervascular carcinoma.
Angiogenesis, a process in which endothelial cells of the
pre-existing capillaries proliferate and migrate to form new
vascular tips or so-called ‘vascular sprouts’ or ‘endothelial buds’
(2), plays an important role in
the progression of HCC and contributes to its malignancy,
invasiveness, and high rates of recurrence and metastasis (3). There is evidence suggesting that
solid tumors do not grow beyond 2–3 mm3 in volume if
vascular sprouts are blocked (2).
Vascular endothelial growth factor (VEGF) is a key factor in tumor
angiogenesis and high levels of VEGF have been identified to be a
determining factor in the HCC grade, clinically correlating with
low rates of overall survival (4–6). As
a result, the development of biomedical research on vascular
biology is paramount to the foundation of genetic engineering and
proteomics technology that may provide a new and effective
angiogenesis-targeting therapy for HCC.
HCCs are tumors with a highly developed vascular
architecture; HCC cells require access to blood vessels for growth
and metastasis. Therefore, the inhibition of angiogenesis
represents a potential therapeutic target for HCC (7). In recent years, research in this area
has been highly active and numerous anti-angiogenic agents have
been developed for specific clinical indications (8). However, anti-angiogenic drugs have
produced modest results in clinical trials despite significant
therapeutic effects demonstrated in vitro or in vivo
(9,10). Overall, these drugs have yet to
contribute to long-term survival benefits (11). Single-chain antibodies (scFv) are
characterized as highly penetrating proteins with low molecular
weight, low immunogenicity and a short half-life. The large-scale
production of scFv is easy to implement by genetic engineering
(12). Therefore, scFv as direct
therapeutic agents or as carriers of cytotoxic agents for specific
targeted therapies are promising for clinical applications,
including HCC therapy.
Tumoral angiogenesis is a complex process closely
regulated by numerous angiogenic factors, among which angiopoietin
and VEGF are the two most significant. VEGF is the most potent
angiogenic factor that promotes endothelial proliferation and
increases vascular permeability by binding to its specific
receptors in endothelial cells, including Flt-1, KDR/Flk-1 and
Flt-4 (13). Angiopoietin-2
(Ang-2) has been found with abnormally high expression levels in
numerous solid tumors, including gastric, ovarian, colorectal and
breast cancers (14–17). Ang-2 is thus considered one of the
most important tumoral angiogenesis promoters. Animal models and
in vitro experiments have shown that Ang-2 and its receptor
Tie2, in association with VEGF, constitute a system that regulates
vascular quiescence and endothelial plasticity, through which a
balanced state of vascular maturity and development of complex
vascular networks are achieved (13). Ang-2 in the presence of VEGF is
important for the initiation of angiogenesis and vascular sprouting
in tumors (18). It has been
reported that VEGF and the angiopoietin/Tie2 system play a key role
in the transformation of normal lung to non-small cell lung
carcinoma (19). Our previous
study (20) indicated that
expression of Ang-2 relative to that of angiopoietin-1 (Ang-1),
through the Tie2 receptor in the presence of VEGF, plays a critical
role in initiating early neovascularization and induces the
transformation of noncancerous liver to HCC. Subsequently, constant
immature neovascularization in HCC further promotes angiogenesis
and tumor progression. Therefore, we suggest that Ang-2-targeting
therapies may be valuable in the treatment of HCC by intervening in
the remodeling of neovascular networks and changing the
microenvironment of the tumor.
In this study, a prokaryotic expression vector of
Ang-2 was constructed and purified human Ang-2 protein was
isolated. A single-chain antibody against human Ang-2 (scFv-Ang2)
was identified, which was purified with phage display technology.
Finally, the effects of scFv-Ang2 in vitro and in
vivo on HCC in nude mice were evaluated.
Materials and methods
Reagents
The following reagents were obtained: pET32c vector
system from Novogen (Madison, WI, USA); plasmid pCANTAB5E,
Escherichia coli TG1 and Escherichia coli BL21,
M13K07 helper phage, mouse anti-M13 antibody and mouse anti-E tag
antibody from Pharmacia Biotech (Piscataway, NJ, USA); pfuDNA
polymerase from Stratagene (Santa Clara, CA, USA), restriction
endonuclease HindIII, NcoI and T4 DNA Ligase from New
England Biolabs (Ipswich, MA, USA); low melting point agarose from
Promega (Madison, WI, USA); isopropyl β-D-1-thiogalactopyranoside
(IPTG), total RNA kit and Moloney murine leukemia virus (M-MLV)
reverse transcriptase from Takara (Shiga, Japan); protein marker
and FastDigest restriction enzymes from MBI Fermentas Inc.
(Burlington, ON, Canada); Rapid Gel purification kit and PureLink™
HiPure plasmid DNA purification kit from Invitrogen (Carlsbad, CA,
USA); enterokinase and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
from Sigma (St. Louis, MO, USA); M199, fetal bovine serum (FBS) and
trypsin-ethylenediaminetetraacetic acid (EDTA) from Hyclone (Logan,
UT, USA); Dulbecco’s modified Eagle’s medium (DMEM) from PAA
Laboratories GmbH (Linz, Austria); endothelial cell growth
supplement ECGs) from Millipore (Temecula, CA, USA); collagenase
from Worthington Biochemica (Lakewood, NJ, USA); mouse anti-human
CD31 monoclonal antibody from Santa Cruz Biotechnology, Inc.,
(Santa Cruz, CA, USA); EDTA, penicillin and streptomycin from
Invitrogen; and dimethyl sulfoxide (DMSO) and Transwell chambers
with 8-μm pore filters from Corning Incorporated (Corning, NY,
USA). Matrigel was obtained from BD Biosciences (Franklin Lakes,
NJ, USA). Recombinant human VEGF was obtained from R&D Systems
(Minneapolis, MN, USA).
Cell culture
Human umbilical vein endothelial cells (HUVECs) were
isolated from human umbilical cords using collagenase and were
cultured in M199 medium containing inactivated 20% FBS and 25%
ECGs. The cells were maintained in a humidified 5% CO2
atmosphere at 37°C. Cells were divided at a ratio of 1:2 once they
reached 70–90% confluence. The cells were grown into a monolayer
within 2–3 days and continually cultured for 2–3 passages prior to
experimentation. MHCC97 (human liver cancer cell line) cells were
obtained from the Liver Cancer Institute of Fudan University and
incubated in high glucose DMEM with 10% FBS at 37°C in a humidified
atmosphere of 95% air and 5% CO2. Experiments were
conducted on cultures that had gone through 2–3 passages. Written
approval for human umbilical vein endothelial cell derivation,
culture, and experimental use was obtained from the Ethics
Committee, Zhongnan Hospital of Wuhan University.
Human HCC model in nude mice
Male athymic BALB/c nu/nu mice, 4–6 weeks old, were
obtained from The Experimental Animal Center of Wuhan University
(Animal Biosafety Level-III Laboratory; Wuhan, China) and
maintained in specific pathogen-free (SPF) conditions. The study
protocol for the use of mice was approved by The Wuhan Medical
Experimental Animal Care Commission (Wuhan, China).
The metastatic model of human HCC in nude mice was
constructed via orthotopic implantation of histologically intact
metastatic tumor tissue. Briefly, 5×106 (0.2 ml) MHCC97
cells were injected subcutaneously into the nude mice. When the
subcutaneous tumor reached ~1.5 cm in diameter, the mice were
sacrificed and small pieces of tumor tissue, ~1 mm3 in
volume, were implanted into the livers of new recipient mice, which
were maintained in standard facilities.
Construction of human Ang-2 prokaryotic
expression vector
Gene amplification
The total RNA was isolated from HUVECs with RNAiso
reagent (Takara) following the manufacturer’s instructions and was
quantified by absorbance analysis at 260 nm. cDNA was generated by
reverse transcription using an oligo(dT) primer and RNA PCR kit
(Takara). According to the original sequence in Gene Bank
(accession number: AF004327), the method of Maisonpierre et
al (21) was used to
synthesize the Ang-2 gene. NcoI and HindIII
restriction sites were inserted into the 5′ and 3′ ends of primers,
respectively. Quantitative PCR (qPCR) was performed using a GeneAmp
PCR system 2400 (Perkin Elmer, Waltham, MA, USA). After equal
amounts of cDNA and specific primers were added to the master mix,
initial denaturation at 94°C for 30 sec, followed by 35 cycles of
denaturation at 94°C for 3 min, annealing at 59°C for 60 sec and
extension at 72°C was conducted. PCR products (5 μl) were
visualized by electrophoresis on 1% agarose gel, stained with
ethidium bromide and purified using a Rapid Gel purification kit,
and were also sequenced by Davis Sequencing (Davis, CA, USA). The
sequence was compared with the matching portion of that submitted
to the NCBI.
The purified PCR products and empty plasmid pET32c
were digested by NcoI and HindIII restriction
enzymes, harvested by electrophoresis on 1% agarose gel and
purified using a Rapid Gel purification kit. Following ligation to
pET32c by incubation with T4DNA ligase at 16°C overnight, the
vector was then used to transform competent E. coli BL21 by
spreading on agar plates with ampicillin at 37°C overnight. A
single colony of E. coli BL21 containing the recombinant
plasmid pET32c-Ang-2 was inoculated and selection was performed in
LB-Amp medium. The alkaline lysis method was used for plasmid
extraction in the mini-preparation scale and the PureLink™ HiPure
plasmid DNA purification kit was used for mini-preparation DNA
extraction. The accuracy of cloning was confirmed using restriction
enzyme mapping and sequencing. The plasmids were extracted using
PureLink™ HiPure plasmid DNA purification kit and were digested
with the NcoI and HindIII restriction endonuclease
enzymes. For each reaction 8 μl purified plasmid, 2 μl buffer, 2 μl
enzyme (1 μl of each in double digestion) and 8 μl ddH2O
were added. The digestion was performed for 5 min in 37°C with
FastDigest restriction enzymes (New England Biolabs). Final
confirmations of positive clones were performed by PCR and DNA
sequencing.
Expression and purification of target
fusion protein
To examine whether pET32c-Ang-2 overexpressed Ang-2,
pET32c-Ang-2 was transfected into the E. coli strain BL21
(DE3) that encodes a chromosomal T7 RNA polymerase under the
control of a tac promoter. A single colony was then inoculated on
LB medium and grown at 37°C overnight with constant agitation. A
200-μl sample of the overnight products was collected and added to
3 ml LB-Amp medium, which was cultured on a shaking plate at 37°C
until the sample reached an OD600 of 0.6. IPTG was added
at a concentration of 1 mmol/l to induce pET32c-Ang-2-BL21 (DE3)
expression. Bacterial liquid was centrifuged at 3,099 × g for 15
min at 4°C and whole protein was collected for SDS-PAGE gel
analysis, according to Novagen’s pET System Manual. Protein was
recovered by electroelution and dialysis methods, and its
concentration was determined by Lowry protein assay.
Fusion protein of Ang-2, which was expressed
downstream of restriction enzyme site of enterokinase, was digested
by enterokinase at 25°C in a water bath so that pure Ang-2 protein
was obtained. Different concentrations of enterokinase with
different durations of digestion were tested to determine digestion
efficiency. Large quantities of target protein were prepared and
concentrated for later use after the optimal preparation conditions
were determined.
Preparation of scFv against Ang-2 by
phage display peptide panning
A non-immunized mouse phage display antibody library
was constructed and screened as previously described (22). To display scFv as a fusion protein
with E tag and M13 p3 (there is an amber stop codon between E tag
and fd g3 in pCANTAB5E), scFv-Ang2 DNAs were ligated into the
phagemid vector pCANTAB5E. The ligated products were used to
transform competent E. coli TG1 cells: TG1 is an amber
suppressor strain (supE). The panning process was performed
according to the method of Carlos et al (23). Three rounds of panning and
enrichment were performed, and the affinities of recombinant phage
antibodies from pooled or individual colonies following each round
of selection were tested by enzyme-linked immunosorbent assay
(ELISA). Finally, screened scFv-Ang2 was identified by SDS-PAGE and
DNA sequencing.
In vitro experiments
Treatment groups
The HUVECs were planted in culture plates with 3
wells for each group. Five groups were used: Group 1 was set as the
control group with no other external factors added, with the
exception of ECGs for maintaining growth in the M199 medium; group
2 contained VEGF (5 μg/l); group 3 contained Ang-2 (10 μg/l); group
4 contained VEGF (5 μg/l) and Ang-2 (10 μg/l); and group 5
contained VEGF (5 μg/l), Ang-2 (10 μg/l) and scFv-Ang2
(1×1011, 2×1011 and 4×1011 pfu/ml,
respectively).
MTT assay
The cell viability was determined using the MTT
assay. Briefly, HUVECs (2×104/well) were seeded onto
96-well plates and incubated for 24 h for synchronization. After
adherence was attained, the various treatments were added, with 3
wells for each group. Following incubation for 24 h, 20 μl MTT
reagent (5 mg/ml) was added to each well and the cells were
incubated for 4 h. The formazan precipitate was dissolved in 150 μl
DMSO and the absorbance value was measured with a microplate reader
(ELx808; BioTek, Winooski, VT, USA) at a wavelength of 490 nm.
Cell migration assay
Transwell plates of 8-μm pore size were used to
evaluate cell migration ability. The polycarbonate filter of the
inner chamber was coated with 1% gelatin and equilibrated with
serum-free DMEM for 1 h. HUVECs were harvested and resuspended in
DMEM medium supplemented with 20% FBS. The prepared cell suspension
(100 μl; 4×105 cells/ml) was added to the inner chamber
and the outer chamber was filled with 600 μl DMEM containing FBS.
The cells were fixed with 90% methanol for 10 min following
incubation at 37°C for 6 h. Following the removal of cells on the
upper side of the membrane using a cotton swab, the cells were
stained with crystal violet for 10 min and washed with PBS. The
cells were observed and counted using an inverted microscope
(CKX41; Olympus, Tokyo, Japan) at ×400 magnification and the
average value of three fields was calculated.
Tubule formation assay
Growth factor-free Matrigel maintained at 0°C was
added to a 96-well microplate and maintained at 37°C for 1 h.
HUVECs at 50 μl/well (2×104/ml) were seeded onto an
extracellular matrix with different factors as previously grouped.
After culturing for 18 h, three random ×100 fields were
photographed under a phase-contrast microscope (CX41; Olympus) for
each well, and the tubules were counted, averaged and compared.
In vivo anti-tumor experiment
Animal grouping
The animals were grouped as follows: 24 nude mice
were randomly divided into therapy and control groups with twelve
animals in each group. scFv-Ang2 (4×1011 pfu; 1 ml) was
intraperitoneally administered once a day in the therapy group and
saline was administered at the same volume and frequency in the
control group. The injection course started from the second day of
inoculation and continued for the following 3 weeks. The body
weight of the mice was recorded once a week.
Parameters observed
On day 21, the mice were sacrificed. Tumors were
weighed and the longest (a) and the smallest (b) diameters were
measured by slide gauge under an operating microscope. Tumor volume
was calculated as follows: V=a.b2/2. The liver tissues
were carefully anatomized and visible metastases were counted.
Paraffin blocks of 10% buffered formalin-fixed samples of lungs
were prepared. Each lung sample was consecutively cut into 10
slices every 30 μm. Serial sections were cut at 5 μm and stained
with hematoxylin and eosin to determine the presence of lung
metastases. Tumor tissue was embedded in a paraffin block for
advanced immunohistochemistry analysis of CD31.
Immunohistochemical assessment of
vessel density
Paraffin-embedded tumor tissues were sectioned (4
μm), the slides were deparaffinized and washed with Tris-buffered
saline (TBS), and the slices were incubated with 10% normal goat
serum (Zhongshan Bio., Guangzhou, China). The sections were then
incubated with appropriately diluted (1:10) rat-anti-mouse CD31
monoclonal antibody for 24 h at 4°C. The primary antibody was
removed and, after washing the sections with TBS,
peroxidase-labeled goat-anti-mouse IgG (Zhongshan Bio.) was added.
Finally the slices were stained with hematoxylin, and washed with
distilled water. Quantification of blood vessels was carried out as
previously described (24). A
brown-stained endothelial cell cluster distinct from adjacent
microvessels, tumor cells or other stromal cells was considered as
a single countable microvessel. The most vascular areas of tumors
were identified on a low-power field (x100) and vessels were
countered in five high-power fields (x200). The data are expressed
as mean ± standard deviation (SD) for five high-power fields.
Statistical analysis
The data were analyzed for significance with an
unpaired Student’s t-test and ANOVA test. Statistical software SPSS
version 13.0 (SPSS, Inc., Chicago, IL, USA) was used in the
analysis. P<0.05 was considered to indicate a statistically
significant result.
Results
Construction of human Ang-2 prokaryotic
expression vector
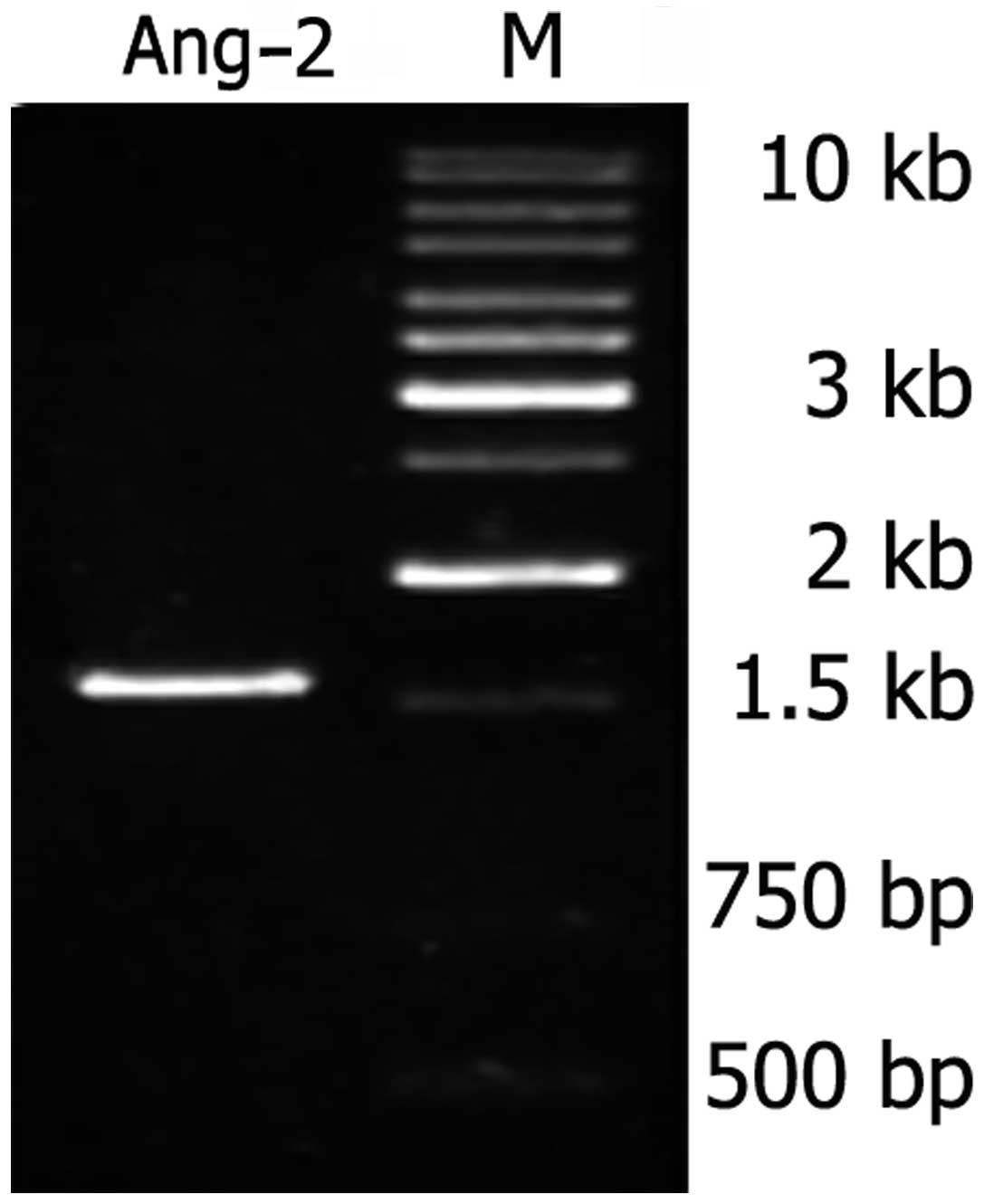
As shown in Fig. 1,
the Ang-2 gene produced a specific band at approximately 1.5 kb in
the agarose gels when it was amplified from the HUVEC cDNA library.
The obtained PCR products had identical cDNA sequences to the gene
bank sequences (data not shown).
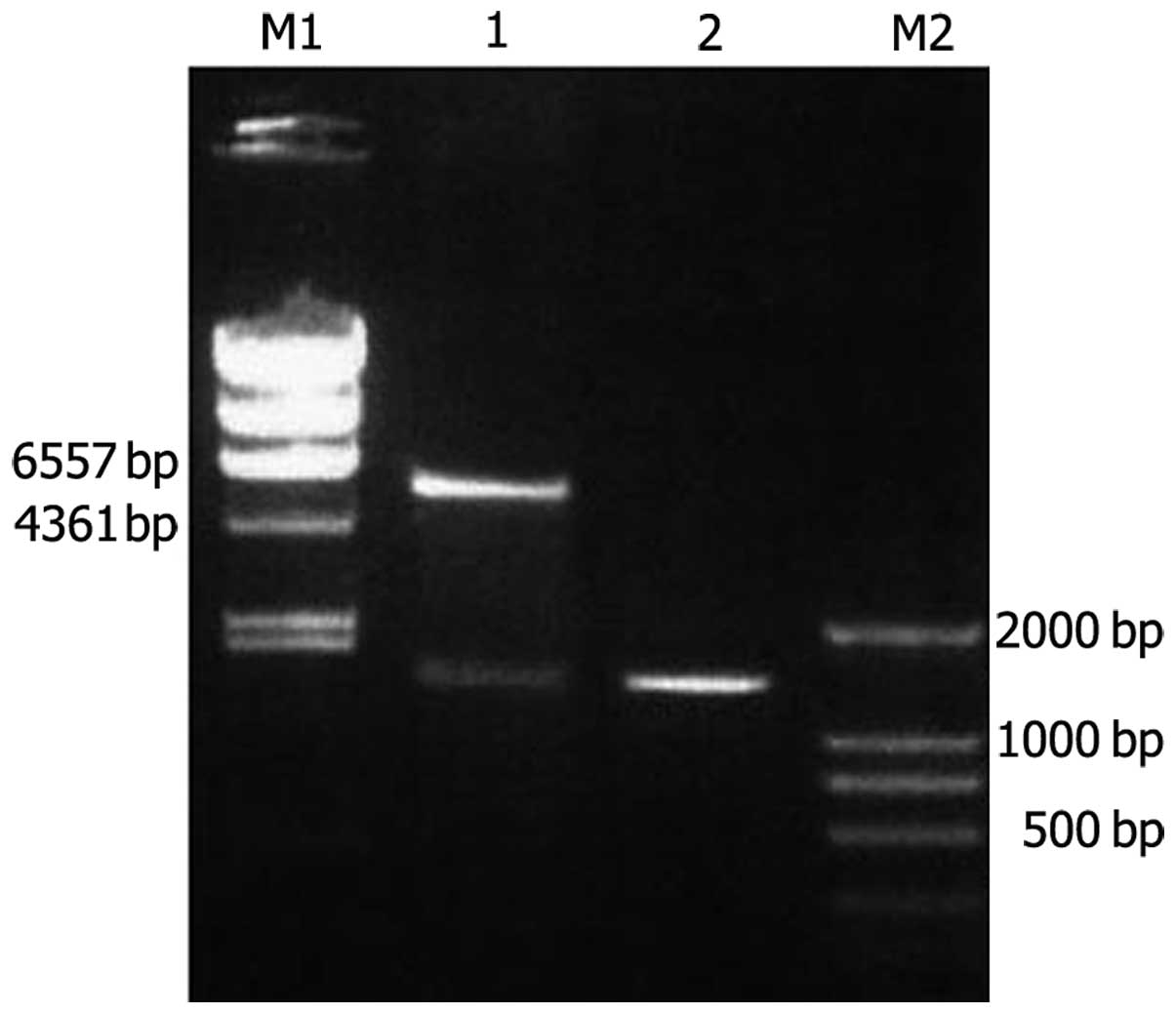
The amplified Ang-2 gene was inserted into the
pET32c vector and was consequently transformed into the
Escherichia coli strain BL21, as confirmed by sequencing and
digestion with NcoI and HindIII (Fig. 2).
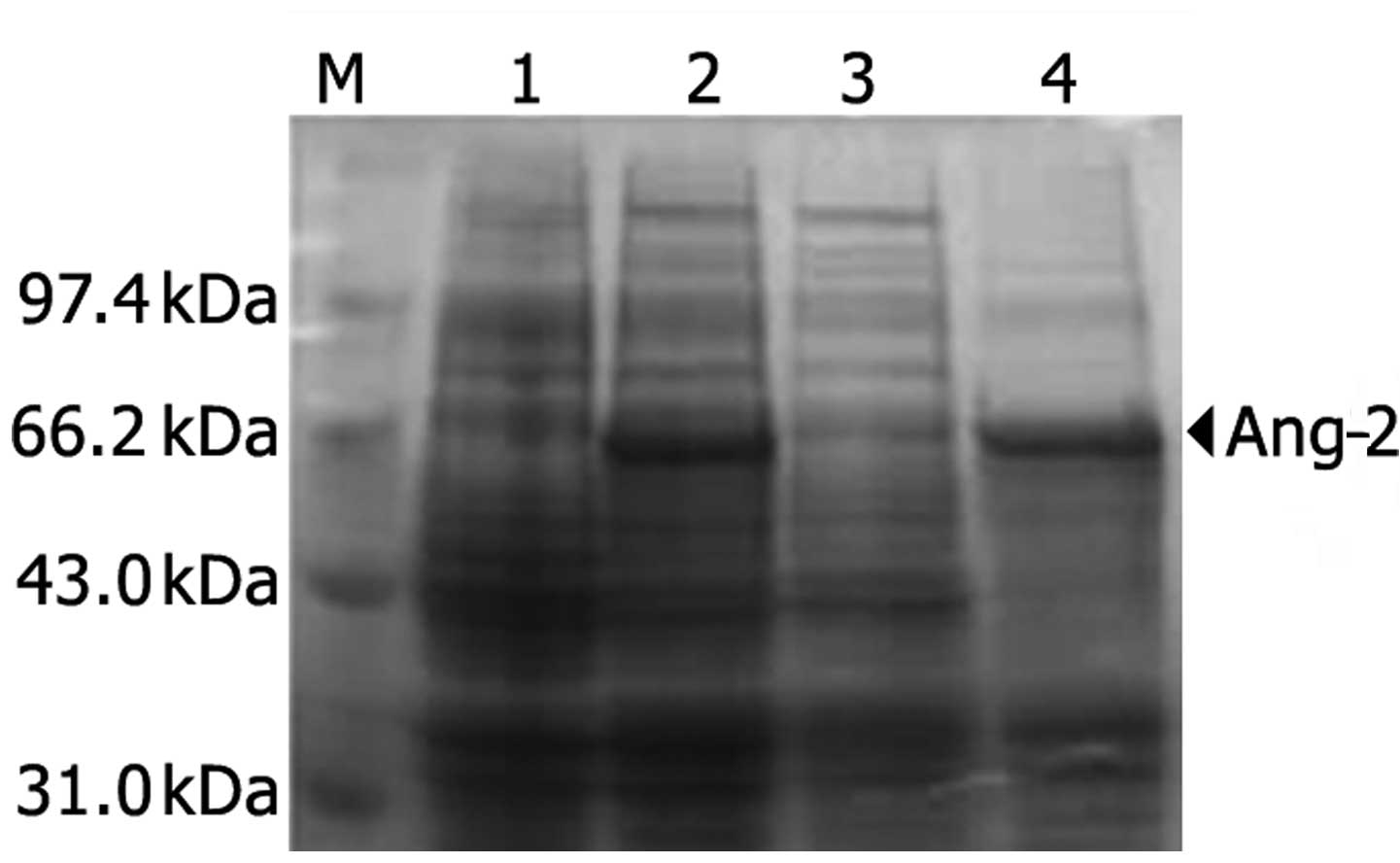
Recombinant plasmid pET32c-Ang-2 was transformed
into Escherichia coli BL21 and was strongly expressed
following IPTG induction at 15°C. The 12% SDS-PAGE electrophoresis
result (Fig. 3) indicated that
Ang-2 protein was solubly expressed in the pET32c system and was
mainly observed in the supernatant. The human Ang-2 consists of 489
amino acids and predicts a molecular weight of 56.9 kDa. Together
with glycosylation of the N terminal amino acid, Ang-2 migrates
approximately to the 60–70 kDa band in SDS-PAGE under reducing
conditions. After optimizing the conditions, Ang-2 protein was
prepared on a large scale and stored for subsequent study.
The complete Ang-2 protein was obtained by
enterokinase digestion at the optimal conditions of 25°C in a water
bath for 4 h. SDS-PAGE of the purified protein products is shown in
Fig. 4. The protein was stored at
-70°C following concentration.
Generation of scFv against Ang-2
Phage display antibody screening
Purified Ang-2 protein was used to screen antibodies
from a phage display library. Three rounds of selection against
Ang-2 showed specific enrichment of phage antibodies. One clone was
obtained with binding activity to Ang-2, as shown in Table I.
 | Table IScreening results of angiopoietin-2
from a phage display antibody library. |
Table I
Screening results of angiopoietin-2
from a phage display antibody library.
| Round of
panning | Input phage
(pfu/ml) | Eluted phage
(pfu/ml) |
|---|
| 1 |
4.25×1013 |
3.01×105 |
| 2 |
3.01×1013 |
2.04×106 |
| 3 |
4.04×1013 |
3.68×106 |
Results of ELISA assays
After 3 rounds of panning, 96 individual clones of
phage-scFv-Ang2 were randomly selected and cultured in a 96-well
plate, 50 μl centrifuged bacteria liquid was obtained and tested by
cell ELISA. As shown in Fig. 5,
positive phage clone A6A7 showed a significantly higher OD value
than the negative (control) group (P<0.05), which demonstrated
the high binding affinity of the phage-scFv-Ang2 clone for Ang-2
protein.
Electrophoresis of the scFv gene
The positive clone was screened and amplified by
PCR. Electrophoresis of the products in agarose gel (15 g/l)
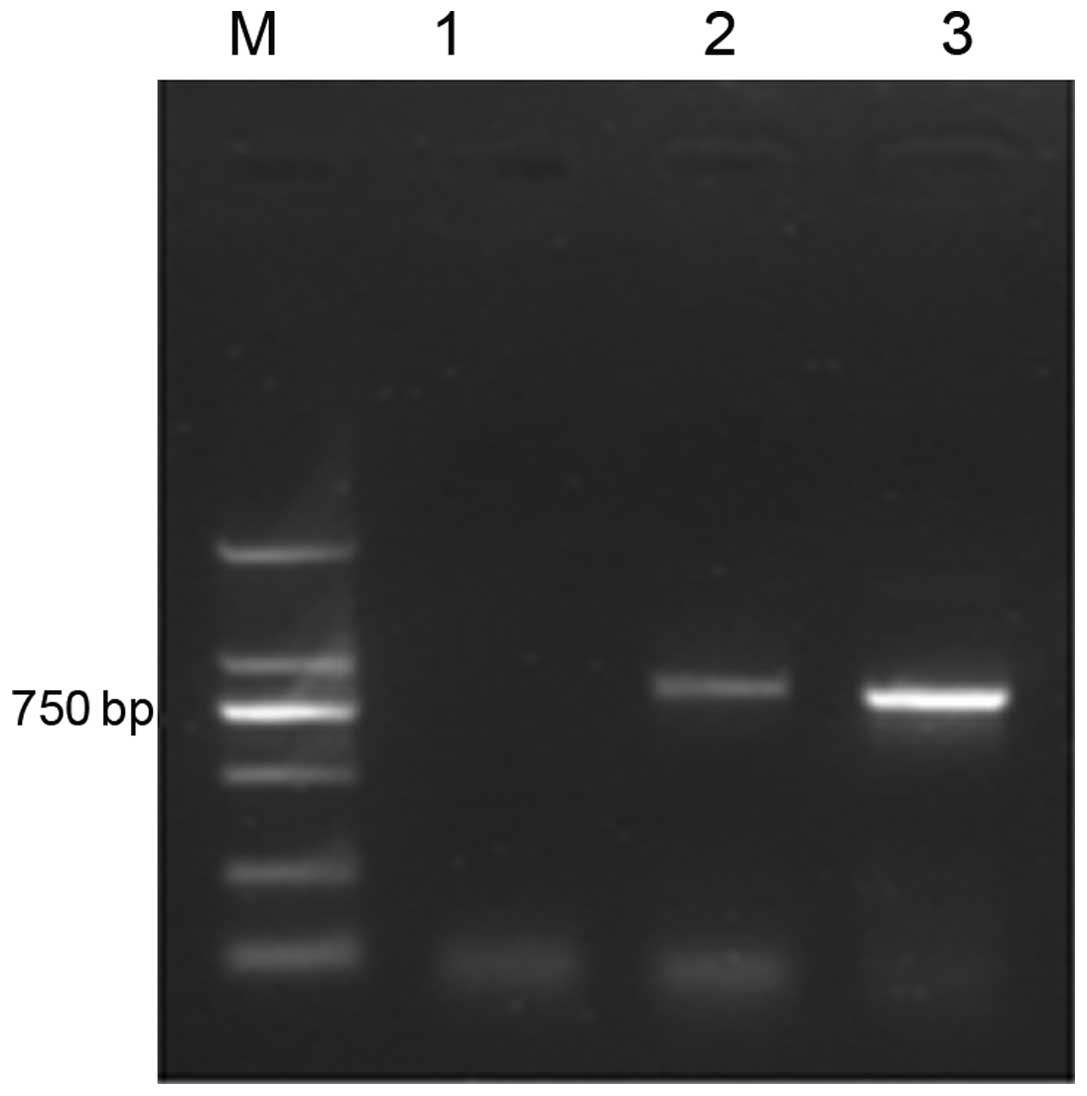
indicated a 750-bp specific scFv-Ang2 fragment (Fig. 6).
DNA sequencing for scFv-Ang2
The result of DNA sequencing (data not shown)
indicated that the obtained scFv gene had an open reading frame
(ORF) of 912 encoding base pairs, which suggests a target protein
with a molecular weight of ~33.7 kDa.
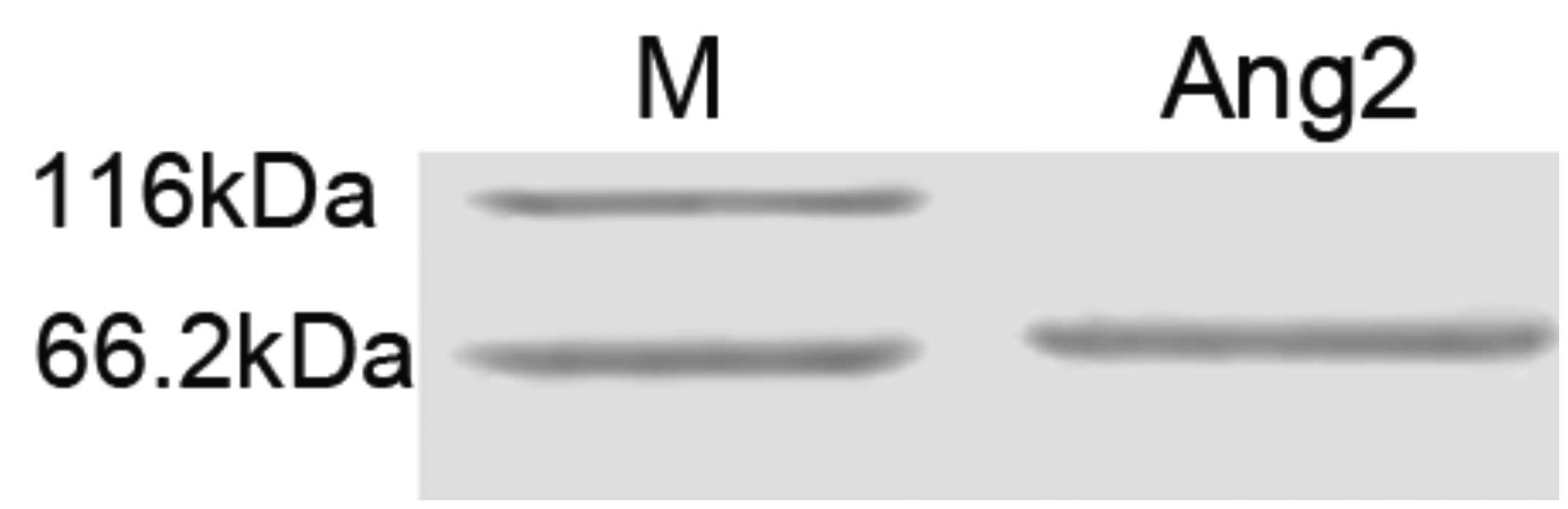
Identification of scFv by
SDS-PAGE
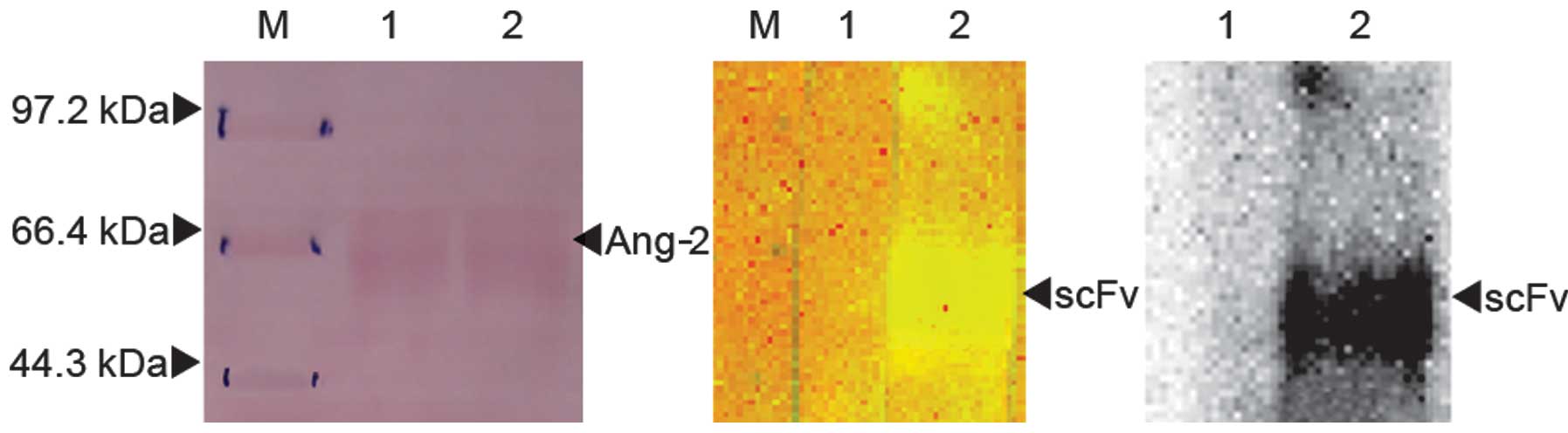
Fig. 7 shows that
the scFv-Ang2 phage specifically recognized Ang-2 protein.
Effects of scFv-Ang2 on angiogenesis and
growth of HCC
Effects on proliferation of
HUVECs
An MTT assay was used to determine the absorbance
value of HUVECs in different groups treated with various
stimulatory factors. The results obtained and comparison between
groups are shown in Table II. The
proliferation capacity of HUVECs significantly increased when VEGF
only was added, which showed a VEGF-dependent enhancement. No
effect was observed when Ang-2 protein only was added. In the VEGF
and Ang-2 combination group, the HUVECs showed the highest
proliferation ability with a significant increase, however, such
ability was significantly inhibited when scFv-Ang2 was added (VEGF
+ Ang-2 + scFv-Ang2), which also showed a marked
concentration-dependent inhibitory effect.
 | Table IIProliferation and migration of HUVECs
in different groups (cultured for 48 h). |
Table II
Proliferation and migration of HUVECs
in different groups (cultured for 48 h).
| | | | |
VEGF+Ang-2+scFv |
|---|
| | | | |
|
|---|
| Assay | Control | VEGF | Ang-2 | VEGF+Ang-2 | 1×1011
pfu/ml | 2×1011
pfu/ml | 4×1011
pfu/ml |
|---|
| MTT | 0.7286±0.0347 |
0.8379±0.0472a | 0.7469±0.0692 |
1.1918±0.1973a |
0.9718±0.0913b |
0.8396±0.1083b |
0.7492±0.1314b |
| Migration | 21.13±2.02 | 41.85±9.10a | 28.74±3.51a | 61.45±10.08a | 43.55±4.76b | 34.56±5.44b | 29.76±1.35b |
Effects on migration of HUVECs
As shown in Table
II, VEGF and Ang-2 promoted HUVEC migration compared with that
in the control group. A combination of the two showed the most
potent migration-promoting effect. Concentration-depend inhibition
on cell migration was observed when scFV-Ang-2 was added.
Effects on tubule formation of
HUVECs
The Matrigel tubule formation assay results showed
that the group treated with a combination of Ang-2 and VEGF
displayed the most increased endothelial cell migration. The HUVECs
were stretched to an increased size and tended towards a tubular
structure formation among endothelial cells and the number of
tubules formed was significantly higher than that of the control
group (P<0.05). However, when scFv-Ang2 was added, these effects
were significantly inhibited, and it was observed that the tubules
formed in the VEGF+Ang-2+scFv group were smaller in number and had
a poorer integrity of lumenal structure than those of the control
group (P<0.05), as shown in Fig.
8. The tubule formation index was calculated according to the
relative ratio of the tubule number in the intervention group to
that in control group; the comparison data are shown in Fig. 8F.
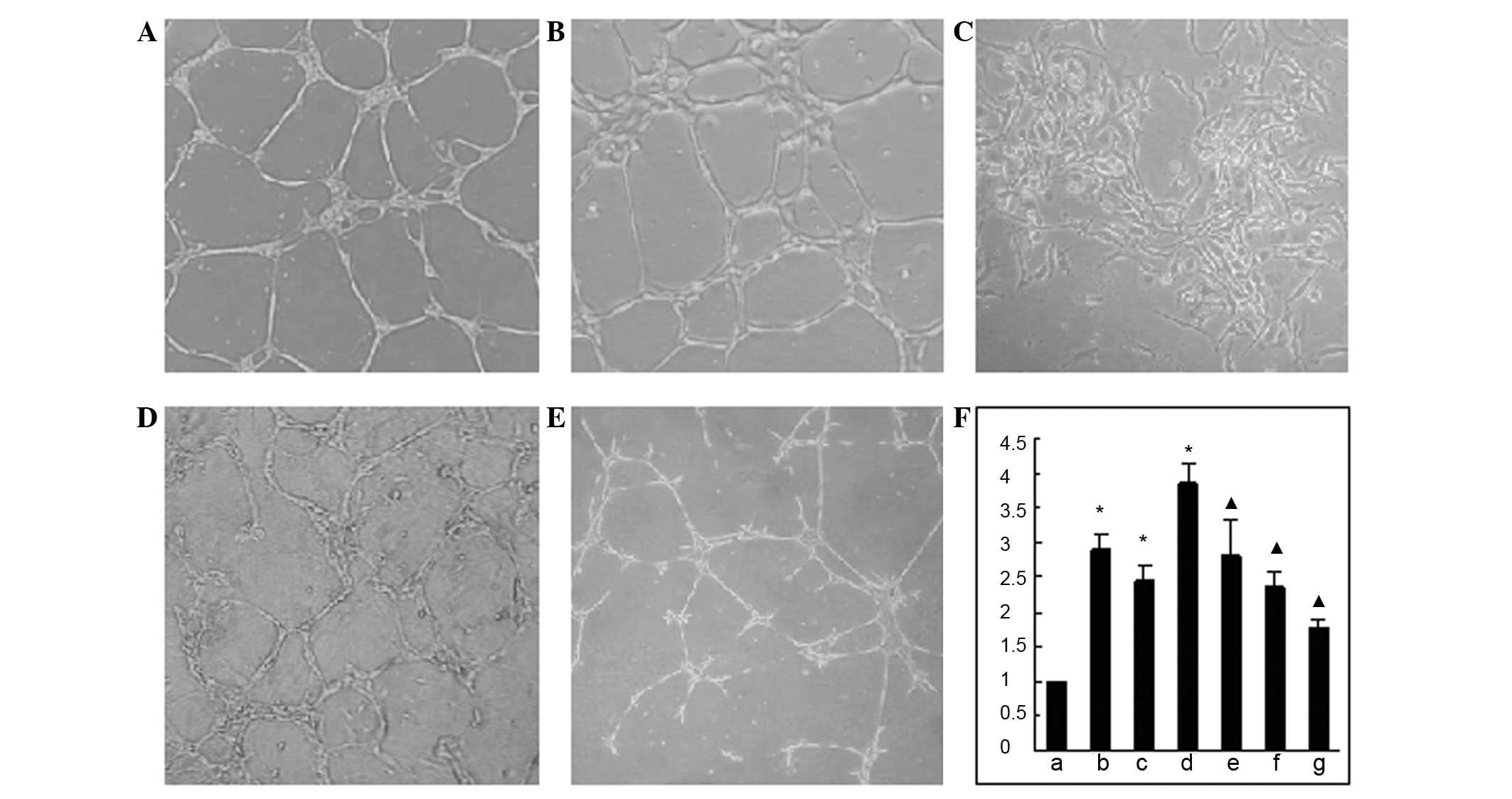 | Figure 8Tubule formation of HUVECs and column
graph of tubule formation index in different groups. (A) Control
group; (B) Ang-2 group; (C) VEGF group; (D) VEGF+Ang-2 group; (E)
VEGF+Ang-2+scFv-Ang2 group; (F) column graph of tubule formation
index in different groups, in which (a) represents control group
data, (b) Ang-2 group, (c) VEGF group, (d) VEGF+Ang-2 group, (e–g)
represents VEGF+Ang-2+scFv-Ang2 groups with scFv-Ang2
concentrations of (e) 1×1011 pfu/ml; (f)
2×1011 pfu/ml; (g) 4×1011 pfu/ml.
▲P<0.05, compared with the VEGF+Ang-2 group;
*P<0.05, compared with the control group. HUVECs,
human umbilical vein endothelial cells; VEGF, vascular endothelial
growth factor; Ang-2, angiopoietin2; scFv, single-chain
antibody. |
Tumor growth in mice
Lumps in the stomach and skin invasion were observed
in the fifth week when the mice were sacrificed. The changes in
tumor weight and volume in the treatment groups were significantly
smaller than those in the control group, and were 0.9301±0.2842 vs.
1.4483±0.3633 g and 0.5981±0.3925 vs. 1.1806±0.3188 cm3,
respectively.
Effects on HCC metastasis
Visible metastases were observed when seven lobes of
liver were carefully anatomized and the number of visible
metastases was recorded. Gross pathological examination of the
lungs identified scattered hemorrhagic spots, which were confirmed
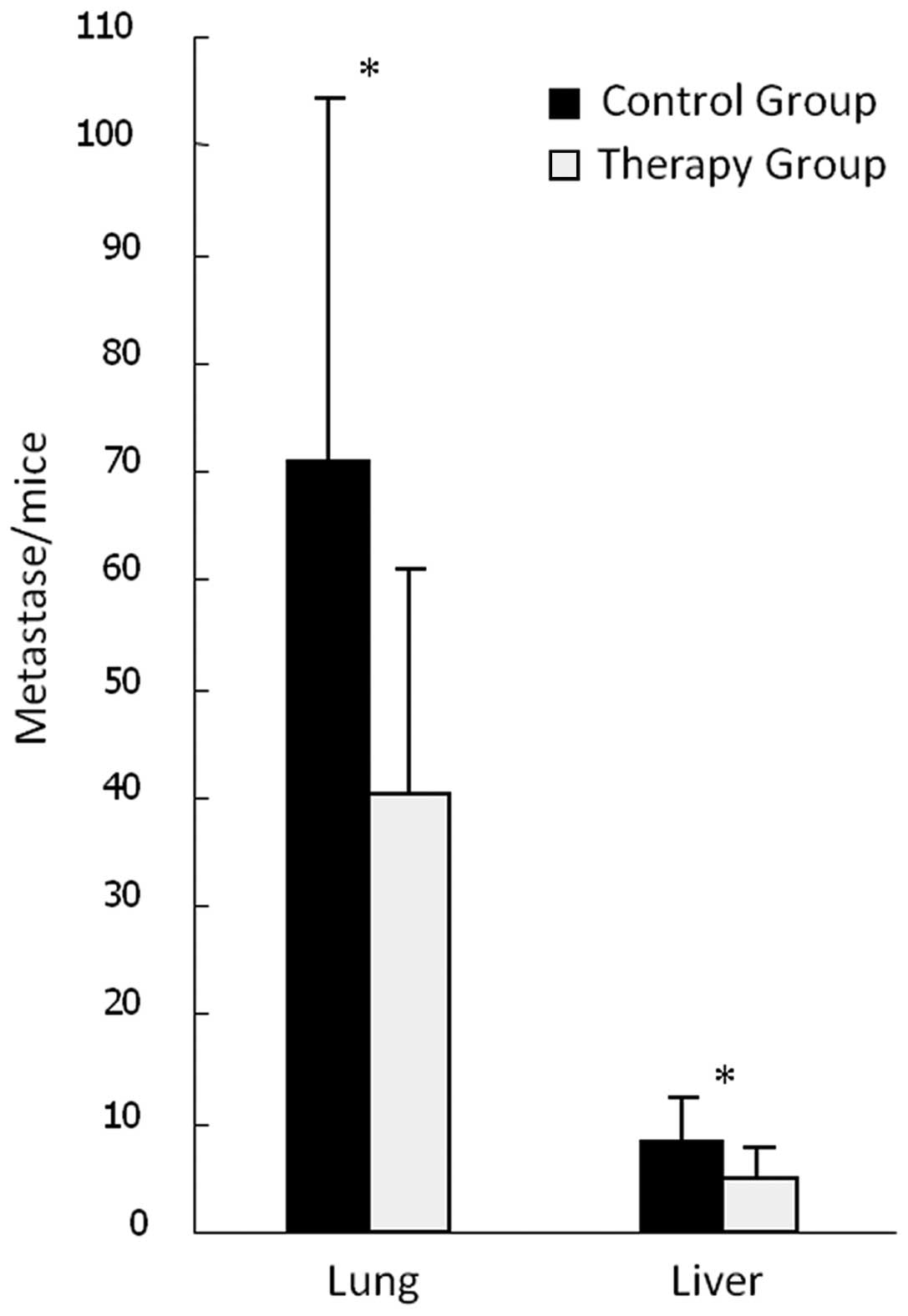
by histopathology to be metastases (Fig. 9). The metastatic rate in the liver
and lungs in the treatment and control groups was 100%, but the
numbers of metastases in both liver and lungs in the therapy group
were significantly reduced compared with those in the control group
(P<0.05, Fig. 10); the numbers
of metastases were 4.75±3.10 vs. 8.25±4.00 in the liver and
40.25±20.79 vs. 70.75±33.60 in the lungs, respectively.
Expression of CD31
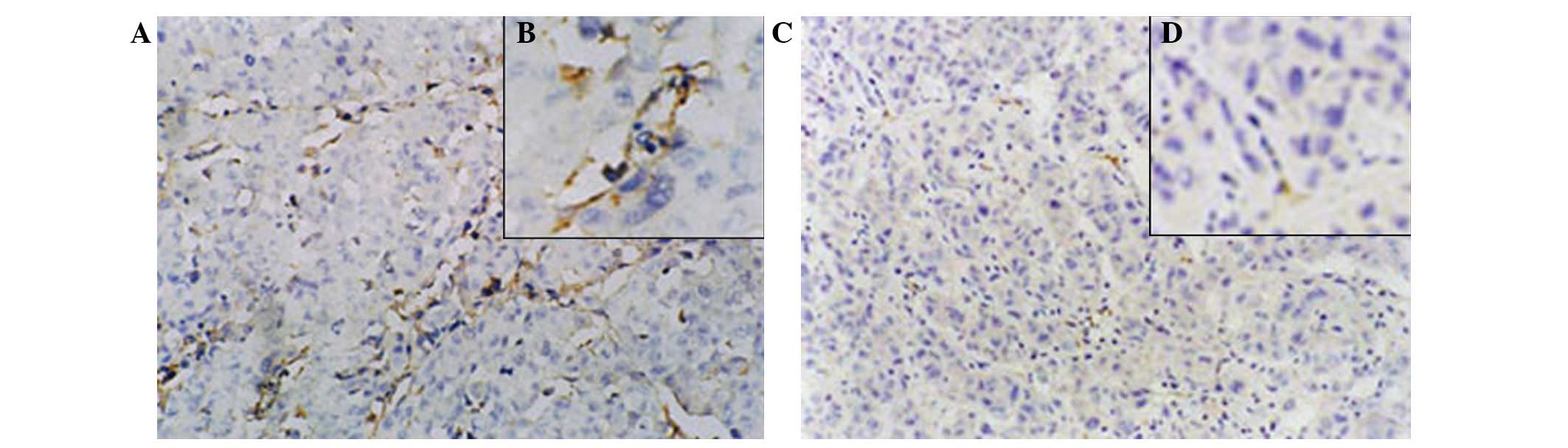
The expression of CD31 in the experimental groups
was poor or even absent, whereas it was strong in the control
group. The newborn endothelial cells were stained brown or yellow
and sinusoidally distributed in the capillary walls of portal area
and fiber interval of the liver tissue (Fig. 11). Microvessel counting revealed
that microvessel density (MVD) in the control group was 70.00±16.27
per high-power field (x200), whereas it was 21.08±6.23 in the
therapy group (P<0.05).
Discussion
Among the various angiogenic regulators, the
vascular endothelial cell-specific receptor tyrosine kinase (RTK)
family plays the most important role in the regulation of the
endothelial cell function (25,26).
Within the family, the function of the angiopoietin/Tie2 system
within the process of neovascularization has gained attention in
recent studies (27). The
angiopoietin family, including Ang-1, -2, -3 and -4, have been
isolated and identified as a group of ligands of the tyrosine
kinase Tie2 receptor (28). Ang-1
and Ang-2 have been demonstrated as the most potent regulators for
neovascularization (29) and are
the activator and antagonist of the Tie2 receptor, respectively,
where binding of Ang-1 causes autophosphorylation of Tie2 whereas
Ang-2 binding suppresses the autophosphorylation (29,30).
It was reported that normal regulation of tyrosine kinase Tie2 is
required for normal vascular development, by regulating vascular
remodeling and maturation (31).
Ang-1 contributes to the maintenance and stabilization of
maturation vessels by promoting interaction between endothelial
cells and support cells, such as pericytes. Knockout mice deficient
in angiopoietin-1 develop severe vascular defects and die in
utero, in a similar manner to Tie2 deficiency in mice (32). Ang-2 acts as an alternative ligand
for Tie2 and binds to Tie2 with similar affinity, but competitively
antagonizes the effects of Ang-1 by inhibiting Tie2 phosphorylation
and activation. Functionally, transgenic mice overexpressing Ang-2
show even more severe vascular defects than the Ang-1 or Tie2
deficient mice (12). In the
presence of VEGF, vessel destabilization caused by Ang-2 has been
hypothesized to induce an angiogenic response, whereas in the
absence of VEGF, Ang-2 leads to vessel regression (21,33).
Previous studies (34,35) suggested that highly expressed Ang-2
in tissues contributed to tumoral angiogenesis and correlated with
tumor growth and poor prognosis. It has also been reported
(36) that Ang-2 may be one of the
most important risk factors for the postoperative recurrence of HCC
following hepatic resection. In addition, patients with high levels
of preoperative Ang-2 mRNA are more likely to have a poorer
survival rate than those with low preoperative Ang-2 mRNA levels
following surgery. Our previous study confirmed that overexpression
of Ang-2 and VEGF was a key promoter of early neovascularization
and consequent carcinogenesis, and contributed greatly to the
invasion and metastasis of HCC, considered as a typical
hypervascular carcinoma. Angiogenesis thus contributes to its poor
prognosis (20). The promotory
effect of Ang-2 on angiogenesis is considered a potential target
for vascular proliferative diseases, such as HCC.
Ang-2-targeting intervention through the
immunological technique of antigen-antibody binding was one of the
most important molecular biological methods. Angiogenic gene
therapy based on a molecular level has been attempted in recent
years; however, due to the complicated process of exogenous genes
entering the body and realizing biologically active protein
expression through transcription and translation, the significant
efficacy of in vitro experiments was not demonstrated in
vivo in the majority of studies, and therapeutic effects in
clinical trials remain difficult to attain (37). Modern phage display peptide library
screening technology allows the use of proteomic methods to achieve
screening and preparation of a high-affinity scFv against a
specific target. Smith first described phage display technology
based on the ability to express foreign (poly)peptides as fusions
to capsid proteins on the surface of bacteriophages in 1985
(38). Surface display is achieved
by inserting a peptide-encoding gene into the gene for a capsid
structural protein. Billions of pooled peptides presented on phage
particles form a phage-displayed peptide library, and in contrast
to regular synthetic small molecule libraries, as many as 1,010
different peptides may be screened simultaneously for the desired
activity (39,40). In the present assay, target phage
with the specific protein that binds with phage peptide sequences
through a ligand-receptor type binding mode was panned out from the
random peptide phage library and further amplification was realized
by transfecting Escherichia coli. The target protein (scFv)
was identified and purified, and its structure and functions were
analyzed. The scFv may be expressed in E. coli as a
single-chain molecule in which the heavy chain (VH) and light chain
(VL) domains of the antibody are joined by a flexible polypeptide
linker (41). Over the past two
decades, phage display has been widely used in numerous scientific
fields including drug discovery/design, such as screening for
receptor agonists and antagonists, drug target validation,
development of vaccines, in vitro selection of new
antibodies, antibody fragments and antibody surrogates as
randomized fragments on diverse scaffold proteins, and discovery of
agents for the targeted delivery of drugs and gene therapy.
Compared with whole antibody molecules, the scFv
contains the variable region of antibody and the complete
antigen-binding site, but does not contain the fragment
crystallizable region (Fc region), which significantly increases
the specificity of the antigen-antibody binding. In addition, its
low molecular weight, low immunogenicity, high penetration and ease
of production on a large scale (12) makes it a particularly promising
therapeutic agent.
The construction of a phage display peptide library
is a complex task. In order to avoid this in preliminary
experiments, a non-immunized mouse phage display antibody library
built by the Institute of Hydrobiology, Chinese Academy of Sciences
(Wuhan, China) with a storage capacity of 109 was used
(22). Therefore, the complex
process of repeatedly constructing an antibody library to target
various antigens was avoided, the majority of antibody genes were
collected in the library and the diversity of antibodies was
significantly increased. It was easy to access a variety of protein
antigens of specific scFv from the antibody library (42). Biologically active Ang-2 protein
was used to reduce interferences during the panning process.
Screening and the identification of scFv-Ang2, which contained 303
amino acids with a molecular weight of ~33.7 kDa was successfully
conducted. The specificity of the intact antibody molecule was
demonstrated in the present study. The obtained scFv-Ang2 was first
used in vitro in primary cultured HUVECs to observe its
effects on vessel formation. The results showed that scFv-Ang2
alone did not promote the proliferation of HUVECs, but was able to
promote endothelial cell migration and tubule formation. When
scFv-Ang2 was combined with VEGF the angiogenic ability of the
latter was significantly enhanced. This confirmed that Ang-2 plays
an important role in the process of angiogenic regulation and may
be valuable as a target for intervention. As Ang-2 itself does not
promote HUVEC proliferation, its function of regulating
neovascularization may be dependent on cooperative action with
other angiogenic factors, such as VEGF. Interactions between Ang-2
and VEGF have also been confirmed in systems other than tumors,
such as a corneal neovascularization model (43). Another important angiogenic factor
angiotensin II (ANGII) has been shown to cause increased expression
of Ang-2 (44,45). However, the mechanism of how these
factors increase Ang-2 expression has not been investigated. We
speculate that Ang-2-mediated downstream signaling pathways may be
the common mechanism of action for numerous angiogenic factors. In
vascular proliferative disorders, such as cancer and diabetic
retinopathy, the blockade of Ang-2 may lead to anti-angiogenesis
from Ang-2 inhibition and the inhibition of other angiogenic
factors whose activities are mediated by Ang-2 (46). When scFv-Ang2 was added to cultured
endothelial cells containing Ang-2, the promotion of endothelial
migration and tubule formation was significantly reduced, which
indicates that scFv-Ang2 was effective in vitro in blocking
Ang-2 and inhibiting its angiogenic activity.
HCC is a typical hypervascular tumor and
angiogenesis accounts for its malignant biological behavior and
poor prognosis. Accordingly studies of anti-angiogenic therapy for
HCC are critically significant. In vivo, the MHCC97 human
HCC cell line was employed to establish a highly metastatic nude
mouse model of liver orthotopic xenografts to observe the effects
of scFv-Ang2 on HCC angiogenesis and tumor growth. The results
revealed that the tumor weight and volume in the treated group were
statistically lower than those of the control group, indicating
that scFv-Ang2 has a significant inhibitory effect on the growth of
HCC. The animal model employed in this study has a 100% metastasis
rate in the liver and lungs under normal growth conditions and is a
common model for intervention in human HCC (47). It was observed in the present study
that scFv-Ang2 significantly inhibited the spread of the metastases
to the lung and the intrahepatic metastasis of HCC in nude mice
(P<0.05), despite the 100% metastasis rate in the liver and lung
in both groups. Expression levels of CD31, which is considered as
an endothelial-specific marker, were examined using monoclonal CD31
antibody. The results suggest that the expression levels of CD31 in
the experimental groups were extremely low, whereas in the control
group CD31 was strongly expressed. Accordingly, MVD in the
experimental group was much lower than that in the control group
(P<0.05). MVD is the most common target reflecting
neovascularization. The reduction of MVD in this study suggests
that scFv-Ang2 had inhibitory effects on angiogenesis. The process
of angiogenesis is a highly complex network of systems. In this
study, the angiopoietin pathway was targeted by purifying human
scFv-Ang2 and testing its effects on the angiogenesis and tumor
growth of HCC in nude mice, which showed that the inhibition of
Ang-2 is an important mechanism of anti-angiogenic action.
Acknowledgements
This study was supported in part by the Special Fund
of Central Universities for Basic Scientific Research, Wuhan
University (Wuhan, China) and Shanghai Sixth People’s Hospital
Medical Group Fund for Scientific Research. The authors would like
to thank Dr Li-Qiao Zhong (Institute of Hydrobiology, the Chinese
Academy of Sciences) for technical assistance.
References
|
1
|
Pisani P, Parkin DM, Bray F and Ferlay J:
Estimates of the worldwide mortality from 25 cancers in 1990. Int J
Cancer. 83:18–29. 1999. View Article : Google Scholar
|
|
2
|
D’Amato RJ, Loughnan MS, Flynn E and
Folkman J: Thalidomide is an inhibitor of angiogenesis. Proc Natl
Acad Sci USA. 91:4082–4085. 1994.
|
|
3
|
Farris AB III, Dursun N, Dhanasekaran R,
Coban I, McIntosh EB, Adsay NV and Kim HS: Tumoral and angiogenesis
factors in hepatocellular carcinoma after locoregional therapy.
Pathol Res Pract. 208:15–21. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chao Y, Li CP, Chau GY, Chen CP, King KL,
Lui WY, Yen SH, Chang FY, Chan WK and Lee SD: Prognostic
significance of vascular endothelial growth factor, basic
fibroblast growth factor, and angiogenin in patients with
resectable hepatocellular carcinoma after surgery. Ann Surg Oncol.
10:355–362. 2003. View Article : Google Scholar
|
|
5
|
Jeng KS, Sheen IS, Wang YC, Gu SL, Chu CM,
Shih SC, Wang PC, Chang WH and Wang HY: Prognostic significance of
preoperative circulating vascular endothelial growth factor
messenger RNA expression in resectable hepatocellular carcinoma: a
prospective study. World J Gastroenterol. 10:643–646. 2004.
|
|
6
|
Poon RT, Ho JW, Tong CS, Lau C, Ng IO and
Fan ST: Prognostic significance of serum vascular endothelial
growth factor and endostatin in patients with hepatocellular
carcinoma. Br J Surg. 91:1354–1360. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Folkman J: Tumor angiogenesis: therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eichholz A, Merchant S and Gaya AM:
Anti-angiogenesis therapies: their potential in cancer management.
Onco Targets Ther. 3:69–82. 2010.PubMed/NCBI
|
|
9
|
Yang JC, Haworth L, Sherry RM, Hwu P,
Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX and
Rosenberg SA: A randomized trial of bevacizumab, an anti-vascular
endothelial growth factor antibody, for metastatic renal cancer. N
Engl J Med. 349:427–434. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cobleigh MA, Langmuir VK, Sledge GW,
Miller KD, Haney L, Novotny WF, Reimann JD and Vassel A: A phase
I/II dose-escalation trial of bevacizumab in previously treated
metastatic breast cancer. Semin Oncol. 30(5 Suppl 16): 117–124.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mayer RJ: Two steps forward in the
treatment of colorectal cancer. N Engl J Med. 350:2406–2408. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Desplancq D, Rinaldi AS, Stoessel A,
Sibler AP, Busso D, Oulad-Abdelghani M, Van Regenmortel MH and
Weiss E: Single-chain Fv fragment antibodies selected from an
intrabody library as effective mono- or bivalent reagents for in
vitro protein detection. J Immunol Methods. 369:42–50. 2011.
View Article : Google Scholar
|
|
13
|
Yamaguchi R, Yano H, Nakashima Y,
Ogasawara S, Higaki K, Akiba J, Hicklin DJ and Kojiro M: Expression
and localization of vascular endothelial growth factor receptors in
human hepatocellular carcinoma and non-HCC tissues. Oncol Rep.
7:725–729. 2000.PubMed/NCBI
|
|
14
|
Ochiumi T, Tanaka S, Oka S, Hiyama T, Ito
M, Kitadai Y, Haruma K and Chayama K: Clinical significance of
angiopoietin-2 expression at the deepest invasive tumor site of
advanced colorectal carcinoma. Int J Oncol. 24:539–547.
2004.PubMed/NCBI
|
|
15
|
Sun XD, Liu XE, Wu JM, Cai XJ, Mou YP and
Li JD: Expression and significance of angiopoietin-2 in gastric
cancer. World J Gastroenterol. 10:1382–1385. 2004.PubMed/NCBI
|
|
16
|
Zhang L, Yang N, Park JW, Katsaros D,
Fracchioli S, Cao G, O’Brien-Jenkins A, Randall TC, Rubin SC and
Coukos G: Tumor-derived vascular endothelial growth factor
up-regulates angiopoietin-2 in host endothelium and destabilizes
host vasculature, supporting angiogenesis in ovarian cancer. Cancer
Res. 63:3403–3412. 2003.
|
|
17
|
Sfiligoi C, de Luca A, Cascone I, Sorbello
V, Fuso L, Ponzone R, Biglia N, Audero E, Arisio R, Bussolino F,
Sismondi P and De Bortoli M: Angiopoietin-2 expression in breast
cancer correlates with lymph node invasion and short survival. Int
J Cancer. 103:466–474. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Glade Bender J, Cooney EM, Kandel JJ and
Yamashiro DJ: Vascular remodeling and clinical resistance to
antiangiogenic cancer therapy. Drug Resist Updat. 7:289–300.
2004.PubMed/NCBI
|
|
19
|
Wong MP, Chan SY, Fu KH, Leung SY, Cheung
N, Yuen ST and Chung LP: The angiopoietins, tie2 and vascular
endothelial growth factor are differentially expressed in the
transformation of normal lung to non-small cell lung carcinomas.
Lung Cancer. 29:11–22. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang ZL, Liu ZS and Sun Q: Significance
of angiopoietins, Tie2 and vascular endothelial growth factor in
the angiogenesis and development of hepatocellular carcinoma. World
J Gastroenterol. 12:4241–4245. 2006.PubMed/NCBI
|
|
21
|
Maisonpierre PC, Suri C, Jones PF,
Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J,
Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN and
Yancopoulos GD: Angiopoietin-2, a natural antagonist for Tie2 that
disrupts in vivo angiogenesis. Science. 277:55–60. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dai H, Gao H, Zhao X, Dai L, Zhang X, Xiao
N, Zhao R and Hemmingsen SM: Construction and characterization of a
novel recombinant single-chain variable fragment antibody against
White Spot Syndrome Virus from shrimp. J Immunol Methods.
279:267–275. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Barbas CF III, Burton DR, Scott JK and
Silverman GJ: Phage Display: A Laboratory Manual. Cold Spring
Harbor Laboratory Press; Cold Spring Harbor: 2001
|
|
24
|
Weidner N, Semple JP, Welch WR and Folkman
J: Tumor angiogenesis and metastasis - correlation in invasive
breast carcinoma. N Engl J Med. 324:1–8. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gale NW and Yancopoulos GD: Growth factors
acting via endothelia cell-spcific receptor tyrosine kinases:
VEGFs, angiopoietins, and ephrins in vascular development. Genes
Dev. 13:1055–1066. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Davis S, Aldrich TH, Jones PF, Acheson A,
Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre
PC and Yancopoulos GD: Isolation of angiopoietin-1, a ligand for
the TIE2 receptor, by secretion-trap expression cloning. Cell.
87:1161–1169. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Saharinen P, Bry M and Alitalo K: How do
angiopoietins Tie in with vascular endothelial growth factors? Curr
Opin Hematol. 17:198–205. 2010.PubMed/NCBI
|
|
28
|
Pham VN, Roman BL and Weinstein BM:
Isolation and expression analysis of three zebrafish angiopoietin
genes. Dev Dyn. 221:470–474. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hata K, Udagawa J, Fujiwaki R, Nakayama K,
Otani H and Miyazaki K: Expression of angiopoietin-1,
angiopoietin-2, and Tie2 genes in normal ovary with corpus luteum
and in ovarian cancer. Oncology. 62:340–348. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mitsuhashi N, Shimizu H, Ohtsuka M,
Wakabayashi Y, Ito H, Kimura F, Yoshidome H, Kato A, Nukui Y and
Miyazaki M: Angiopoietins and Tie-2 expression in angiogenesis and
proliferation of human hepatocellular carcinoma. Hepatology.
37:1105–1113. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sugimachi K, Tanaka S, Taguchi K, Aishima
S, Shimada M and Tsuneyoshi M: Angiopoietin switching regulates
angiogenesis and progression of human hepatocellular carcinoma. J
Clin Pathol. 56:854–860. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Suri C, Jones PF, Patan S, Bartunkova S,
Maisonpierre PC, Davis S, Sato TN and Yancopoulos GD: Requisite
role of angiopoietin-1, a ligand for the TIE2 receptor, during
embryonic angiogenesis. Cell. 87:1171–1180. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Teichert-Kuliszewska K, Maisonpierre PC,
Jones N, Campbell AI, Master Z, Bendeck MP, Alitalo K, Dumont DJ,
Yancopoulos GD and Stewart DJ: Biological action of angiopoietin-2
in a fibrin matrix model of angiogenesis is associated with
activation of Tie2. Cardiovasc Res. 49:659–670. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang J, Wu K, Zhang D, Tang H, Xie H, Hong
L, Pan Y, Lan M, Hu S, Ning X and Fan D: Expression and clinical
significances of angiopoietin-1, -2 and Tie2 in human gastric
cancer. Biochem Biophy Res Commun. 337:386–393. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nakayama T, Hatachi G, Wen CY, Yoshizaki
A, Yamazumi K, Niino D and Sekine I: Expression and significance of
Tie-1 and Tie-2 receptors, and angiopoietins-1, 2 and 4 in
colorectal adenocarcinoma: Immunohistochemical analysis and
correlation with clinicopathological factors. World J
Gastroenterol. 11:964–969. 2005. View Article : Google Scholar
|
|
36
|
Wada H, Nagano H, Yamamoto H, Yang Y,
Kondo M, Ota H, Nakamura M, Yoshioka S, Kato H, Damdinsuren B, Tang
D, Marubashi S, Miyamoto A, Takeda Y, Umeshita K, Nakamori S, Sakon
M, Dono K, Wakasa K and Monden M: Expression pattern of angiogenic
factors and prognosis after hepatic resection in hepatocellular
carcinoma: importance of angiopoietin-2 and hypoxia-induced
factor-1 alpha. Liver Int. 26:414–423. 2006. View Article : Google Scholar
|
|
37
|
Jain RK: Normalization of tumor
vasculature: an emerging concept in antiangiogenic therapy.
Science. 307:58–62. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Smith GP: Filamentous fusion phage: novel
expression vectors that display cloned antigens on the virion
surface. Science. 228:1315–1317. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Smith GP and Petrenko VA: Phage Display.
Chem Rev. 97:391–410. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bratkovic T: Progress in phage display.
Cell Mol Life Sci. 67:749–767. 2010. View Article : Google Scholar
|
|
41
|
Huston JS, Levinson D, Mudgett-Hunter M,
Tai MS, Novotný J, Margolies MN, Ridge RJ, Bruccoleri RE, Haber E,
Crea R, et al: Protein engineering of antibody binding sites:
recovery of specific activity in an anti-digoxin single-chain Fv
analogue produced in Escherichia coli. Proc Natl Acad Sci
USA. 85:5879–5883. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rao Y, Zhong L, Liao T, Jin S, Wang Y,
Song B, Li J, Zhang X, Hemmingsen SM, Xu Y and Dai H: Novel
recombinant monoclonal antibodies for vitellogenin assays in
cyprinid fish species. Dis Aquat Organ. 93:83–91. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lobov IB, Brooks PC and Lang RA:
Angiopoietin-2 displays VEGF-dependent modulations of capilliary
structure and endothelial cell survival in vivo. Proc Natl Acad Sci
USA. 99:11205–11210. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ji Y, Wang Z, Li Z, Li K, Le X and Zhang
T: Angiotensin II induces angiogenic factors production partly via
AT1/JAK2/STAT3/SOCS3 signaling pathway in MHCC97H cells. Cell
Physiol Biochem. 29:863–874. 2012. View Article : Google Scholar
|
|
45
|
Imai N, Hashimoto T, Kihara M, Yoshida S,
Kawana I, Yazawa T, Kitamura H and Umemura S: Roles for host and
tumor angiotensin II type 1 receptor in tumor growth and
tumor-associated angiogenesis. Lab Invest. 87:189–198. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
White RR, Shan S, Rusconi CP, Shetty G,
Dewhirst MW, Kontos CD and Sullenge BA: Inhibition of rat corneal
angiogenesis by a nuclease-resistant RNA aptamer specific for
angiopoietin-2. Proc Natl Aead Sci USA. 100:5028–5033. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li Y, Tang Y, Ye L, Liu B, Liu K, Chen J
and Xue Q: Establishment of a hepatocellular carcinoma cell line
with unique metastatic characteristics through in vivo selection
and screening for metastisis-related genes through cDNA microarray.
J Cancer Res Clin Oncol. 129:43–51. 2003.
|