Introduction
Idiopathic macular holes (IMHs), which lack a
precise ocular pathogenesis that is likely to indicate a primary
disease of the fundus, have been identified as a cause of visual
loss in the elderly. The macula lutea is the most important area
for central vision. A hallmark inciting event for IMH formation is
considered to be focal shrinkage of the vitreous cortex and
traction in the foveal area. Subsequently, central visual acuity
usually deteriorates. Kelly and Wendel were the first to report
improved results for vitreous surgery of an IMH in 1991 (1). Since then, vitrectomy for IMHs has
rapidly become a commonly performed procedure worldwide. Currently,
the majority of scholars consider that a combined application of
vitrectomy with internal limiting membrane (ILM) peeling is able to
improve anatomical closure and the recovery of vision. However, a
series of studies have shown that combined surgery results in IMH
closure in 90% of eyes, which indicates that 10% of eyes failed to
achieve IMH closure (2–6). The development of methods to prevent
failure and promote anatomical closure is currently a challenging
issue in clinical treatment (7,8). Our
research group has developed a surgical procedure for the
enlargement of ILM peeling combined with vitrectomy following a
previous failed correction of IMH. The present study investigates
the efficacy of this secondary surgery for macular hole
closure.
Materials and methods
Clinical data
Between January 2009 and June 2011, patients who
received a pars plana vitrectomy (PPV) combined with ILM peeling
surgery were studied in the Department of Ophthalmology of the
Ninth People’s Hospital affiliated with Shanghai Jiaotong
University (Shanghai, China). All patients provided informed
consent prior to surgery. The present study was approved by the
Ethics Committee of the Ninth People’s Hospital affiliated with
Shanghai Jiaotong University. A slit lamp with a handheld or fixed
lens and optical coherence tomography (OCT; Cirrus™ HD-OCT 4000;
Carl Zeiss Meditec, Inc., Jena, Germany) were used to examine the
periphery and the center of the retina. All patients were diagnosed
as having an IMH. The exclusion criteria included myopia of >6
diopters, ocular trauma and other systematic or ocular issues
associated with IMHs. The series included 134 eyes of 130 patients,
of which 42 patients were male and 88 were female with 42 and 92
eyes being treated, respectively. The age range was 46–81 years,
with a mean of 63.75±7.45 years. Patient clinical course ranged
between 1 and 36 months, with a mean of 4.4±5.3 months. The eyes
were divided into stages according to the Gass categories (9). There were 23 eyes (17.1%) in stage
II, 47 eyes (35.1%) in stage III and 64 eyes (47.8%) in stage IV.
Retinal defects ranged between 200 and 800 μm, in diameter with a
mean of 457±129 μm. In total, 134 eyes underwent the standard
three-port full PPV using a 23-gauge incision, combined with ILM
peeling. This was followed by phacoemulsification and artificial
lens implantation. Following the application of 0.025% indocyanine
green (ICG) dye (Dandong Medical and Pharmaceutical Ltd. Liability
Corporation, Dandong, China) during surgery, ILM of 2 disk
diameters (DD) around the hole was peeled off. Among these eyes, 14
retinal defects were not closed, of which 11 IMHs remained open at
week 1. In addition, three reopened 3 months postoperatively. Among
the 14 patients, one declined secondary surgery and the remaining
13 cases agreed to have a secondary vitrectomy with surgical
enlargement of ILM peeling.
The 13 eyes that underwent secondary ILM peeling
surgery (four male and nice female) each had an artificial lens.
Patient age ranged between 43 and 79 years, with a mean of
64.65±6.18 years and the clinical course ranged between 6 and 30
months with an mean of 13.4±5.4 months. IMHs ranged between 450 and
800 μm in diameter and the mean was 584±89 μm. The 13 eyes
comprised five in stage III and nine in stage IV.
Surgery methods
Primary and secondary surgeries were performed by
one surgeon. All surgeries were performed under local anesthesia.
The primary surgeries were a standard three-port full PPV using
23-gauge incisions, phacoemulsification and artificial lens
implantation in phakic eyes. The posterior cortical vitreous was
removed using triamcinolone acetonide to aid visualization. ICG
(0.1 ml, 0.025%) was applied to stain the macula lutea for 15 sec,
and was then removed by suctioning. The extent of the ILM peeling
was 2–4 DD and 14% C3F8 gas was introduced
into the vitreous cavity during surgery. Patients were instructed
to maintain a strict face-down position for 3 h/day for 7 days
postoperatively. Secondary surgeries to enlarge the peeling of the
ILM were performed using a standard three-port 23-gauge incision
following local anesthesia; 0.1 ml ICG (0.025%) was injected into
the vitreous cavity and later removed by suctioning. Following this
procedure, the ILM edge remaining from the previous surgery was
viewable. This edge was engaged with retinal forceps tangentially
to the retinal surface and the ILM was torn annularly to the
vascular arcades of the posterior retina.
C3F8 (14%) was then administered to the
vitreous cavity. Patients were instructed to remain face-down for 3
h/day for 7 days.
Clinical observations
Eyes were examined at week 1, months 1, 3 and 6 and
1 year following surgery. Snellen chart assessments, as well as
slit lamp examinations combined with a three-mirror contact lens
and a 120-diopter lens were performed to examine vision, the
intraocular anterior segment and the ocular fundus. OCT was used to
observe the macular configuration and determine if there were any
complications. In addition, patients were closely observed for 6
months to check for the development of a retinal hole. An
anatomical success was defined as an OCT examination showing the
complete disappearance of the hole and the recovery of continuity
in the neural epithelium. If the hole was not completely closed or
the retinal neurosensory layer lacked integrity, the surgery was
determined to have failed, even if the neurosensory layer was
anatomically closed and the hole had become smaller.
Statistical analysis
Visual acuity scores from pre- and postsurgery were
transformed to logMAR and subsequently the vision of index and hand
motion corresponded to 2.0 and 3.0, respectively. A t-test was
performed to compare variables among various groups, including age,
duration of the disease, size of the IMH and the time of follow-up
between the first surgery with a vitrectomy and ILM peeling surgery
and the second with a vitrectomy and surgery to enlarge ILM
peeling. The Gass stages were analyzed with a χ2 test.
P<0.05 was considered to indicate a statistically significant
difference. The results presented in this study are expressed as
the mean ± standard deviation (SD).
Results
Postsurgical ocular conditions
Table I shows the
characteristics of gender, age, follow-up time, Gass stage, size of
the IMH and pre and postsurgical acuity of patients at the primary
surgery, involving PPV combined with ILM peeling, and the secondary
surgery to enlarge the ILM peeling. There was no significant
difference in gender, age and follow-up time between the primary
and secondary surgeries. The duration of the clinical course in the
secondary surgery group was 13.3±4.0 months, which was
significantly different from the duration course of 4.5±5.0 months
for the primary surgery group. In addition, the IMHs in the
secondary surgery patients were larger, with a mean size of 584±89
μm, compared with those in the patients treated with the first
surgery, which had a mean size of 457±129 μm.
 | Table IBaseline characteristics and
postoperative functional outcomes in eyes following the first PPV
surgery and the second surgery to enlarge ILM peeling following
first surgery failure. |
Table I
Baseline characteristics and
postoperative functional outcomes in eyes following the first PPV
surgery and the second surgery to enlarge ILM peeling following
first surgery failure.
| Characteristics | First surgery | Second surgery | t-value | P-value |
|---|
| Gender |
| Male | 42 | 4 | | |
| Female | 88 | 9 | 0.013 | 0.91 |
| Age, years | 64.2±7.1 | 64.6±6.1 | 1.54 | 0.14 |
| Duration, months | 4.5±5.0 | 13.3±4.0 | 6.13 | <0.001 |
| Gass stage |
| II | 23 | 0 | | |
| III | 47 | 5 | | |
| IV | 64 | 9 | 1.64 | 0.10 |
| Macular hole size,
μm | 443.0±122.6 | 588.5±108.3 | 4.12 | <0.001 |
| Follow-up,
months | 12.3±0.88 | 13.5±5.8 | 0.70 | 0.497 |
| LogMAR,
presurgery | 0.94±0.19 | 1.03±0.18 | 1.60 | 0.08 |
| LogMAR,
postsurgery | 0.61±0.28 | 0.92±0.16 | 6.16 | <0.001 |
For all the primary surgery patients, the rate of
anatomical closure of the IMH was 89.6% (120/134). In addition, IMH
closure was achieved in 100% (23/23), 89.4% (42/47) and 85.9%
(55/64) of the patients in stages II, III and IV, respectively.
There were 14 eyes that did not heal in 14 patients, of which five
were in stage III and nine were in stage IV. Excluding the one
patient in stage IV who declined the secondary surgery, the rate of
closure was 61.5% (8/13) in the group of 13 patients that underwent
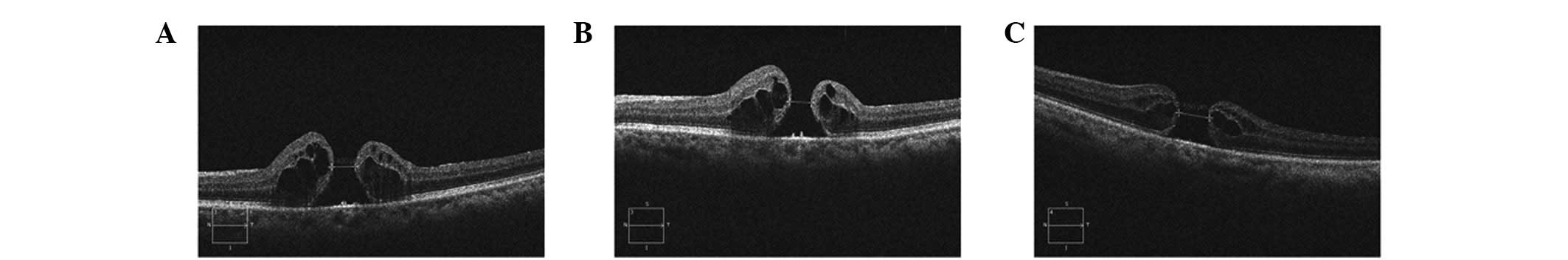
the secondary surgery to enlarge the ILM peeling (Fig. 1). In this group, the success rate
was 80% (4/5) in stage III and 50% (4/8) in stage IV. There were
five eyes in five patients in which the IMHs did not close
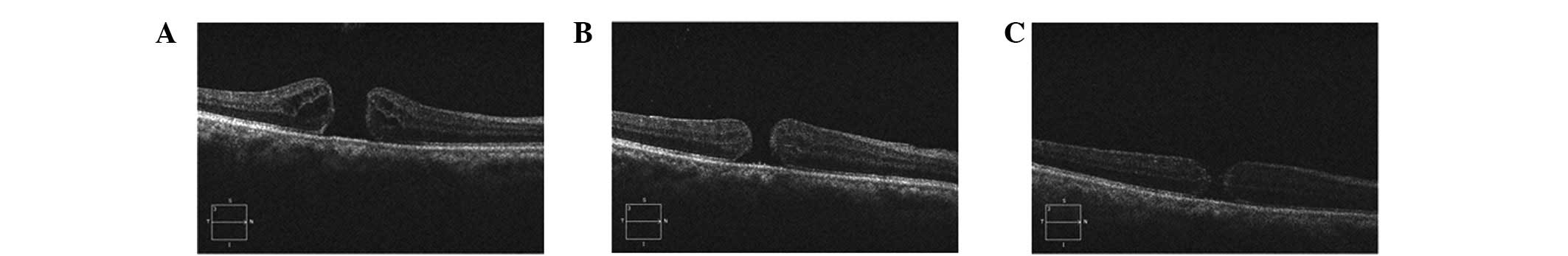
completely (Fig. 2), of which
three had similar morphology and size postoperatively. The
remaining two eyes had a retinal neurosensory layer attached to the
retinal pigment epithelium (RPE), but a smaller hole (Fig. 3).
Postsurgical visual acuity
The mean logMAR visual acuity following the primary
surgery increased from 0.94±0.19 to 0.61±0.28 (P<0.001), which
was considered to be statistically significant. Following the
secondary surgery, the mean visual acuity increased from 1.03±0.18
to 0.92±0.16 (P=0.021), a statistically significant difference.
Symptoms of metamorphopsia disappeared or were alleviated in the
eight eyes with successful anatomical closure and visual acuity was
0.98 prior to surgery and 0.84 six months following surgery, a
statistically significant difference (P=0.013). The five unclosed
eyes showed slightly improved vision 6 months following the
surgical enlargement of ILM peeling; the mean visual acuity of
1.026 was improved to 1.010. This difference was not found to be
significantly different (P=0.186).
Complications following surgery
The secondary surgeries to enlarge ILM peeling were
completed in the 13 eyes, during which there was bleeding in eight
eyes. However, the bleeding stopped spontaneously. Retinal tears or
active hemorrhage were not present during the course of the
surgeries. Patients were followed-up for 6–28 months, with a mean
follow-up duration of 13.5±1.3 months. During the follow-up period,
no complications, including bleeding or retinal tears, were
observed.
Discussion
ILM peeling surgery began in 1992 with the
hypothesis that it may be used to close IMHs and prevent them from
reopening (10). The ILM is a
layer of transparent film, located between the retina and the
vitreous humor and has been hypothesized to be composed of a
basement membrane of Müller cells (11). The ILM is thick in the area of the
macula, but extremely thin in the central fovea of the macula. It
is closely attached to the vitreous cortex. Pathologically, the ILM
supports the proliferation of RPE cells and fibroblasts. ILM
peeling surgery removes the ILM and tissue that is overexpressed
and loosens the traction in a tangential direction around the hole,
which promotes the healing of the IMH (4). This also clears support for the
proliferation of RPE and fibroblasts, avoiding the emergence of the
epiretinal membrane, which may prevent the recurrence of an IMH
(5). In addition, the trauma
produced by ILM peeling may stimulate the proliferation of
gliocytes, including Müller cells, in favor of closing the IMH
(6). However, other methods to
promote the closure of IMHs in patients who have undergone failed
surgeries must be identified to further improve vision.
In 2008, Valldeperas and Wong performed secondary
surgery with autonomous plasma and gas as a filler in patients with
failed closure of an IMH. Anatomical closure was achieved in 76%
(40/52) of the patients (7). The
difference between the authors’ findings and those of the present
study may be due to technique. In the 2008 study, ILM peeling was
rarely carried out in the initial and secondary surgeries. In 2011,
D’Souza et al reported the rate of anatomical closure as
46.7% (14/30) following secondary surgery with PPV and ILM peeling
in cases where the first surgery had failed (8). The study by D’Souza et al
described the surgeries for a number of cases that were performed
by three surgeons, but provided no detailed description of the size
of the area that was peeled in the primary and secondary surgeries.
The success rate of the surgeries to enlarge ILM peeling performed
in the present study, which was 61.5% (8/13) for IMH closure,
markedly exceeded the success rate in the study by D’Souza et
al. This difference may be associated with the extent of the
enlargement in the peeling surgery; in the current study, the
peeling extended to the vascular arcades of the posterior fundus.
It has been hypothesized that enlargement of the peeling by surgery
may loosen the tangentially directed traction coming from the
peripheral retina, which acts on the retina around the IMH. In
addition, the absence of traction for the posterior fundus is
beneficial for the neuroepithelium attached to the RPE. Also, the
trauma produced by peeling the ILM promotes the proliferation of
gliacytes, including Müller cells, which promotes the healing of
the IMH.
It was observed that visual acuity markedly
increased following the secondary closure; however, the acuity was
considerably less than that prior to IMH closure following the
primary surgery, which is consistent with a previous study by
Christensen et al (12).
Restoration of the anatomy and function of the macular
neuroepithelium is likely to result in the gradual improvement of
visual acuity between 6 and 12 months following surgery (13–15).
This indicates that the primary focus should be on closing the hole
in IMH surgeries, rather than seeking a 50:50 chance of closing the
hole without ILM peeling in the search for a slightly improved
functional outcome. In addition, certain studies have shown that
the removal of the ILM may have a possible risk of mechanical
retinal damage and toxic damage due to the use of dyes or
illumination (16–20). However, ILM removal was not
identified to have an effect on visual acuity in IMH surgery
(21).
Following the first ILM peeling surgery, the
unhealed eyes were in stage III or IV, their course of disease was
>12 months and the size of the IMH was >450 μm. These
observations were significantly different to those of the healed
eyes following the primary surgery. This indicates that the closure
of the IMH is closely associated with the stage and duration of the
disease and the size of the IMH. These results are consistent with
the study by Kumagai et al in which the rate of macular hole
closure in Asian individuals was inversely associated with the
duration of disease when the duration was >6 months and the size
of the IMH was >400 μm (22).
It remains controversial whether there is a correlation between the
success of IMH closure and the course of the disease with the size
of IMH (23,24). Further studies are required to
determine if the duration and size of the IMH affects the closing
of IMHs in Caucasians or Asians. The method in the present study
promoted the closure of IMHs in two-thirds of the unhealed IMHs and
no complications were observed during the surgeries. Therefore, it
remains questionable whether there is a need to increase the extent
of the peel from 2 DD to the size of the vascular arcades of the
posterior fundus in stage III or IV patients with a clinical course
of >12 months and an IMH size of >450 μm.
In conclusion, in cases where an IMH fails to close
following vitrectomy combined with ILM peeling surgery, a secondary
surgery to extend the peeling of the ILM to the vascular arcades of
the posterior fundus is recommended. The secondary surgery
effectively promotes the closure of the IMH and also anatomical
reset. The secondary surgery is relatively safe. The results
obtained indicate that for the patients with a clinical course of
>12 months and a macular hole size >450 μm in stages III or
IV, it may be favorable to increase the extent of the peel from 2
DD to the size of the vascular arcades during the primary surgery.
Due to the current study being a retrospective study with only a
small sample size, a prospective study is currently being conducted
to evaluate the clinical results and the safety of primary PPV
combined with enlargement of ILM peeling for patients with
long-term, large IMHs in stages III or IV.
Acknowledgements
The study was supported by grants from the National
Nature Science Foundation of China (no. 81070757) and Key
Discipline of Shanghai (no. 993020).
References
|
1
|
Kelly NE and Wendel RT: Vitreous surgery
for idiopathic macular holes. Results of a pilot study. Arch
Ophthalmol. 109:654–659. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sheidow TG, Blinder KJ, Holekamp N, Joseph
D, Shah G, Grand MG, Thomas MA, Bakal J and Sharma S: Outcome
results in macular hole surgery: an evaluation of internal limiting
membrane peeling with and without indocyanine green. Ophthalmology.
110:1697–1701. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mester V and Kuhn F: Internal limiting
membrane removal in the management of full-thickness macular holes.
Am J Ophthalmol. 129:769–777. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brooks HL Jr: Macular hole surgery with
and without internal limiting membrane peeling. Ophthalmology.
107:1939–1949. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yoshida M, Otsubo A and Kishi S: Incidence
of reopening of macular hole after successful surgery with removal
of internal limiting membrane. Jap J Clin Ophthal. 56:999–1003.
2002.
|
|
6
|
de Nie KF, Crama N, Tilanus MA, Klevering
BJ and Boon CJ: Pars plana vitrectomy for disturbing primary
vitreous floaters: clinical outcome and patient satisfaction.
Graefes Arch Clin Exp Ophthalmol. 251:1373–1382. 2013.PubMed/NCBI
|
|
7
|
Valldeperas X and Wong D: Is it worth
reoperating on macular holes? Ophthalmology. 115:158–163. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
D’Souza MJ, Chaudhary V, Devenyi R, Kertes
PJ and Lam WC: Re-operation of idiopathic full-thickness macular
holes after initial surgery with internal limiting membrane peel.
Br J Ophthalmol. 95:1564–1567. 2011.PubMed/NCBI
|
|
9
|
Meleth AD, Toy BC, Nigam D, et al:
Prevalence and progression of pigment clumping associated with
idiopathic macular telangiectasia type 2. Retina. 33:762–770. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rezende FA and Kapusta MA: Internal
limiting membrane: ultrastructural relationships, with clinical
implication for macular hole healing. Can J Ophthalmol. 39:251–259.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Steel DH and Lotery AJ: Idiopathic
vitreomacular traction and macular hole: a comprehensive review of
pathophysiology, diagnosis, and treatment. Eye (Lond). 27(Suppl 1):
S1–S21. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Christensen UC, Krøyer K, Sander B, Larsen
M, Henning V, Villumsen J and la Cour M: Value of internal limiting
membrane peeling in surgery for idiopathic macular hole stage 2 and
3: a randomised clinical trial. Br J Ophthalmol. 93:1005–1015.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Passemard M, Yakoubi Y, Muselier A, Hubert
I, Guillaubey A, Bron AM, Berrod JP and Creuzot-Garcher C:
Long-term outcome of idiopathic macular hole surgery. Am J
Ophthalmol. 149:120–126. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Leonard RE II, Smiddy WE, Flynn HW Jr and
Feuer W: Long-term visual outcomes in patients with successful
macular hole surgery. Ophthalmology. 104:1648–1652. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sanisoglu H, Sevim MS, Aktas B, Sevim S
and Nohutcu A: Outcomes of 23-gauge pars plana vitrectomy and
internal limiting membrane peeling with brilliant blue in macular
hole. Clin Ophthalmol. 5:1177–1183. 2011.PubMed/NCBI
|
|
16
|
Iriyama A, Uchida S, Yanagi Y, Tamaki Y,
Inoue Y, Matsuura K, Kadonosono K and Araie M: Effects of
indocyanine green on retinal ganglion cells. Invest Ophthalmol Vis
Sci. 45:943–947. 2004. View Article : Google Scholar
|
|
17
|
Yip HK, Lai TY, So KF and Kwok AK: Retinal
ganglion cell toxicity caused by photosensitising effects of
intravitreal indocyanine green with illumination in rat eyes. Br J
Ophthalmol. 90:99–102. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jackson TL, Hillenkamp J, Knight BC, Zhang
JJ, Thomas D, Stanford MR and Marshall J: Safety testing of
indocyanine green and trypan blue using retinal pigment epithelium
and glial cell cultures. Invest Ophthalmol Vis Sci. 45:2778–2785.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stanescu-Segall D and Jackson TL: Vital
staining with indocyanine green: a review of the clinical and
experimental studies relating to safety. Eye (Lond). 23:504–518.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Beutel J, Dahmen G, Ziegler A and Hoerauf
H: Internal limiting membrane peeling with indocyanine green or
trypan blue in macular hole surgery: a randomized trial. Arch
Ophthalmol. 125:326–332. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Terasaki H, Miyake Y, Nomura R, Piao CH,
Hori K, Niwa T and Kondo M: Focal macular ERGs in eyes after
removal of macular ILM during macular hole surgery. Invest
Ophthalmol Vis Sci. 42:229–234. 2001.PubMed/NCBI
|
|
22
|
Kumagai K, Furukawa M, Ogino N, Uemura A,
Demizu S and Larson E: Vitreous surgery with and without internal
limiting membrane peeling for macular hole repair. Retina.
24:721–727. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Freeman WR, Azen SP, Kim JW, el-Haig W,
Mishell DR III and Bailey I: Vitrectomy for the treatment of
full-thickness stage 3 or 4 macular holes. Results of a
multicentered randomized clinical trial. The Vitrectomy for
Treatment of Macular Hole Study Group. Arch Ophthalmol. 115:11–21.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Christensen UC, Krøyer K, Sander B,
Jorgensen TM, Larsen M and la Cour M: Macular morphology and visual
acuity after macular hole surgery with or without internal limiting
membrane peeling. Br J Ophthalmol. 94:41–47. 2010. View Article : Google Scholar : PubMed/NCBI
|