Introduction
Chondrocytes in articular cartilage are
differentiated from mesenchymal cells during embryonic development
(1–3). The differentiated chondrocytes are
able to proliferate and undergo hypertrophic maturation. Cartilage
is composed of a dense extracellular matrix, made up from
macromolecules such as type II collagen, sulfated proteoglycan and
fibronectin (4). This biosynthetic
composition of chondrocytes is maintained during complex biological
processes, including cartilage development, differentiation and
repair. However, the differentiated chondrocyte phenotype is
unstable in culture and destroyed in degenerative diseases, such as
osteoarthritis (OA) and rheumatoid arthritis (RA) (5–9).
Cyclooxygenase (COX) is an enzyme that catalyzes the
conversion of arachidonic acid to prostaglandin H2, the
precursor of a variety of biologically active mediators such as
prostaglandin E2 (PGE2), prostacyclin and
thromboxane A2 (10,11). Two isoforms of COX are COX-1 and
COX-2. COX-1 is constitutively expressed in a wide variety of
tissues, is ubiquitous in its distribution, and is thought to be
involved in tissue homeostasis and maintenance of the levels of
prostaglandins. COX-2 is an enzyme induced by pro-inflammatory
cytokines, tumor promoters, oncogenes and growth factors, and is
involved mainly in the regulation of inflammatory responses in
numerous types of cell, such as monocytes, fibroblasts and
endothelial cells (2,12).
Resveratrol
(C14H12O3;
3,5,4′-trihydroxy-trans-stilbene) was first identified in
the roots of white hellebore (Veratrum grandiflorum) in 1940
(13). Resveratrol is a natural
polyphenolic compound that is also found in the skin of red grapes,
cranberries and peanuts, and the root extracts of the weed
Polygonum cuspidatum (14–16).
Numerous signaling pathways involving resveratrol have been
evaluated and a number of its targets and mechanisms of action have
been identified. It has been reported that resveratrol has
antitumor activity and immunomodulatory, antioxidative and
anti-inflammatory functions, as well as numerous biological
activities. Resveratrol has been shown to exhibit in vitro
as well as in vivo chemopreventive and chemotherapeutic
activities (15,17–20).
Elmali et al observed a significant
protective effect of resveratrol injections on articular cartilage
degradation in rabbit models for OA and RA via histological
analysis in vivo (21).
Resveratrol has been demonstrated to suppress aging by activating
the SIRT1 gene, which suppresses cell apoptosis (22–26).
In human articular chondrocytes, Czaki et al elucidated
anti-apoptotic and anti-inflammatory regulatory mechanisms mediated
by resveratrol (27). In human
articular chondrocytes, resveratrol together with curcumin was
shown to suppress the apoptosis induced by IL-1β through
stimulation of the mitogen-activated protein kinase (MAPK)
signaling pathway (27).
However, the effects of resveratrol on
differentiation and the inflammatory response in normal cells,
including chondrocytes, and the mechanism by which resveratrol acts
are not clearly understood. As a result, the present study was
conducted to investigate the effects of resveratrol on
differentiation and the inflammatory response of rabbit
chondrocytes and to analyze the subsequently regulated
intracellular signal transduction pathways.
Materials and methods
Reagents and antibodies
Resveratrol was purchased from Sigma-Aldrich (St.
Louis, MO, USA). The resveratrol was diluted in sterile
dimethylsulfoxide (Sigma-Aldrich; final concentration in the medium
was >1%) and stored at −20°C. Dulbecco’s modified Eagle’s medium
(DMEM) and fetal bovine serum (FBS) were purchased from Invitrogen
(Burlington, ON, Canada). Streptomycin, penicillin and SP600125
were obtained from Sigma-Aldrich. SB203580 (SB), PD98059 (PD),
LY294002 (LY) and triciribine (TB) were purchased from Calbiochem
(San Diego, CA, USA). Type II collagen, actin, COX-2 and pERK
antibodies were obtained from Santa Cruz Biotechnology Inc. (Santa
Cruz, CA, USA) and pAkt, p38 and pJNK were from Cell Signaling
Technology (Danvers, MA, USA).
Cell culture
Rabbit articular chondrocytes were isolated from the
cartilage of two-week-old New Zealand white rabbits (KOATECH,
Pyeongtaek-si, Gyeonggi-do, Korea) using enzymatic digestion, as
described previously (28). The
cartilage slices were dissociated enzymatically for 6 h in 0.2%
collagenase type II (381 U/mg solid; Sigma-Aldrich) in DMEM.
Following collection of individual cells by brief centrifugation at
230 × g for 10 min and 20°C, the cells were suspended in DMEM
supplemented with 10% (v/v) FBS, 50 μg/ml streptomycin and 50 U/ml
penicillin. The cells were then plated on culture dishes at a
density of 5×104 cells/cm2. The medium was
changed every two days, and the cells reached confluence after
approximately five days. After three days the cell cultures were
treated with resveratrol. The following pharmacological agents were
added 1 h prior to the addition of resveratrol: SB to inhibit p38
kinase, PD to inhibit ERK, and LY and TB to inhibit
phosphoinositide 3-kinase (PI3K) and Akt, respectively. The study
was approved by the Ethics Committee of Kongju National University
(Gongju, Republic of Korea; IRB no. 2011–2).
Western blot analysis
Whole cell lysates were prepared by extracting
proteins using a cold radioimmunoprecipitation assay buffer [50 mM
Tris-HCl, pH 7.4; 150 mM NaCl; 1% Nonidet P-40; and 0.1% sodium
dodecylsulfate (SDS); supplemented with protease inhibitors (10
μg/ml leupeptin, 10 μg/ml pepstatin A, 10 μg/ml aprotinin and 1 mM
4-(2-aminoethyl)benzenesulfonyl fluoride and phosphatase inhibitors
(1 mM NaF and 1 mM Na3VO4)] obtained from
Sigma-Aldrich. The lysates were size-fractionated by
SDS-polyacrylamide gel electrophoresis and transferred to a
nitrocellulose (NC) membrane (Whatman Schleicher and Schuell,
Dassel, Germany). The NC sheet was then blocked with 5% non-fat dry
milk in Tris-buffered saline. Antibodies against type II collagen,
COX-2, pp38, pERK, pJNK and Akt were used for probing corresponding
NC blots overnight at 4°C. The membranes were then washed three
times with Tris-buffered saline/Tween-20 and incubated with
horseradish peroxidase-conjugated secondary antibody
(Sigma-Aldrich) for 2 h followed by exposure in an LAS-4000 imager
(Fujifilm Corp., Tokyo, Japan) according to the manufacturer’s
instructions. The experimental results were transformed into in
numerical values using Image J 1.41 (Software Inquiry, Quebec,
Canada).
PGE2 assay
PGE2 production in the articular
chondrocytes was determined by measuring the levels of cellular and
secreted PGE2 with an assay kit purchased from Assay
Design Inc. (Ann Arbor, MI, USA). A PGE2 linked
immunosorbent assay kit was purchased from Amersham Pharmacia
Biotech (Piscataway, NJ, USA). Briefly, the chondrocytes were
seeded in standard 96-well microtiter plates at a density of
2×104 cells/well and treated with various reagents, such
as resveratrol, for 1 h prior to treatment with SB, PD, LY and TB
for 3 h after treatment with resveratrol in the absence or presence
of inhibitors (SB, PD, LY and TB). The amount of PGE2
present in total cell lysates was quantified according to the kit
manufacturer’s instructions. Levels were calculated against a
standard curve of PGE2.
Immunofluorescence staining
The expression levels and distribution of type II
collagen and COX-2 in the rabbit articular chondrocytes were
determined by indirect immunofluorescence microscopy, as described
previously (28). Rabbit
chondrocytes were fixed with 3.5% paraformaldehyde in
phosphate-buffered saline (PBS) for 15 min at room temperature, and
permeabilized and blocked with 0.1% Triton X-100 and 5% fetal calf
serum in PBS for 30 min. The fixed cells were washed three times
with PBS and incubated for 2 h with antibodies against type II
collagen (Santa Cruz Biotechnology, Inc.) or COX-2 (Cayman
Chemical, Ann Arbor, MI, USA). The cells were washed and incubated
with rhodamine or fluorescein-conjugated secondary antibodies for 1
h, washed with PBS, and observed under a fluorescence microscope
(Olympus, Tokyo, Japan).
Immunohistochemical staining
The cartilage explants (125 mm3) were
fixed in 4% paraformaldehyde in PBS for 24 h at 4°C, washed with
PBS, dehydrated with graded ethanol, embedded in paraffin and
sectioned into 4-μm slices as described previously (28). The sections were stained by the
standard procedures using antibodies against type II collagen or
COX-2, and visualized by development with an EnVisionTM+ kit
purchased from Dako (Carpinteria, CA, USA) following the
manufacturer’s instructions.
Determination of chondrocyte
phenotype
The cells were fixed with 95% methanol at −20°C for
2 min and stained with 0.1% Alcian blue (Sigma Aldrich) in 0.1 M
HCl overnight. The chondrocytes were washed three time with PBS
buffer and 6 M guanidine HCl was added for 6 h. Production of
sulfated proteoglycan was measured at 620 nm by an enzyme-linked
immunosorbent assay.
Data analysis and statistics
The results are expressed as the mean ± standard
deviation. The values were calculated from the specified number of
determinations. The significance of the differences between the
experimental and control groups was assessed by one-way ANOVA. A
value of P<0.05 was considered to indicate a statistically
significant difference.
Results
Resveratrol regulates the differentiation
of chondrocytes
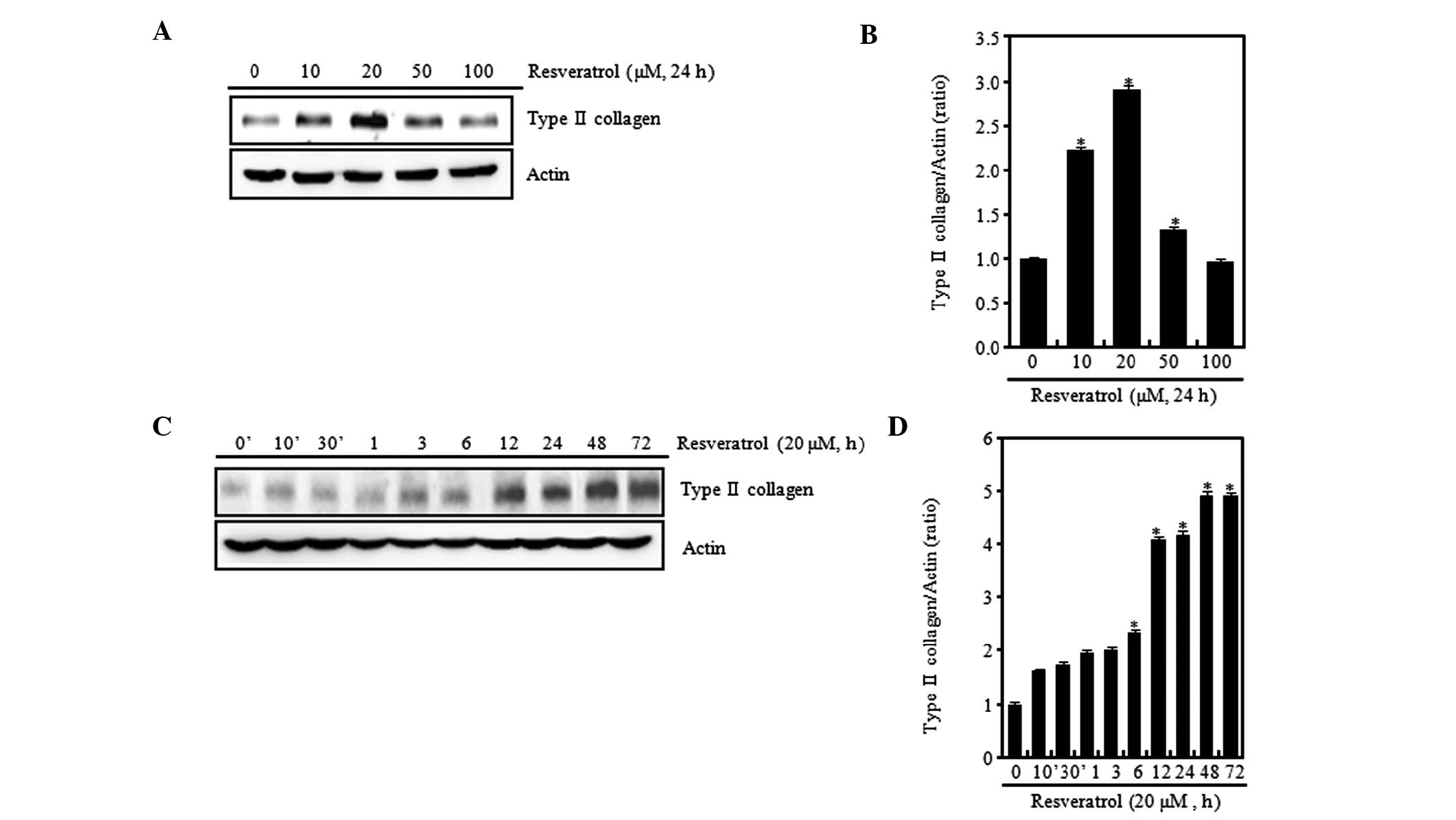
An aim of this study was to determine whether
resveratrol regulates the expression of type II collagen and
sulfated proteoglycan in rabbit articular chondrocytes (Fig. 1). Various concentrations of
resveratrol were tested, and at low concentrations (≤20 μM) it was
found that the expression levels of type II collagen gradually
increased in a concentration-dependent manner when compared with
those in the control chondrocytes without resveratrol treatment.
However, at a high concentration (100 μM), the expression level of
type II collagen was observed to be reduced (Fig. 1A). When cells were treated with 20
μM resveratrol, type II collagen expression levels were increased
in a time-dependent manner (Fig.
1C). The expression levels were determined using ImageJ
software (Fig. 1B and D). Alcian
blue staining was used to identify the levels of sulfated
proteoglycan, which is an extracellular substrate molecule commonly
used as another marker protein for differentiation of chondrocytes.
Similar to type II collagen expression, the Alcian blue staining
results showed that the levels of proteoglycan increased compared
with those in the control when the chondrocytes were treated with
20 μM resveratrol, while they were less elevated when the
chondrocytes were treated with 100 μM resveratrol (Fig. 2A). Moreover, it was observed that
the changes in the proteoglycan levels in relation to time also
started to increase from 24 h after the addition of resveratrol,
similar to the effect on the expression of type II collagen
(Fig. 2B). In order to verify the
aforementioned results at chondrocytic cellular and tissue levels,
immunofluorescence and immunohistochemical staining assays were
performed to identify the levels of type II collagen and
proteoglycan. As a result of performing immunofluorescence staining
using type II collagen antibody following treatment with 20 μM
resveratrol for 24 h, increased expression levels of type II
collagen in resveratrol-treated cells were confirmed (Fig. 2C). Moreover, at the cartilaginous
tissue level, increased expression levels of type II collagen were
confirmed (Fig. 2D, upper panel).
In addition, the results of performing Alcian blue staining
following treatment of the cartilaginous tissue with 20 μM
resveratrol for 24 h showed increased levels of proteoglycan in the
resveratrol-treated tissues (Fig.
2D, lower panel). In general reference to the aforementioned
results, it was found that chondrocytic differentiation was
regulated differentially according to the concentration of
resveratrol, and inducement of chondrocytic differentiation was
identified after 24 h of treatment with resveratrol at a low
concentration (20 μM).
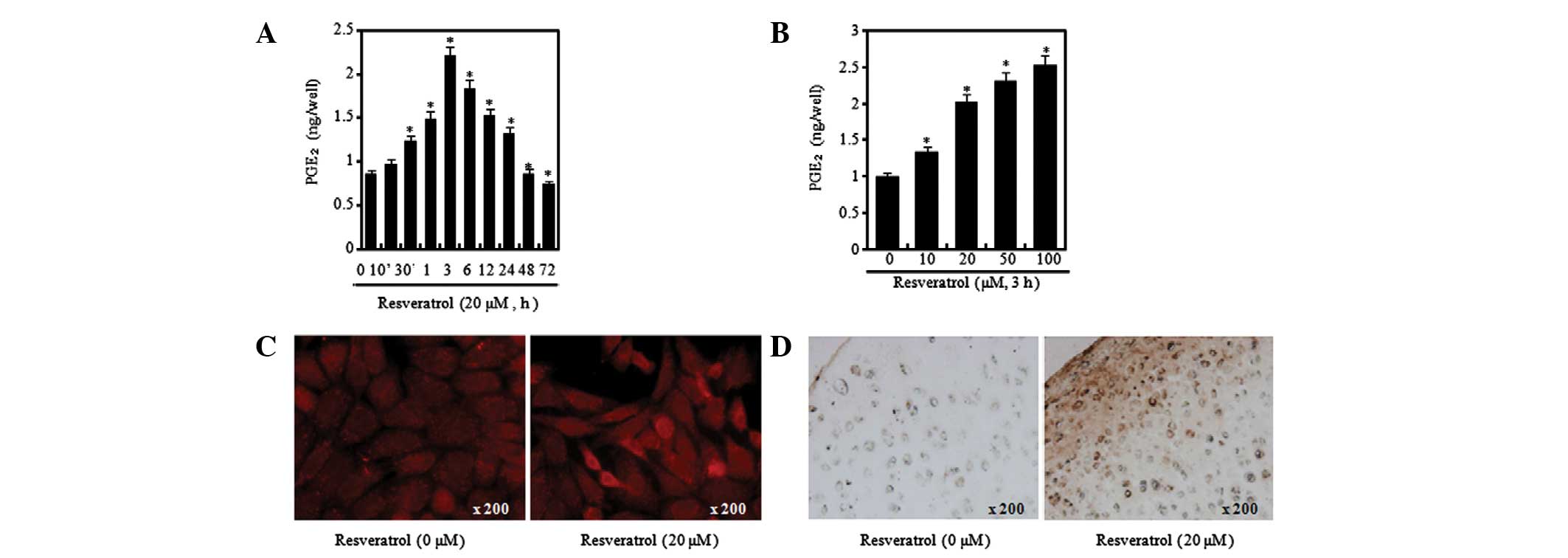
Resveratrol induces an inflammatory
response in chondrocytes
Previous studies have shown that the expression of
COX-2 increases the levels of PGE2 and that
PGE2 induces various inflammatory reactions (10,29).
In the present study, chondrocytes were treated with resveratrol at
20 μM for different time periods (Fig.
3A and B) or with various concentrations of resveratrol for 3 h
(Fig. 3C and D). Stimulation of
cells with resveratrol induced a marked increase in COX-2
expression levels, which was apparent within 3 h after treatment
with resveratrol. The COX-2 expression levels peaked at 3 h and the
subsequent reduction was detectable for up to 72 h (Fig. 3A and B). Concentration-dependent
increases in COX-2 expression levels were measured by western blot
analysis and densitometric analysis (Fig. 3C and D). In order to find out more
clearly whether or not resveratrol induces an inflammatory response
in chondrocytes, a PGE2 assay was performed to evaluate
the levels of PGE2, which is a product of COX-2, and
changes in the expression levels of COX-2 at chondrocytic cellular
and tissue levels were identified through immunofluorescence and
immunohistochemical staining (Fig.
4). As a result of performing the PGE2 assay
following the treatment of chondrocytes with resveratrol at
different concentrations and time periods, it was possible to
verify that the levels of PGE2 greatly increased at 3 h
after treatment with 20 μM resveratrol but had begun to decrease by
6 h, in a similar manner to COX-2 (Fig. 4A). Moreover, it was confirmed that
the PGE2 production levels were increased by resveratrol
in a concentration-dependent manner (Fig. 4B). In order to identify the levels
of COX-2 expression at the chondrocytic cell and tissue levels,
immunofluorescence and immunohistochemical staining was conducted.
Increased COX-2 expression levels were observed in the cells and
tissues treated with resveratrol (Fig.
4C and D). The aforementioned results signify that resveratrol
induces an inflammatory response in chondrocytes.
Resveratrol increases the activation of
MAPK and Akt
To investigate through which signal transduction
system the chondrocytic differentiation and inflammatory response
due to resveratrol are regulated, the activation of MAPK and PI3K
was examined. The MAPK and PI3K signal transduction pathways are
closely associated with the regulation of chondrocytic
differentiation and the inflammatory response (Fig. 5). The results confirmed an increase
in activation of MAPK-related proteins (ERK, p38 and JNK) and Akt
due to resveratrol (Fig. 5A). To
study the changes in the expression levels of the proteins
associated with cellular signal transduction according to treatment
time, western blot analysis was performed following treatment with
20 μM of resveratrol for various time periods up to 72 h. The
results showed that the activation levels of MAPK-related proteins
(ERK, p38 and JNK) started to increase from 6 h and those of Akt
started to increase from 24 h after the resveratrol treatment
(Fig. 5B). Such results signify
that chondrocytic differentiation and the inflammatory response
induced by resveratrol are associated with activation of MAPK and
Akt. Accordingly, inhibitors of MAPK-related proteins (ERK
inhibitor, PD; p38 inhibitor, SB; JNK inhibitor, SP), TB (an
inhibitor of Akt), and LY (an inhibitor of PI3K; the PI3K pathway
is an upstream signal transduction pathway for Akt), were used to
clearly identify the signal transduction pathways that are
regulated by resveratrol. The chondrocytes first underwent
pretreatment 1 h prior to resveratrol treatment to block the ERK,
p38, JNK and Akt signaling pathways and were then treated with
resveratrol, following which changes to chondrocytic
differentiation and the inflammatory response proteins were studied
through western blot analysis, immunofluorescence staining, Alcian
blue staining and PGE2 assay (Fig. 6 and 7). The results of these assays showed
that the resveratrol-induced increases in the expression levels of
type II collagen and COX-2 were attenuated by treatment with SB,
PD, LY and TB (Fig. 6). However,
no changes to the levels of type II collagen and COX-2 expression
due to SP treatment were observed (data not shown). Following
verification using Alcian blue staining, immunofluorescence
staining and PGE2 assay methods, the resveratrol-induced
increases in the levels of proteoglycan and expression levels of
type II collagen and COX-2 were observed to be similarly attenuated
by PD, SB, TB and LY treatment (Fig.
7). Such results indicate that the differentiation and
inflammatory response induced by resveratrol are mediated through
the ERK, p38 and Akt signal transduction pathways.
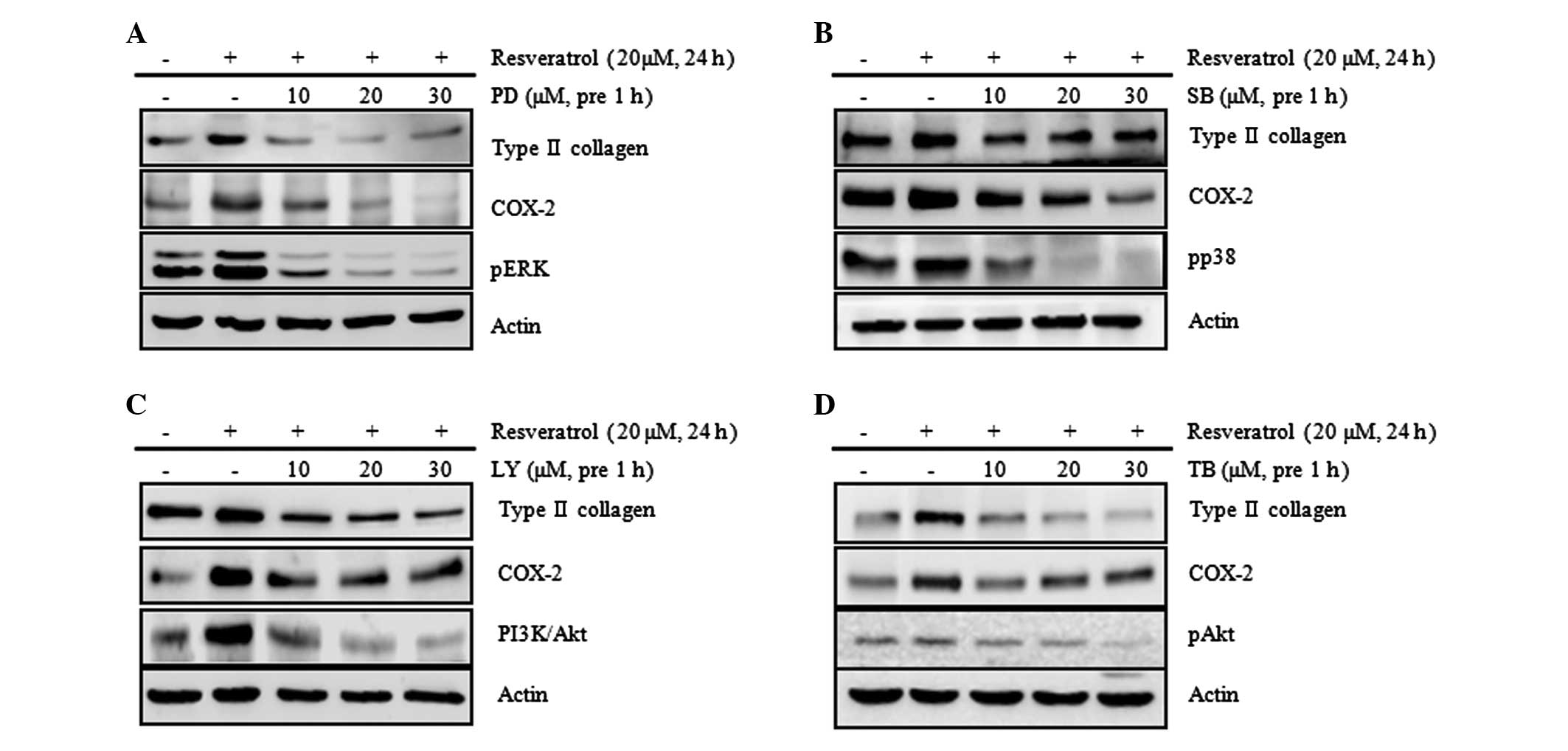 | Figure 6Resveratrol induces type II collagen
and COX-2 expression via the ERK, p38 and Akt signaling pathways in
rabbit articular chondrocytes. Rabbit articular chondrocytes were
untreated or treated with the indicated concentrations of the
inhibitors: (A) PD, an inhibitor of ERK; (B) SB, an inhibitor of
p38; (C) LY, an inhibitor of PI3K/Akt; or (D) TB, an inhibitor of
Akt for 1 h and then treated with 20 μM resveratrol for 24 h.
Expression of pERK, pp38, pAkt, type II collagen and COX-2 was
detected by western blot analysis. Expression of actin was used as
the loading control. The data represent the results of a typical
experiment from at least four independent experiments. PD, PD98059;
SB, SB203580; LY, LY294002; TB, triciribine; COX-2,
cyclooxygenase-2; ERK, extracellular signal-regulated kinase; PI3K,
phosphoinositide 3-kinase. |
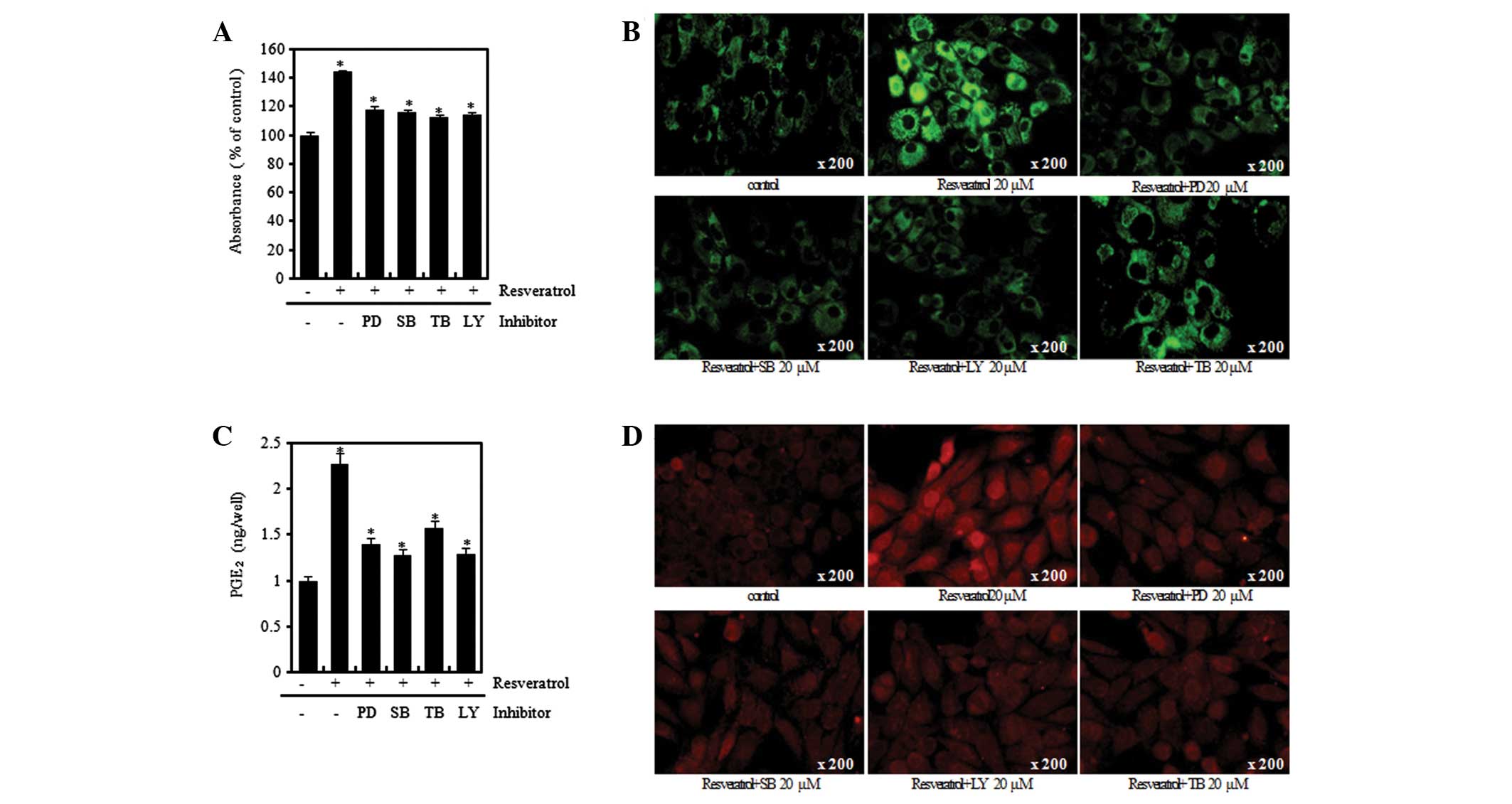 | Figure 7Resveratrol (Res) induces
differentiation and inflammation in rabbit articular chondrocytes.
(A) Rabbit articular chondrocytes were untreated or treated with
the 20 μM of inhibitors (PD98059, SB203580, LY294002) and
triciribine (TB) for 1 h and then treated with 20 μM Res for 24 h.
Accumulation of sulfated proteoglycan was determined by Alcian blue
staining. Expression of (B) type II collagen and (D) COX-2 was
determined by immunofluorescence staining (magnification, ×200).
(C) Rabbit articular chondrocytes were untreated or treated with
the 20 μM of inhibitors (PD98059, SB203580, LY294002) and
triciribine (TB) for 1 h and then treated with 20 μM Res for 3 h.
*P<0.05, compared with untreated cells. PD, PD98059;
SB, SB203580; LY, LY294002; TB, triciribine; COX-2,
cyclooxygenase-2; PGE2, prostaglandin E2. |
Discussion
Resveratrol, one of the major stilbenes, has a
structure that is related to the synthetic estrogen
diethylstilbestrol. It comprises two phenol rings linked by a
styrene double bond and exists in two isoforms (19,30).
Resveratrol has been demonstrated to process anticancer,
anti-aging, anti-inflammatory and neuroprotective activities
(31). Resveratrol has also been
found to exhibit diverse biological effects; it induces MMP-9
expression and cell migration via the p38 kinase and PI3K pathway
in HT1080 human fibrosarcoma cells (32) and induces differentiation via
reduction of the expression of MMPs; this regulation is mediated by
the p38 and JNK pathway in HTB94 human chondrosarcoma cells.
However, the essential cellular and molecular
targets and a signaling mechanism for resveratrol have not been
completely defined. Although type II collagen and sulfated
proteoglycan are important for differentiation and COX-2 is
significant in the inflammatory response, the underlying regulatory
mechanisms of type II collagen and COX-2 in articular chondrocytes
are not yet understood. In the present study, the effects of
resveratrol on the expression of type II collagen and COX-2 in
rabbit articular chondrocytes were investigated and the regulatory
mechanisms involved in these effects were investigated.
Resveratrol induces apoptosis or anti-proliferative
effects in a variety of cell types, including prostate, breast,
lung, leukemia, bladder and ovarian cancer cells (33). It prevents the proliferation of
tumor cells by inhibiting DNA synthesis and cell cycle progression
and by modulating a series of signaling molecules (17). Resveratrol reduces cell apoptosis
and the inflammatory response induced by inflammatory cytokines and
inhibits dedifferentiation in arthritic chondrocytes (30,34).
In the present study, it was found that resveratrol inhibited the
proliferation of rabbit articular chondrocytes (data not
shown).
Studies have identified that the diverse effects of
resveratrol are regulated differentially according to numerous
conditions, such as the concentration of resveratrol and the
treatment period (16–18). At a higher dose, resveratrol is
pro-apoptotic, inducing apoptosis in cancer cells by exerting a
death signal. In addition, at a higher dose, resveratrol depresses
cardiac function, elevates the levels of apoptotic protein
expression, which results in an unstable redox environment, and
increases myocardial infarct size and the number of apoptotic cells
(17). The expression levels of
proteins associated with cell survival are increased, which results
in anti-apoptotic effects, when cells are treated with a low dose
of resveratrol (16). Studies have
indicated that it may be possible to use resveratrol to prevent and
treat OA. For example, Shakibaei et al characterized the
effects of IL-1β-induced suppression of collagen type II and
β1-integrin signal receptor synthesis, and observed that the
activation of caspase-3 and PARP cleavage were blocked by
resveratrol (35,36). A study has suggested that
resveratrol directly blocks caspase-3 and the subsequent cleavage
of PARP and reverses the IL-1β-induced upregulation of ROS in
chondrocytes (35). Furthermore,
resveratrol inhibits the activation of NF-κB and thus downregulates
NF-κB-regulated pro-inflammatory gene products such as COX-2, IL-1β
and IL-6, which are important in the pathogenesis of OA (37). In the present study, it was
demonstrated that a low concentration of resveratrol promotes
differentiation, but treatment with a high concentration of
resveratrol results in inducement of dedifferentiation (Figs. 1A and 2A), and resveratrol significantly induces
the expression of type II collagen in a time-dependent manner
(Figs. 1C and 2B). Treatment of rabbit articular
chondrocytes with resveratrol was shown to induce the expression of
COX-2 and increase PGE2 production in an dose-dependent
manner, and the highest expression levels of COX-2 and
PGE2 production were observed at 3 h after treatment
with resveratrol (Figs. 3 and
4).
MAPK cascades have been shown to be key in the
transduction of extracellular signals to cell responses. The MAPK
signaling pathways relay, amplify and integrate signals from a wide
range of stimuli prior to eliciting an appropriate physiological
response that may include cell growth, proliferation,
differentiation, development, inflammatory responses, apoptosis and
invasion in mammalian cells (38,39).
The PI3K/Akt signaling pathway is important for cell growth,
differentiation and survival (40). In previous studies, chondrocyte
differentiation and the inflammatory response were demonstrated to
be associated with the MAPK and PI3K/Akt signaling pathways
(41,29). Although the precise mode of
resveratrol action has not yet elucidated, a few signaling pathways
and molecular targets have been suggested. In several types of
tumor cell line, resveratrol has inhibited the activation of JNK
and its upstream MAPK/ERK and MEK (14). It has been reported that apoptosis
through activation of p53, which is one of the chemotherapeutic
effects of resveratrol, occurs through ERK/p38 (42). In addition, studies have
demonstrated that resveratrol inhibits IL-1β-induced expression of
COX-2 and production of PGE2, causing inhibition of the
expression of cartilage-specific collagen type II (43,44).
Resveratrol has been shown to induce apoptotic cell death, and
suppression of pro-survival PI-3K/Akt signaling may be an important
mediator in this process (25). In
the present study, resveratrol activated all the ERK, p38, JNK and
Akt signaling pathways that belong to the MAPK signaling system
(Fig. 5). Therefore, in order to
elucidate the association of these signaling systems with cell
differentiation and the inflammatory response due to resveratrol,
the ERK, p38, JNK and Akt signal transduction pathways were
attenuated with their respective inhibitors, PD, SB, SP, TB and LY,
following which the expression levels of type II collagen and COX-2
and the synthesized levels of proteoglycan and PGE2 were
observed. The results showed that, with the exception of SP
treatment, the increased type II collagen and COX-2 expression
levels and increased levels of proteoglycan and PGE2
were attenuated following treatment with PD, SB, TB and LY
(Figs. 6 and 7).
These results suggest that the differentiation and
inflammatory response induced by resveratrol in rabbit articular
chondrocytes are regulated through the ERK, p38 and Akt signaling
pathways. Since various signaling pathways in addition to the MAPK
and Akt signaling pathways, such as the PKC pathway, are associated
with the regulation of intrachondrocytic reactions, further
detailed studies are required. In addition, as the mechanisms
behind the dedifferentiation induced by treatment with a high
resveratrol concentration and the suppressed inflammatory response
following exposure to resveratrol for long time periods are not
known, these also require further investigation. Such study results
may be used as fundamental data for the therapy of chondrocytic
illnesses such as arthritis.
Acknowledgements
This study was supported by the Korean Health
Technology R&D Project, Ministry of Health & Welfare,
Republic of Korea (A120960-1201-0000300).
References
|
1
|
Abramson SB, Attur M, Amin AR and Clancy
R: Nitric oxide and inflammatory mediators in the perpetuation of
osteoarthritis. Curr Rheumatol Rep. 3:535–541. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Araki E, Forster C, Dubinsky JM, Ross ME
and Iadecola C: Cyclooxygenase-2 inhibitor NS-398 protects neuronal
cultures from lipopolysaccharide-induced neurotoxicity. Stroke.
32:2370–2375. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arichi H, Kimura Y, Okuda H, Baba K,
Kozawa M and Arichi S: Effects of stilbene components of the roots
of Polygonum cuspidatum Sieb. et Zucc. on lipid metabolism.
Chem Pharm Bull (Tokyo). 30:1766–1770. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eyre D: Collagen of articular cartilage.
Arthritis Res. 4:30–35. 2002. View
Article : Google Scholar
|
|
5
|
Choy EH and Panayi GS: Cytokine pathways
and joint inflammation in rheumatoid arthritis. N Engl J Med.
344:907–916. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Héraud F, Héraud A and Harmand MF:
Apoptosis in normal and osteoarthritic human articular cartilage.
Ann Rheum Dis. 59:959–965. 2000.
|
|
7
|
Kim SJ, Ju JW, Oh CD, et al: ERK-1/2 and
p38 kinase oppositely regulate nitric oxide-induced apoptosis of
chondrocytes in association with p53, caspase-3, and
differentiation status. J Biol Chem. 277:1332–1339. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sandell LJ and Adler P: Developmental
patterns of cartilage. Front Biosci. 4:D731–D742. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sandell LJ and Aigner T: Articular
cartilage and changes in arthritis. An introduction: cell biology
of osteoarthritis. Arthritis Res. 3:107–113. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hawkey CJ: COX-2 inhibitors. Lancet.
353:307–314. 1999. View Article : Google Scholar
|
|
11
|
Pecchi E, Priam S, Mladenovic Z, et al: A
potential role of chondroitin sulfate on bone in osteoarthritis:
inhibition of prostaglandin E2 and matrix
metalloproteinases synthesis in interleukin-1β-stimulated
osteoblasts. Osteoarthritis Cartilage. 20:127–135. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim SJ, Im DS, Kim SH, et al: Beta-catenin
regulates expression of cyclooxygenase-2 in articular chondrocytes.
Biochem Biophys Res Commun. 296:221–226. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Khanna D, Sethi G, Ahn KS, et al: Natural
products as a gold mine for arthritis treatment. Curr Opin
Pharmacol. 7:344–351. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shakibaei M, Harikumar KB and Aggarwal BB:
Resveratrol addiction: to die or not to die. Mol Nutr Food Res.
53:115–128. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liao PC, Ng LT, Lin LT, Richardson CD,
Wang GH and Lin CC: Resveratrol arrests cell cycle and induces
apoptosis in human hepatocellular carcinoma Huh-7 cells. J Med
Food. 13:1415–1423. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mukherjee S, Dudley JI and Das DK:
Dose-dependency of resveratrol in providing health benefits. Dose
Response. 8:478–500. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hwang JT, Kwak DW, Lin SK, Kim HM, Kim YM
and Park OJ: Resveratrol induces apoptosis in chemoresistant cancer
cells via modulation of AMPK signaling pathway. Ann NY Acad Sci.
1095:441–448. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin HY, Sun M, Tang HY, et al: Resveratrol
causes COX-2- and p53-dependent apoptosis in head and neck squamous
cell cancer cells. J Cell Biochem. 104:2131–2142. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin HY, Tang HY, Davis FB and Davis PJ:
Resveratrol and apoptosis. Ann NY Acad Sci. 1215:79–88. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu W, Fu YC and Wang W: Cellular and
molecular effects of resveratrol in health and disease. J Cell
Biochem. 113:752–759. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Elmali N, Esenkaya I, Harma A, Ertem K,
Turkoz Y and Mizrak B: Effect of resveratrol in experimental
osteoarthritis in rabbits. Inflamm Res. 54:158–162. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Meeran SM and Katiyar SK: Cell cycle
control as a basis for cancer chemoprevention through dietary
agents. Front Biosci. 13:2191–2202. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bäckesjö CM, Li Y, Lindgren U and Haldosén
LA: Activation of Sirt1 decreases adipocyte formation during
osteoblast differentiation of mesenchymal stem cells. J Bone Miner
Res. 21:993–1002. 2006.
|
|
24
|
Banerjee Mustafi S, Chakraborty PK and
Raha S: Modulation of Akt and ERK1/2 pathways by resveratrol in
chronic myelogenous leukemia (CML) cells results in the
downregulation of Hsp70. PLoS One. 5:e87192010.PubMed/NCBI
|
|
25
|
Jiang H, Shang X, Wu H, et al: Combination
treatment with resveratrol and sulforaphane induces apoptosis in
human U251 glioma cells. Neurochem Res. 35:152–161. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Weng CJ, Wu CF, Huang HW, Wu CH, Ho CT and
Yen GC: Evaluation of anti-invasion effect of resveratrol and
related methoxy analogues on human hepatocarcinoma cells. J Agric
Food Chem. 58:2886–2894. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Csaki C, Mobasheri A and Shakibaei M:
Synergistic chondroprotective effects of curcumin and resveratrol
in human articular chondrocytes: inhibition of IL-1beta-induced
NF-kappaB-mediated inflammation and apoptosis. Arthritis Res Ther.
11:R1652009. View
Article : Google Scholar
|
|
28
|
Yoon YM, Kim SJ, Oh CD, et al: Maintenance
of differentiated phenotype of articular chondrocytes by protein
kinase C and extracellular signal-regulated protein kinase. J Biol
Chem. 277:8412–8420. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Blanco FJ, Guitian R, Moreno J, de Toro FJ
and Galdo F: Effect of antiinflammatory drugs on COX-1 and COX-2
activity in human articular chondrocytes. J Rheumatol.
26:1366–1373. 1999.PubMed/NCBI
|
|
30
|
Lei M, Liu SQ and Liu YL: Resveratrol
protects bone marrow mesenchymal stem cell derived chondrocytes
cultured on chitosan-gelatin scaffolds from the inhibitory effect
of interleukin-1beta. Acta Pharmacol Sin. 29:1350–1356. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tang FY, Su YC, Chen NC, Hsieh HS and Chen
KS: Resveratrol inhibits migration and invasion of human
breast-cancer cells. Mol Nutr Food Res. 52:683–691. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gweon EJ and Kim SJ: Resveratrol induces
MMP-9 and cell migration via the p38 kinase and PI-3K pathways in
HT1080 human fibrosarcoma cells. Oncolgy Rep. 29:826–834.
2013.PubMed/NCBI
|
|
33
|
Hashimoto S, Ochs RL, Komiya S and Lotz M:
Linkage of chondrocyte apoptosis and cartilage degradation in human
osteoarthritis. Arthritis Rheum. 41:1632–1638. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shakibaei M, Mobasheri A and Buhrmann C:
Curcumin synergizes with resveratrol to stimulate the MAPK
signaling pathway in human articular chondrocytes in vitro. Genes
Nutr. 6:171–179. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Csaki C, Keshishzadeh N, Fischer K and
Shakibaei M: Regulation of inflammation signalling by resveratrol
in human chondrocytes in vitro. Biochem Pharmacol. 75:677–687.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shakibaei M, John T, Seifarth C and
Mobasheri A: Resveratrol inhibits IL-1 beta-induced stimulation of
caspase-3 and cleavage of PARP in human articular chondrocytes in
vitro. Ann NY Acad Sci. 1095:554–563. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang J, Gao JS, Chen JW, Li F and Tian J:
Effect of resveratrol on cartilage protection and apoptosis
inhibition in experimental osteoarthritis of rabbit. Rheumatol Int.
32:1541–1548. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Widmann C, Gibson S, Jarpe MB and Johnson
GL: Mitogen-activated protein kinase: conservation of a
three-kinase module from yeast to human. Physiol Rev. 79:143–180.
1999.PubMed/NCBI
|
|
39
|
Zhang W and Liu HT: MAPK signal pathways
in the regulation of cell proliferation in mammalian cells. Cell
Res. 12:9–18. 2002. View Article : Google Scholar
|
|
40
|
Foster FM, Traer CJ, Abraham SM and Fry
MJ: The phosphoinositide (PI) 3-kinase family. J Cell Sci.
116:3037–3040. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
DeLise AM, Fischer L and Tuan RS: Cellular
interactions and signaling in cartilage development. Osteoarthritis
Cartilage. 8:309–334. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
She QB, Bode AM, Ma WY, Chen NY and Dong
Z: Resveratrol-induced activation of p53 and apoptosis is mediated
by extracellular-signal-regulated protein kinases and p38 kinase.
Cancer Res. 61:1604–1610. 2001.PubMed/NCBI
|
|
43
|
Wight RD, Tull CA, Deel MW, et al:
Resveratrol effects on astrocyte function: relevance to
neurodegenerative diseases. Biochem Biophys Res Commun.
426:112–115. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shakibaei M, Csaki C, Nebrich S and
Mobasheri A: Resveratrol suppresses interleukin-1beta-induced
inflammatory signaling and apoptosis in human articular
chondrocytes: potential for use as a novel nutraceutical for the
treatment of osteoarthritis. Biochem Pharmacol. 76:1426–1439. 2008.
View Article : Google Scholar
|