Introduction
Mouse testes have been established as a useful model
for studies on andrology and reproductive toxicology (1,2).
Under normal conditions, the mouse testis is comprised of a mixture
of Sertoli and germ cells that work together to accomplish
reproductive functions.
Spermatogenesis is a complex process of germ cell
proliferation and differentiation. A large number of factors affect
the process of spermatogenesis, including pathological changes of
the seminiferous epithelium, aberrant gene expression and
environmental factors (3). A
previous study indicated that pathological changes of spermatogonia
lead to impaired stretching of spermatids and damaged production of
the axoneme in rats (4).
Pathological changes of the seminiferous epithelium may cause the
disruption of Sertoli and germ cells, which results in impaired
spermatogenesis (5). Disruption of
Sertoli cell function may also lead to germ cell loss (6). Moreover, an analysis of the
seminiferous epithelium in mutant male mouse testis indicated that
spermiogenesis may undergo arrest at various steps (7). Hence, pathological changes of the
seminiferous epithelium may result in decreased spermatogenesis.
However, whether normal adult male mouse testes exhibit
pathological changes has not, to the best of our knowledge, been
reported.
The aims of the present study were to investigate
whether normal adult mouse testes exhibit pathological changes and
to evaluate the incidence of testicular abnormalities in normal
adult mice. A retrospective analysis of 720 adult male Kunming mice
testicular tissues, used in previous studies as controls, was
performed.
Materials and methods
Animals
A total of 720 healthy adult Kunming male mice (body
weight, 29–36 g; age, 9–10 weeks) were purchased from the Medical
Laboratory Animal Center (Guangzhou, China). These mice had all
been used as normal controls in previous experiments between July
2006 and October 2011, and the testicular tissue samples taken in
the previous experiments were analyzed in this retrospective study.
Mice were maintained at a controlled temperature (23–25°C) and
raised in a light-controlled room on a 12/12 h light-dark cycle.
The mice were housed in metal cages and fed a standard laboratory
diet. The protocol was approved by the Ethics Committee of Jinan
University, Guangzhou, China
Histological analysis
Mice were sacrificed by cervical dislocation and the
testes were removed and placed in a Petri dish containing
physiological saline. After washing, sections of the bilateral
testicular tissues were quickly excised and then fixed in Bouin’s
solution (Sigma, Louisville, KY, USA) for 24 h.
The samples were dehydrated and embedded in paraffin
and 4-μm thick sections were cut and placed on glass slides, which
were kept at 37°C for >12 h. The sections were immersed in xylol
to remove the paraffin and then dehydrated with a descending
alcohol series and deionized water. Finally, the sections were
stained with hematoxylin and eosin prior to histological
analysis.
Results
In total, the testes of nine mice (1.3%) exhibited
pathological changes in the study. Among the nine adult mice with
abnormal testes, two of the mice had bilateral microrchidia
(22.2%), whilst the others showed a normal testicular size. In
these abnormal mouse testes, bilateral testicular tissues showed
similar pathological changes.
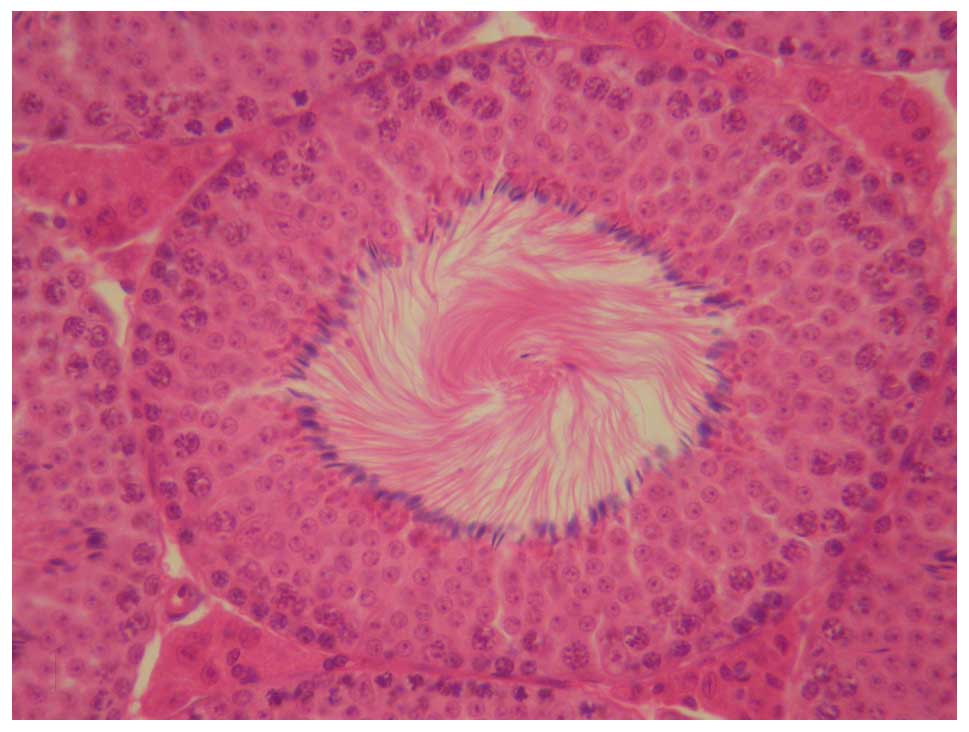
In normal testes, histological examinations
demonstrated a normal arrangement of cellular components (Fig. 1). In the mice with microrchidia,
testicular tissue showed that seminiferous epithelial vacuolation
and the absence of sperm existed in all tubules and that
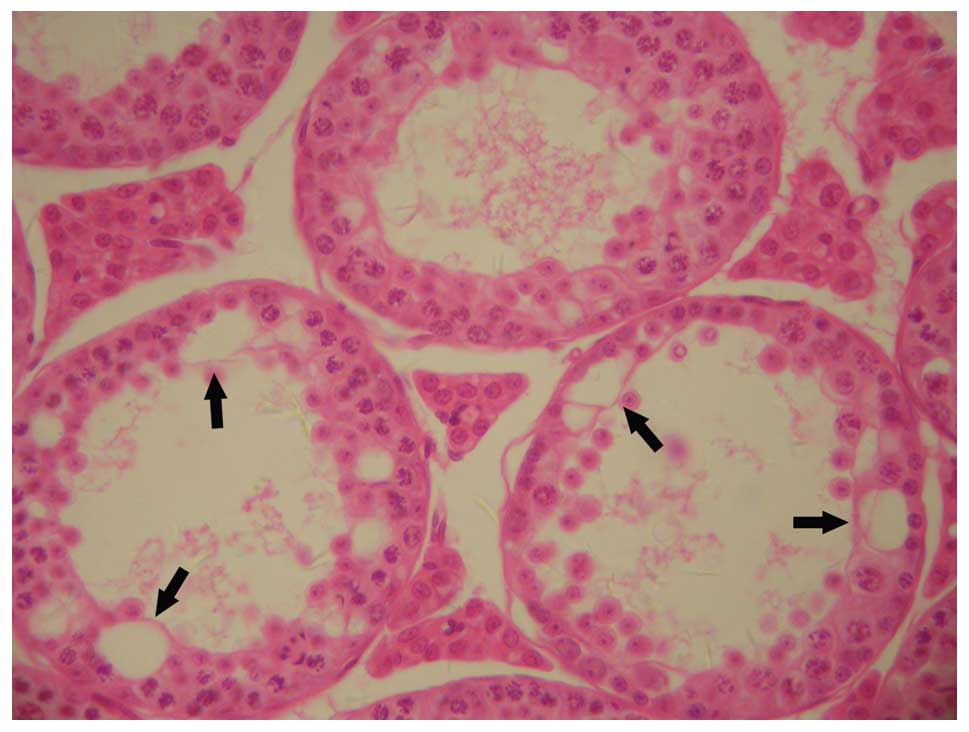
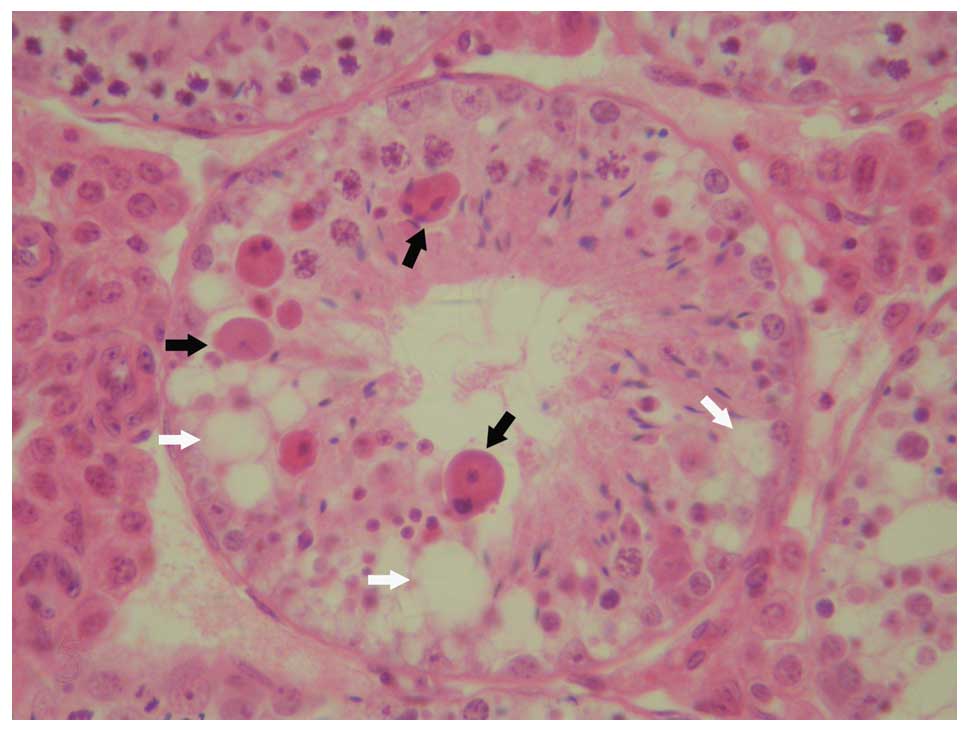
spermatogenesis had arrested at the spermatocyte stage (Fig. 2). In other abnormal testes,
testicular pathological changes included seminiferous epithelial
vacuolation, severe hypospermatogenesis, the rare presence of sperm
and the absence of seminiferous epithelium and Sertoli cells in
tubules (Fig. 3). Moderate
hypospermatogenisis was observed in tubules (Fig. 4), as well as seminiferous
epithelial vacuolation and a small number of symplasts composed of
collapsed spermatids (Fig. 5).
Discussion
The results of the present study are, to the best of
our knowledge, the first to show that normal adult male mouse
testes exhibit pathological changes, with an incidence of
testicular abnormality of 1.3%. The histological changes may induce
spermatogenic disorders and an absence of sperm and pathological
changes of the male mouse testes may cause male infertility.
Abnormalities in spermatogenesis promote germ cell
apoptosis, which results in spermatogenetic arrest (8). The spermatogenetic arrest, at various
stages of spermatogenesis, causes subfertility or infertility and
may be associated with genetic abnormalities (9). Various aspects of spermatogenetic
arrest have also been reported and correlated with various possible
mechanisms of meiotic abnormalities (10). Moreover, there are a variety of
factors that have been demonstrated to cause spermatogenetic
arrest, including genetic mutation, environmental factors and
hormone deficiency (11,12). Testis weight is also an important
indicator of overall testicular health, reflecting changes in germ
cell loss (13). In the present
study, in which the mice were reared in the same environment and
conditions, histological examination showed that spermatogenesis
was arrested at the spermatocyte stage. Additionally, microrchidia
may also cause spermatogenetic arrest in the adult male mouse.
Autosomal recessive mutation of the microrchidia (morc) gene
results in the complete arrest of spermatogenesis at an early
meiotic stage (14). Hence, we
hypothesized that a spontaneous mutation is a possibility but morc
mutation is more likely. In addition, other possibilities require
consideration, including acute febrile disease, hyperthermia and
other systemic insults/diseases.
Vacuolar changes of the seminiferous epithelia were
observed to occur in normal mouse testicular tissues in the present
study. Similar pathological changes of testicular tissues have also
been reported in previous toxicological studies (15,16).
Spermatogenic epithelial vacuolation in testis has been observed to
decrease the number of testicular sperm (17). Moreover, seminiferous epithelial
vacuolation is common in affected tubules, particularly near the
rete, indicative of a breakdown in Sertoli-germ cell junctions
(18). These factors indicate that
pathological changes of the seminiferous epithelia in testes may
lead to hypospermatogenesis. However, the etiology of vacuolation
in testicular tissue is unknown. Vacuolation may be associated with
abnormal gene expression (19,20).
Mouse reproductive tract diseases may also cause seminiferous
epithelial vacuolation (21).
Therefore, abnormal testes should be identified when using normal
adult mouse testes for studies of seminiferous epithelia.
The potential limitations of the present study
require consideration. Firstly, this study was a retrospective
analysis. It was not possible to examine whether the pathological
changes of testicular tissues correlated with changes in other
reproductive organs in normal adult mice, including the epididymis,
prostate, seminal vesicles and urethral anatomy. Secondly, there
were no surplus samples of abnormal testicular tissues for further
analysis at the molecular and genetic levels. If possible, more
studies of testicular tissues are likely to not only aid the
understanding of pathogenesis of abnormal testes, but also provide
new information concerning the etiology of developmental
defects.
In conclusion, the results show that normal adult
male mice exhibit testicular pathological changes. Therefore, more
attention should be paid to the possibility of abnormal testes when
using normal adult male mice to establish a testicular experimental
model.
References
|
1
|
Muczynski V, Cravedi JP, Lehraiki A, et
al: Effect of mono-(2-ethylhexyl) phthalate on human and mouse
fetal testis: In vitro and in vivo approaches. Toxicol Appl
Pharmacol. 261:97–104. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nagano M, Patrizio P and Brinster RL:
Long-term survival of human spermatogonial stem cells in mouse
testes. Fertil Steril. 78:1225–1233. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Beissbarth T, Borisevich I, Hörlein A, et
al: Analysis of CREM-dependent gene expression during mouse
spermatogenesis. Mol Cell Endocrinol. 212:29–39. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Merker HJ and Günther T: Testis damage
induced by zinc deficiency in rats. J Trace Elem Med Biol.
11:19–22. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vernet N, Dennefeld C, Guillou F, Chambon
P, et al: Prepubertal testis development relies on retinoic acid
but not rexinoid receptors in Sertoli cells. EMBO J. 25:5816–5825.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moffit JS, Bryant BH, Hall SJ and
Boekelheide K: Dose-dependent effects of sertoli cell toxicants
2,5-hexanedione, carbendazim, and mono-(2-ethylhexyl) phthalate in
adult rat testis. Toxicol Pathol. 35:719–727. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nantel F, Monaco L, Foulkes NS, et al:
Spermiogenesis deficiency and germ-cell apoptosis in CREM-mutant
mice. Nature. 380:159–162. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shaha C, Tripathi R and Mishra DP: Male
germ cell apoptosis: regulation and biology. Philos Trans R Soc
Lond B Biol Sci. 365:1501–1515. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shivanandappa T and Krishnakumari MK:
Hexachlorocyclohexane-induced testicular dysfunction in rats. Acta
Pharmacol Toxicol (Copenh). 52:12–17. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Johannisson R, Schulze W and Holstein AF:
Megalospermatocytes in the human testis exhibit asynapsis of
chromosomes. Andrologia. 35:146–151. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Carrell DT, De Jonge C and Lamb D: The
genetics of male infertility: a field of study whose time is now.
Arch Androl. 52:269–274. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Toshimori K, Ito C, Maekawa M, Toyama Y,
et al: Impairment of spermatogenesis leading to infertility. Anat
Sci Int. 79:101–111. 2004. View Article : Google Scholar
|
|
13
|
Elkis Y, Bel S, Lerer-Goldstein T, et al:
Testosterone deficiency accompanied by testicular and epididymal
abnormalities in TMF(−/−) mice. Mol Cell Endocrinol. 365:52–63.
2013.PubMed/NCBI
|
|
14
|
Watson ML, Zinn AR, Inoue N, et al:
Identification of morc (microrchidia), a mutation that results in
arrest of spermatogenesis at an early meiotic stage in the mouse.
Proc Natl Acad Sci USA. 95:14361–14366. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Singh SK and Chakravarty S:
Antispermatogenic and antifertility effects of
20,25-diazacholesterol dihydrochloride in mice. Reprod Toxicol.
17:37–44. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mahmoud YI: Effect of extract of Hibiscus
on the ultrastructure of the testis in adult mice. Acta Histochem.
114:342–348. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hentrich A, Wolter M, Szardening-Kirchner
C, et al: Reduced numbers of Sertoli, germ, and spermatogonial stem
cells in impaired spermatogenesis. Mod Pathol. 24:1380–1389. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liao W, Cai M, Chen J, et al: Hypobaric
hypoxia causes deleterious effects on spermatogenesis in rats.
Reproduction. 139:1031–1038. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chuma S, Hosokawa M, Kitamura K, et al:
Tdrd1/Mtr-1, a tudor-related gene, is essential for male germ-cell
differentiation and nuage/germinal granule formation in mice. Proc
Natl Acad Sci USA. 103:15894–15899. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Paiardi C, Pasini ME, Gioria M and Berruti
G: Failure of acrosome formation and globozoospermia in the wobbler
mouse, a Vps54 spontaneous recessive mutant. Spermatogenesis.
1:52–62. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Seachrist DD, Johnson E, Magee C, et al:
Overexpression of follistatin in the mouse epididymis disrupts
fluid resorption and sperm transit in testicular excurrent ducts.
Biol Reprod. 87:412012. View Article : Google Scholar : PubMed/NCBI
|