Introduction
Adult-onset hypothyroidism leads to
hippocampus-dependent cognitive dysfunction, in which several
neurotransmitter systems and synaptic proteins are involved
(1–4). Neurotransmitters, which are stored in
synaptic vesicles in presynaptic neurons, are the material
foundation of synaptic transmission. The release of a
neurotransmitter requires the assistance of a variety of synaptic
proteins (5,6). Acetylcholine (ACh), which is involved
in learning and memory, is a significant neurotransmitter in the
brain and has a close relationship with thyroid hormones (THs)
(2). Studies using gene
recombination technology have revealed that the synaptic proteins
syntaxin-1 and munc-18 are involved in the release of ACh in mouse
brains (7,8). Syntaxin-1, abundantly expressed in
the presynaptic membrane, has been implicated in synaptic vesicle
docking, which is the initial association of synaptic vesicles with
the plasma membrane (9). Munc-18
is a neuronal protein that binds tightly to an N-terminal peptide
sequence in syntaxin-1 and accelerates the fusion of
neurotransmitter-containing synaptic vesicles and the plasma
membrane (10).
Thyroxin (T4) replacement therapy is a validated
treatment for hypothyroidism. However, for patients with cognitive
dysfunction, the data regarding treatment with T4 are ambiguous. In
certain cases, T4 replacement therapy has been found to restore the
levels of triiodothyronine (T3), T4 and thyroid-stimulating hormone
(TSH) and fully remedy molecular impairments exhibited in the
hypothyroid brain. However, in other patients, these effects were
not observed (11,12). In addition, the concentrations of
Ca2+/calmodulin-independent protein kinase (CaMKII),
neurogranin, SNAP-25 and calmodulin, in which changes were induced
by hypothyroidism, have been found to return to basal levels
following T4 replacement therapy. However, the levels of protein
kinase C-γ and synaptotagmin-1 in the hippocampus were not restored
in adult hypothyroid rats receiving T4 replacement therapy
(11,13,14).
These observations indicate that it is necessary to identify new
alternative therapeutic methods for treating hypothyroidism.
Donepezil (DON), a potent acetylcholinesterase
(AChE) inhibitor, has demonstrated clinical efficacy, increasing
the levels of ACh at synapses and thereby ameliorating memory and
cognition impairments (15). At
present, DON is widely administered for the treatment of mild
cognitive impairment (16,17). In the present study, the ability of
DON to treat the neurocognitive parameter impairments in
hypothyroidism was investigated. Therefore, the expression levels
of munc-18 and syntaxin-1, as well as the ACh content, were
observed in the dorsal hippocampi of rats with adult-onset
hypothyroidism. In addition, the efficacies of T4 and DON in the
treatment of the altered parameters were investigated.
Materials and methods
Animals
Three-month-old adult male Sprague-Dawley rats
(n=55) were obtained from the Nanjing Experimental Animal Center
(Nanjing, China). The animals were maintained at room temperature
under natural light-dark cycle conditions and received a standard
rodent diet and water ad libitum. The body weight (BW) of
the rats was recorded weekly to monitor growth inhibition, which is
a marker of hypothyroidism. Procedures involving animals and their
care were performed in accordance with the Animal Care and Use
Committee of Anhui Medical University (Hefei, China).
The rats were randomly classified into five groups:
Control, hypothyroid, hypothyroid receiving T4 replacement therapy,
hypothyroid receiving DON therapy and hypothyroid receiving T4 plus
DON therapy. Hypothyroidism was induced in the hypothyroid group
(Hypo group) by adding 6-n-propyl-2-thiouracil (PTU; Sigma-Aldrich,
St. Louis, MO, USA) to the drinking water at a concentration of
0.05% (w/v) for six weeks (n=11). The DON group was treated with
PTU for six weeks, as described for the Hypo group and from the
fifth week, 0.005% (w/v) DON (Sigma-Aldrich) was added to the
drinking water every day for two weeks (n=11). The T4 group was
treated with PTU for six weeks, as described for the Hypo group and
from the fifth week, T4 (dissolved in saline solution, 6 μg/100 g
BW) was injected intraperitoneally for two weeks to restore the
hypothyroid animals to euthyroid status (n=10). The T4 plus DON
group (T4 + DON group) was treated according to the same protocol
as the T4 group for six weeks with the modification that 0.005%
(w/v) DON was added to the drinking water from the fifth week
(n=11). The control group (C group) was administered the same
volume of saline solution for six weeks (n=12).
Thyroid hormones
Rats were anesthetized using chloral hydrate (350
mg/kg BW), following the delivery of the final dose. Next, blood
collected from the abdominal aorta (1.5 ml), underwent
centrifugation at 14,000 × g for 15 min. Prior to subsequent
analysis, the serum was rapidly frozen at −20ºC. T3 and T4 serum
concentrations were obtained using a radioimmunoassay kit (North
Institute of Biological Technology, Beijing, China). The detection
ranges of the assay were: T3, 0.92–2.78 nmol/l; T4, 58–140 nmol/l;
and TSH, 0.5–4.7 μIU/ml
Tissue preparation
Rats were sacrificed and the brains were dissected
on ice following blood collection. For immunohistochemistry, the
right brains were isolated and then fixed in 4% paraformaldehyde
for 7 days at 4ºC. The hippocampus from the left brain was stored
at −80ºC prior to determining the content of ACh.
Determination of ACh content
Rats were sacrificed, and the hippocampus was
separated. After weighting, 9 ml of normal saline was added to 1 g
of hippocampus, followed by homogenation in a glass homogenizer.
ACh content in hippocampus homogenates was measured by the modified
method of Hestrin (18) to compare
the amounts of this key neurotransmitter in the hippocampus among
the groups, as previously described. Briefly, 0.2 ml supernatant
was mixed with 0.35 ml distilled water followed by addition of 0.05
ml calabarine sulfate (1.5 mmol/l) and 0.2 ml trichloroacetic acid
(1.84 mol/l). The mixture was centrifuged at 5,000 × g for 5 min.
Next, 0.1 ml ultimate supernatant was added to 0.1 ml alkaline
hydroxylamine hydrochloride (equal volumes of 2.0 mol/l
hydroxylamine hydrochloride and 3.5 mol/l sodium hydroxide),
incubated at room temperature for 15 min and reacted with 0.05 ml
HCl (4.0 mol/l) and 0.05 ml ferric chloride (0.37 mol/l, containing
0.1 mol/l HCl). Next, 0.2 ml medium and tissue homogenates were
spotted in duplicate onto 96-well microplates. Physostigmine (1.5
mmol/l) was added to the reaction mixture to inhibit the activity
of AChE. Following an additional 2 min incubation, the intensity of
the brown ferric complex was read at 540 nm on a Take3™ plate
reader (BioTek Instruments Inc., Winooski, VT, USA). ACh levels
were expressed in micrograms per milligram of hippocampal protein
(μg/mg prot).
Protein assay
Protein in the hippocampal homogenates was detected
using a BCA Protein Assay kit (Thermo Fisher Scientific, Waltham,
MA, USA) according to the manufacturer’s instructions.
Immunohistochemistry
The fixed right hemispheres were embedded in
paraffin and sectioned coronally using a microtome to produce
6-μm-thick sections. Five sections (1/20 serial sections) of the
dorsal hippocampus were selected from each rat and mounted on
polylysine-coated slides. Following deparaffinization, each section
underwent antigen retrieval, by heating in 10 mM citrate buffer (pH
6.0) at 100ºC for 10 min. Non-specific binding was blocked using 5%
normal goat serum in PBS. The sections were then incubated with
mouse anti-munc-18 (1:200; BD Biosciences, Franklin Lakes, NJ, USA)
or rabbit anti-syntaxin-1 (1:400; Millipore, Temecula, CA, USA)
primary polyclonal antibodies at 37ºC for 1 h and overnight at 4ºC.
Next, sections were washed in PBS, incubated with biotinylated
secondary antibody [rabbit anti-mouse or goat anti-rabbit IgG
(Bioss-Bio Ltd., Beijing, China)] for 15 min at 37ºC and washed
again in PBS. Sections were incubated further with HRP for 10 min
at 37ºC, washed in PBS and colored with diaminobenzidine (Bioss-Bio
Ltd.) at room temperature for 7 min. Finally, hematoxylin was
applied for 3 min to counterstain the sections which were then
dehydrated, rinsed and coverslipped with glycerin. Negative
controls were treated in the absence of primary antibodies.
Quantitative analysis was performed using an image analysis system.
The system included MetaMorph image acquisition and processing
software (JADA 801D; JEDA Science-Technology Development Co., Ltd.,
Nanjing, China) and a Nikon 80i microscope (Nikon, Tokyo, Japan)
equipped with a HP computer. Layers were analyzed from various
subfields of the dorsal hippocampus, including the stratum oriens
(SO), stratum radiatum (SR) and stratum lacunosum-moleculare (SLM)
in CA1; the SO, stratum lucidum (SL) and SR in CA3; and the
polymorphic layer (PL) and molecular layer (ML) in the dentate
gyrus (DG). An image of the complete hippocampal formation was
obtained initially at a low magnification of ×40 and then images at
a higher magnification of ×200, in various subfields of the
hippocampus, were acquired according to the size of each subfield:
three images in CA1 for SO and SR; one image in CA3 and DG-PL; and
two images in DG-ML and CA1-SLM. Digital data were exported into
MetaMorph software for analysis and processing. The average optical
density (OD) represented the intensity of immunohistochemical
staining.
Statistical analysis
Data were analyzed using SPSS 17.0 for Windows
(SPSS, Inc., Chicago, IL, USA) and are presented as mean ± SEM. One
way analysis of variance, using least-significant difference for
post hoc analysis, was used to determine the total serum
concentrations of T3, T4 and TSH, as well as the immunoreactivity
of syntaxin-1 and munc-18 for all treatment groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Serum concentrations of the hormones
Serum T3, T4 and TSH concentrations are presented in
Table I. The serum T3 and T4
levels were significantly lower (P<0.01) and TSH levels were
significantly higher (P<0.01) in the SD rats of the Hypo and DON
groups than in those in the C group. T4 and T4 + DON treatment
restored T3, T4 and TSH levels to values that were not
significantly different from those in the control group
(P>0.05).
 | Table ISerum T3, T4 and TSH levels in the
five groups. |
Table I
Serum T3, T4 and TSH levels in the
five groups.
| Group | Number | T3, nmol/l | T4, nmol/l | TSH, μIU/ml |
|---|
| C | 12 | 0.83±0.03 | 49.81±1.08 | 1.02±0.14 |
| Hypo | 11 | 0.60±0.03a | 18.19±1.72a | 19.78±3.01a |
| DON | 11 | 0.57±0.02a | 18.58±0.91a | 19.55±3.29a |
| T4 | 10 | 0.83±0.08 | 52.42±1.92 | 1.21±0.32 |
| T4 + DON | 11 | 0.77±0.07 | 52.71±2.04 | 1.07±0.15 |
Protein levels of syntaxin-1 and munc-18
in the hippocampus
Representative photomicrographs of the immunolabeled
munc-18 and syntaxin-1 proteins in the different groups are
presented in Figs. 1 and 2, respectively. The distributions of
syntaxin-1 and munc-18 in the dorsal hippocampus were similar among
the five groups. Each layer in the CA1, CA3 and DG subfields
exhibited punctate spots of reaction product and the CA3-SL
subfield was observed to exhibit large spots of munc-18, where
large terminals of mossy fiber were located (Figs. 1 and 2).
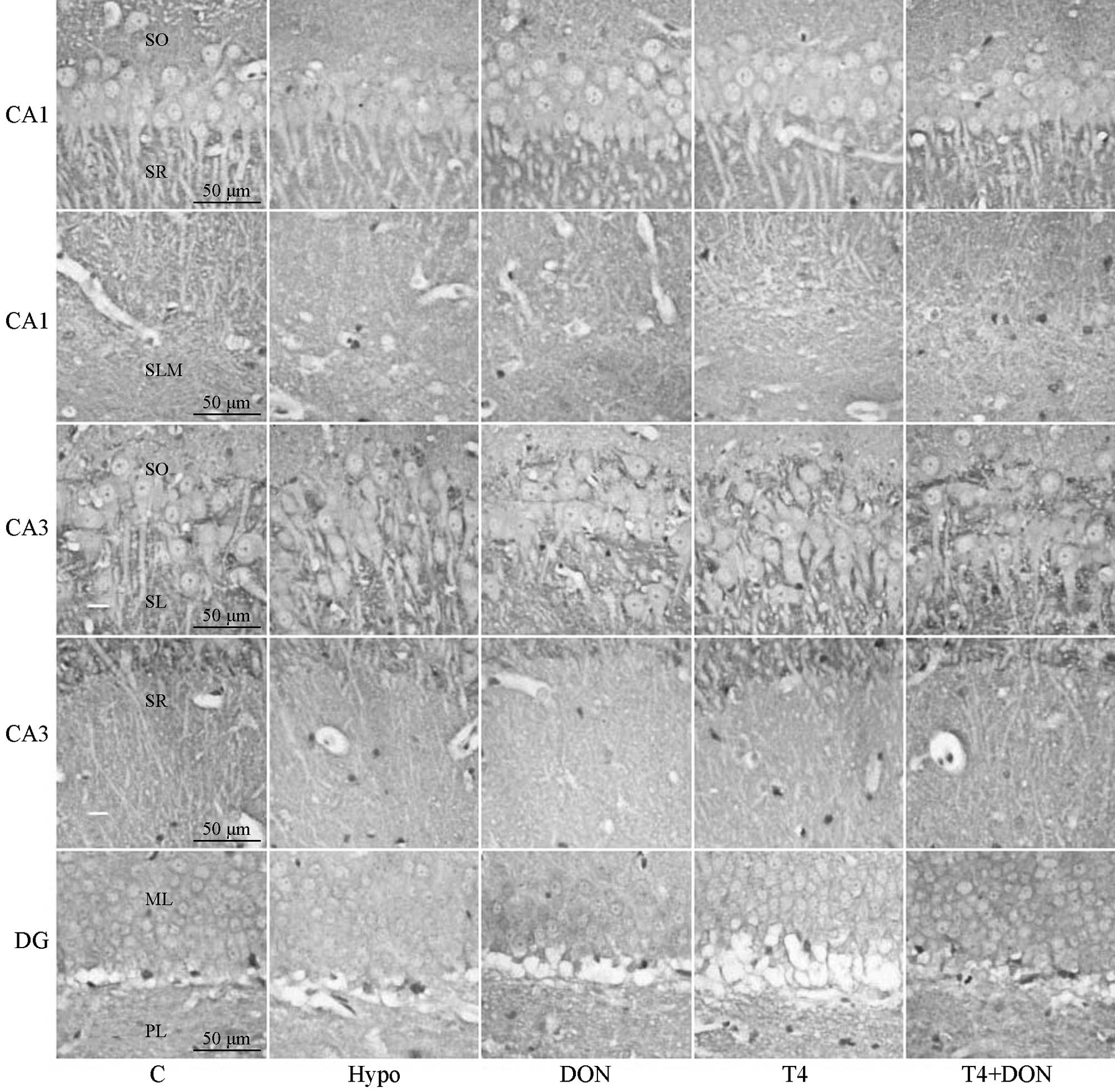 | Figure 1Photomicrographs of coronal sections
showing munc-18 immunoreactivity in CA1, CA3 and DG subregions of
the hippocampi of rats from the Hypo, T4, DON, T4 + DON and C
groups (n=10–12). Distinct punctate spots of reaction product were
observed in every layer of CA1, CA3 and DG subregions; note a
slight reduction in overall staining intensity of CA3-SR, DG-ML and
DG-PL in the Hypo, DON and T4 groups (magnification, ×400; scale
bar, 50 μm). C, control group; Hypo, hypothyroid group; DON,
hypothyroid rats treated with 0.005% (w/v) DON in drinking water;
T4, hypothyroid rats treated with 6 μg T4/100 g BW; T4 + DON,
hypothyroid rats treated with 6 μg T4/100 g BW plus 0.005% (w/v)
donepezil in drinking water. SO, stratum oriens; SR, stratum
radiatum; SLM, stratum lacunosum-moleculare; SL, stratum lucidum;
ML, molecular layer; PL, polymorphic layer; T4, thyroxine; DON,
donepezil; BW, body weight. |
 | Figure 2Photomicrographs of coronal sections
showing syntaxin-1 immunoreactivity in CA1, CA3 and DG subregions
of the hippocampi of rats from the Hypo, T4, DON, T4 + DON and C
groups (n=10–12). Distinct punctate spots of reaction product were
observed in every layer of CA1, CA3 and DG subregions; note that
the staining for syntaxin-1 was more intense in DG-PL and in all
layers of CA1 and CA3 of Hypo and DON groups and that the overall
staining intensity was equal in the DG-ML of each of the five
groups (magnification, ×400; scale bar, 50 μm). Hypo, hypothyroid
group; DON, hypothyroid rats treated with 0.005% (w/v) DON in
drinking water; T4, hypothyroid rats treated with 6 μg T4/100 g BW;
T4 + DON, hypothyroid rats treated with 6 μg T4/100 g BW and 0.005%
(w/v) DON in drinking water; C, control group. SO, stratum oriens;
SR, stratum radiatum; SLM, stratum lacunosum-moleculare; SL,
stratum lucidum; ML, molecular layer; PL, polymorphic layer; DON,
donepezil; T4, thyroxine; BW, body weight. |
Tables II and
III present the analyzed OD
values of munc-18 and syntaxin-1 immunoreactivity in each stratum
of the hippocampal subfields. The OD values of munc-18 in three
layers of the CA3 and DG subfields, i.e., CA3-SR, DG-PL and DG-ML,
in the Hypo, DON and T4 groups were significantly lower compared
with those of the corresponding layers in the C group (P<0.05).
In the T4 + DON group, the OD values in all layers were similar to
those in the C group (P=0.170, 0.863 and 0.600 respectively). The
OD values of syntaxin-1 in all layers of CA1 and CA3, and in DG-PL
were observed to be significantly higher in the Hypo group compared
with those in the corresponding layers in the C group (P<0.01).
No significant differences were identified between the T4 group and
the C group, but the absolute values of the OD of syntaxin-1 in the
T4 group were larger than those of the control (P>0.05). In the
T4 + DON group, the OD values in these layers were more similar to
those in the C group (P>0.05).
 | Table IIMunc-18 expression in various layers
of each subfield in the hippocampus. |
Table II
Munc-18 expression in various layers
of each subfield in the hippocampus.
| Subfield | Stratum | C | Hypo | DON | T4 | T4 + DON |
|---|
| CA1 | SO | 4.63±0.90 | 4.43±0.88 | 4.43±1.02 | 4.53±0.66 | 4.67±0.97 |
| SR | 3.82±0.85 | 3.53±0.56 | 3.61±0.66 | 3.69±0.97 | 3.91±0.77 |
| SLM | 4.37±0.76 | 3.93±0.87 | 4.06±0.74 | 4.09±0.76 | 4.21±0.78 |
| CA3 | SO | 4.62±0.72 | 3.63±0.72 | 3.91±0.88 | 4.35±0.68 | 4.68±0.70 |
| SL | 3.55±0.88 | 3.12±0.79 | 3.19±0.60 | 3.21±0.67 | 3.67±0.77 |
| SR | 4.88±0.76 | 3.42±0.53b | 3.69±0.63b | 4.19±0.55a | 4.59±0.69 |
| DG | ML | 4.61±0.70 | 3.34±0.93b | 3.61±1.31b | 3.80±0.91a | 4.67±0.59 |
| PL | 4.11±0.50 | 3.31±0.78b | 3.42±0.46b | 3.54±0.64a | 4.26±0.65 |
 | Table IIISyntaxin-1 expression in various
layers of each subfield in the hippocampus. |
Table III
Syntaxin-1 expression in various
layers of each subfield in the hippocampus.
| Subfield | Stratum | C | Hypo | DON | T4 | T4+DON |
|---|
| CA1 | SO | 0.36±0.11 | 1.15±0.38b | 1.19±0.29b | 0.39±0.10 | 0.36±0.09 |
| SR | 0.45±0.50 | 1.32±0.17b | 1.24±0.33b | 0.49±0.10 | 0.45±0.78 |
| SLM | 0.42±0.13 | 1.13±0.41b | 0.96±0.29b | 0.48±0.14 | 0.40±0.11 |
| CA3 | SO | 0.89±0.14 | 1.21±0.32b | 1.14±0.35a | 1.00±0.33 | 0.90±0.25 |
| SL | 1.02±0.19 | 1.55±0.59b | 1.20±0.14b | 1.07±0.20 | 1.01±0.11 |
| SR | 0.71±0.95 | 1.31±0.33b | 1.23±0.43b | 0.73±0.12 | 0.70±0.14 |
| DG | ML | 1.70±0.67 | 1.91±0.60 | 1.77±0.42 | 1.81±0.62 | 1.55±0.67 |
| PL | 2.06±0.49 | 2.87±0.53b | 2.76±0.37b | 2.12±0.36 | 2.07±0.44 |
Content of ACh in the hippocampus
Alkaline hydroxylamine colorimetry was performed to
detect the content of ACh in the hippocampi of the rats in the
different groups. The ACh content in the hippocampus is illustrated
in Fig. 3. The results show that
the amount of ACh was significantly decreased by 24% in the
hypothyroid rats (P=0.016) and the content was observed to be
restored to control values by treatment with DON, T4 or T4 + DON
(P=0.382, 0.265 and 0.411, respectively).
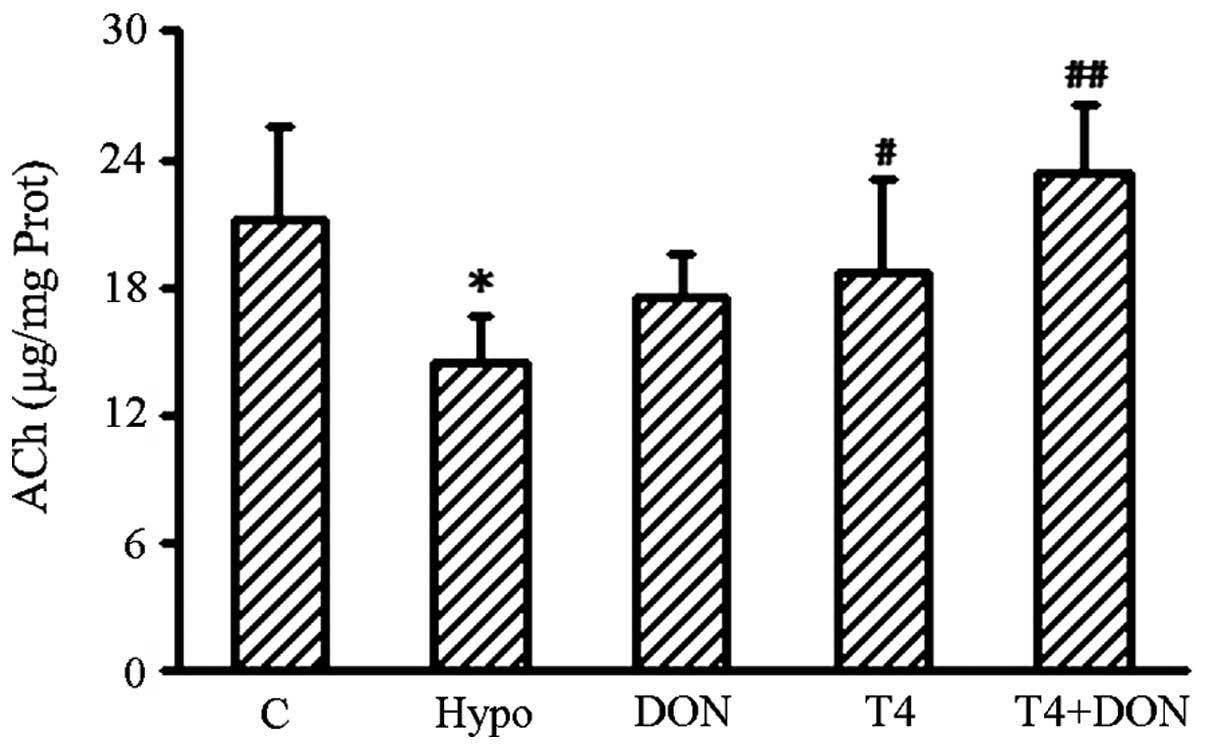 | Figure 3Concentration of hippocampal ACh in
the Hypo, T4, DON, T4 + DON and C groups (n=10–12). Homogenates
were extracted from the hippocampus of each rat. Hypothyroidism
induced a significant reduction in ACh content in the hippocampus
and the DON (0.005%), thyroxine (T4; 6 μg/100 g BW) or combined
treatment (T4 + DON) restored the ACh levels to control values.
Data shown represent mean ± SEM of three independent experiments.
Hypo, hypothyroid group; DON, hypothyroid rats treated with 0.005%
(w/v) DON in drinking water; T4, hypothyroid rats treated with 6 μg
T4/100 g BW; T4 + DON, hypothyroid rats treated with 6 μg T4/100 g
BW and 0.005% (w/v) DON in drinking water; C, control group.
*P<0.05, vs. control group; #P<0.05 and
##P<0.01, vs. hypothyroid group. ACh, acetylcholine;
T4, thyroxine; DON, donepezil; BW, body weight. |
Discussion
In the present study, immunohistochemical analysis
revealed that the expression of munc-18 and syntaxin-1 was
significantly altered in the hippocampus of adult-onset hypothyroid
rats compared with that in the controls. Munc-18 in the Hypo group
was expressed at a significantly lower level in the SR of CA3 and
in the DG in the hippocampus. The results obtained in this study
are consistent with a previous study reporting decreased munc-18
levels in the dorsal hippocampus of rats with adult-onset
hypothyroidism (13). As it has
been confirmed that TH regulates protein synthesis in the brain
(19), the reduced expression of
munc-18 may be associated with the lower TH neuronal levels in the
hippocampus associated with hypothyroidism. Under the same
conditions, the present study also observed that syntaxin-1 levels
in the dorsal hippocampus were increased. Previous studies have
shown that the expression of syntaxin-1 is upregulated in the
adrenal gland in rats with secondary hypothyroidism, induced by
hypophysectomy (20). In
thyroidectomized rats, levels of syntaxin-1 have been shown to be
downregulated in the adenohypophysis (21) and reductions in the expression of
syntaxin-1 were also observed in the prefrontal cortex of rats with
PTU-induced hypothyroidism (22).
The regulation mechanism of syntaxin-1 is unknown. Previous studies
have indicated that hypothyroidism induces various quantitative
distributions of THs (23), as
well as unidentically changing the isoforms of the thyroid receptor
(TR) in various regions of the brain; for example, in the
hippocampus and cerebral cortex, the relative expression of TRα1
was shown to increase, whereas the expression of TRα2 was decreased
(24). It is possible that various
TR isoforms, in different nervous tissues, regulate syntaxin-1.
In the current study, a significant decrement of ACh
content in the hippocampus of adult-onset hypothyroid rats was
observed. Decreased ACh content has also been identified in the
spinal cords of methimazole-induced adulthood hypothyroid rats
(25). It has been reported that a
deficit in THs yields cholinergic neurons with a small somata and
decreased numbers (26), thus
leading to an insufficient synthesis of neurotransmitters. In
addition, evidence from tissue culture experiments indicates that
the enzymes responsible for the synthesis of ACh are under direct
TH control (27); in the absence
of THs, the enzymatic activity is weakened, hence the synthesis of
ACh is decreased. In the current study, DON treatment ameliorated
the reduction of ACh content in the Hypo group. This phenomenon
also occurs when cholinesterase inhibitors, including DON,
neostigmine and galantamine, are used to treat other diseases; for
example, with the oral administration of DON to treat mild
cognitive impairment (16,17) and the oral or intramuscular
injection of neostigmine treatment for myasthenia gravis (28). The mechanism of this phenomenon is
consistent with the hypothesis that cholinesterase inhibitors
increase ACh levels by preventing the enzymatic degradation of ACh,
thus prolonging its availability (15,29).
In addition, galantamine and other AChE inhibitors may act as
agonists at nicotinic receptors and enhance the release of ACh via
a nicotinic mechanism, particularly under conditions of impaired
cholinergic function (30).
T4 replacement therapy has been shown to
re-establish plasma TH euthyroidism in adult-onset hypothyroidism,
thereby attenuating reductions in the levels of ACh (31,32)
and the impaired expression of synaptic proteins associated with
cognitive function (33). In the
present study, the synaptic proteins syntaxin-1 and munc-18 were
restored by T4 replacement therapy; however, munc-18 levels did not
reach those in the control. Previous animal studies have also
reported that normal ranges of hormone substitution restored
CaMKII, calmodulin, SNAP-25 and neurogranin but not protein kinase
C-γ and syt-1 in hypothyroid rats (1,13),
indicating that T4 replacement therapy causes asynchronous recovery
of adult-onset hypothyroidism-induced molecular impairments in the
brain. The asynchronous recovery may be associated with the
different distributions and properties of these proteins in neurons
(34). With regard to the failure
to fully restore munc-18 expression, it is possible that the
recovery of munc-18 in the hypothyroid hippocampus requires a
different dose of exogenous T4. Studies using the isotopic
equilibrium technique identified that the concentration of T4 in
plasma greatly exceeded that present in the central nervous system
(35). Despite T4 replacement
therapy enabling serum THs to reach euthyroidism, the hormone
substitution in the brain may still be insufficient. Indeed, this
concept is supported by a study in which munc-18 in the brain was
fully restored by a large dose of T4 (20 μg/100g BW) (13). However, large doses of T4 therapy
result in marked increases in serum TH leves that may be
detrimental to health. Therefore, the present study explored the
effect of DON upon hypothyroidism.
In the DON + T4 group, munc-18 was found to be
restored to control values in all layers, although the exact
mechanisms underlying this regulation remain uncharacterized.
Accumulated evidence from previous studies suggests that DON
possesses neuroprotective properties in the suppression of
neurodegeneration (15,36). Studies have reported that the
neuroprotective effects of DON slow the progression of hippocampal
atrophy in Alzheimer’s disease (37), protect cortical neurons in models
of oxygen-glucose deprivation and glutamate-induced toxicity,
protect against the effects of hippocampal mitochondrial
dysfunction in transgenic mouse models (38), and increase the total dendritic
length and spine density of neurons in aged mice (39). In addition, DON treatment has been
shown to be effective in preserving presynaptic protein in the
hippocampus and spinal cord in a tauopathy mouse model (40). Although the mechanisms concerning
the neuroprotective effect of DON are not currently explicit, the
neuroprotection observed upon the administration of DON is unlikely
to be associated with AChE inhibition, as the neuroprotection
afforded by DON is not achieved by other cholinesterase inhibitors,
including neostigmine, galantamine or rivastigmine (38). DON may induce its neuroprotective
effect by activating the neurotrophin receptors in the hippocampus
(41). In addition, it has been
shown that DON protects neurons by upregulating nicotinic
acetylcholine receptor subtypes to decrease the glutamate toxicity
that is involved in a number of neuronal degenerative diseases
(36,39,42).
In this context, the recovery of synaptic protein munc-18 in the
co-administration group may occur as a result of DON-induced
neuroprotection against hippocampal neuronal impairment, leading to
an altered synthesis of the synaptic proteins.
In conclusion, the present study showed that
adult-onset hypothyroidism induced alterations of munc-18,
syntaxin-1 and ACh levels in the hippocampus. The expression of
syntaxin-1 and ACh content was restored by T4 monotherapy while the
expression of munc-18 was not. Co-administration of T4 and DON
resulted in more effective restorations than either alone. The
thyroid hormone has a direct effect on metabolism of hippocampal
ACh in adult rats, and DON is helpful for treatment of synaptic
protein impairment induced by hypothyroidism. Further research is
required to investigate the efficacy of DON treatment and the
molecular mechanism underlying this regulation, particularly, the
long-term effects of acetylcholinesterase inhibitors on behavior
and synaptic proteins in mouse models of hypothyroidism.
References
|
1
|
Alzoubi KH, Gerges NZ, Aleisa AM and
Alkadhi KA: Levothyroxin restores hypothyroidism-induced impairment
of hippocampus-dependent learning and memory: Behavioral,
electrophysiological, and molecular studies. Hippocampus. 19:66–78.
2009. View Article : Google Scholar
|
|
2
|
Smith JW, Evans AT, Costall B and Smythe
JW: Thyroid hormones, brain function and cognition: a brief review.
Neurosci Biobehav Rev. 26:45–60. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu CL, Xu YX, Zhan Y, et al: Effect of
thyroxine on synaptotagmin 1 and SNAP-25 expression in dorsal
hippocampus of adult-onset hypothyroid rats. J Endocrinol Invest.
34:280–286. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhu DF, Wang ZX, Zhang DR, et al: fMRI
revealed neural substrate for reversible working memory dysfunction
in subclinical hypothyroidism. Brain. 129:2923–2930. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Weimer RM, Richmond JE, Davis WS, Hadwiger
G, Nonet ML and Jorgensen EM: Defects in synaptic vesicle docking
in unc-18 mutants. Nat Neurosci. 6:1023–1030. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shen J, Tareste DC, Paumet F, Rothman JE
and Melia TJ: Selective activation of cognate SNAREpins by
Sec1/Munc18 proteins. Cell. 128:183–195. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gengyo-Ando K, Kitayama H, Mukaida M and
Ikawa Y: A murine neural-specific homolog corrects cholinergic
defects in Caenorhabditis elegans unc-18 mutants. J
Neurosci. 16:6695–6702. 1996.PubMed/NCBI
|
|
8
|
Sakisaka T, Yamamoto Y, Mochida S, et al:
Dual inhibition of SNARE complex formation by tomosyn ensures
controlled neurotransmitter release. J Cell Biol. 183:323–337.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
de Wit H, Cornelisse LN, Toonen RF and
Verhage M: Docking of secretory vesicles is syntaxin dependent.
PLoS One. 1:e1262006.PubMed/NCBI
|
|
10
|
Han GA, Malintan NT, Collins BM, Meunier
FA and Sugita S: Munc18–1 as a key regulator of neurosecretion. J
Neurochem. 115:1–10. 2010.
|
|
11
|
Alzoubi KH, Gerges NZ and Alkadhi KA:
Levothyroxin restores hypothyroidism-induced impairment of LTP of
hippocampal CA1: electrophysiological and molecular studies. Exp
Neurol. 195:330–341. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Leentjens AF and Kappers EJ: Persistent
cognitive defects after corrected hypothyroidism. Psychopathology.
28:235–237. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu Y, Ning D, Wang F, Liu C, Xu Y, Jia X
and Zhu D: Effect of thyroxine on munc-18 and syntaxin-1 expression
in dorsal hippocampus of adult-onset hypothyroid rats. Eur J
Histochem. 56:e222012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vallortigara J, Alfos S, Micheau J,
Higueret P and Enderlin V: T3 administration in adult hypothyroid
mice modulates expression of proteins involved in striatal synaptic
plasticity and improves motor behavior. Neurobiol Dis. 31:378–385.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Akasofu S, Kimura M, Kosasa T, Sawada K
and Ogura H: Study of neuroprotection of donepezil, a therapy for
Alzheimer’s disease. Chem Biol Interact. 175:222–226. 2008.
|
|
16
|
Giacobini E: Cholinesterase inhibitors
stabilize Alzheimer’s disease. Ann NY Acad Sci. 920:321–327.
2000.
|
|
17
|
Nordberg A and Svensson AL: Cholinesterase
inhibitors in the treatment of Alzheimer’s disease: a comparison of
tolerability and pharmacology. Drug Saf. 19:465–480. 1998.
|
|
18
|
Hestrin S: The reaction of acetylcholine
and other carboxylic acid derivatives with hydroxylamine, and its
analytical application. J Biol Chem. 180:249–261. 1949.PubMed/NCBI
|
|
19
|
Sokoloff L and Klee CB: The effect of
thyroid on protein synthesis in brain and other organs. Res Publ
Assoc Res Nerv Ment Dis. 43:371–386. 1966.PubMed/NCBI
|
|
20
|
Hepp R, Grant NJ, Chasserot-Golaz S, Aunis
D and Langley K: The hypophysis controls expression of SNAP-25 and
other SNAREs in the adrenal gland. J Neurocytol. 30:789–800. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Quintanar JL and Salinas E: Effect of
hypothyroidism on synaptosomal-associated protein of 25 kDa and
syntaxin-1 expression in adenohypophyses of rat. J Endocrinol
Invest. 25:754–758. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang HY, Sun CP, Jia XM, Gui L, Zhu DF and
Ma WQ: Effect of thyroxine on SNARE complex and synaptotagmin-1
expression in the prefrontal cortex of rats with adult-onset
hypothyroidism. J Endocrinol Invest. 35:312–316. 2012.PubMed/NCBI
|
|
23
|
Broedel O, Eravci M, Fuxius S, Smolarz T,
Jeitner A, Grau H, et al: Effects of hyper- and hypothyroidism on
thyroid hormone concentrations in regions of the rat brain. Am J
Physiol Endocrinol Metab. 285:E470–E480. 2003.PubMed/NCBI
|
|
24
|
Constantinou C, Margarity M and Valcana T:
Region-specific effects of hypothyroidism on the relative
expression of thyroid hormone receptors in adult rat brain. Mol
Cell Biochem. 278:93–100. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Molinengo L, Cassone MC and Oggero L:
Action of hypo- and hyperthyroidism on the postmortal decay of
acetylcholine in the rat spinal cord. Neuroendocrinology. 42:28–31.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gould E and Butcher LL: Developing
cholinergic basal forebrain neurons are sensitive to thyroid
hormone. J Neurosci. 9:3347–3358. 1989.PubMed/NCBI
|
|
27
|
Ahmed MT, Sinha AK, Pickard MR, Kim KD and
Ekins RP: Hypothyroidism in the adult rat causes brain
region-specific biochemical dysfunction. J Endocrinol. 138:299–305.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mehndiratta MM, Pandey S and Kuntzer T:
Acetylcholinesterase inhibitor treatment for myasthenia gravis.
Cochrane Database Syst Rev. 2:CD0069862011.
|
|
29
|
Kroker KS, Rast G, Giovannini R, Marti A,
Dorner-Ciossek C and Rosenbrock H: Inhibition of
acetylcholinesterase and phosphodiesterase-9A has differential
effects on hippocampal early and late LTP. Neuropharmacology.
62:1964–1974. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Barnes CA, Meltzer J, Houston F, Orr G,
McGann K and Wenk GL: Chronic treatment of old rats with donepezil
or galantamine: effects on memory, hippocampal plasticity and
nicotinic receptors. Neuroscience. 99:17–23. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shahrara S, Drvota V and Sylvén C: Organ
specific expression of thyroid hormone receptor mRNA and protein in
different human tissues. Biol Pharm Bull. 22:1027–1033. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ladinsky H, Consolo S, Peri G and
Garattini S: Acetylcholine, choline and choline acetyltransferase
activity in the developing brain of normal and hypothyroid rats. J
Neurochem. 19:1947–1952. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wekking EM, Appelhof BC, Fliers E, et al:
Cognitive functioning and well-being in euthyroid patients on
thyroxine replacement therapy for primary hypothyroidism. Eur J
Endocrinol. 153:747–753. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
de Wit H, Walter AM, Milosevic I,
Gulyás-Kovács A, Riedel D, Sørensen JB and Verhage M:
Synaptotagmin-1 docks secretory vesicles to syntaxin-1/SNAP-25
acceptor complexes. Cell. 138:935–946. 2009.PubMed/NCBI
|
|
35
|
van Doorn J, Roelfsema F and van der Heide
D: Concentrations of thyroxine and 3,5,3′-triiodothyronine at 34
different sites in euthyroid rats as determined by an isotopic
equilibrium technique. Endocrinology. 117:1201–1208. 1985.
|
|
36
|
Dong H, Yuede CM, Coughlan CA, Murphy KM
and Csernansky JG: Effects of donepezil on amyloid-beta and synapse
density in the Tg2576 mouse model of Alzheimer’s disease. Brain
Res. 1303:169–178. 2009.PubMed/NCBI
|
|
37
|
Mori E, Hashimoto M, Krishnan KR and
Doraiswamy PM: What constitutes clinical evidence for
neuroprotection in Alzheimer disease: support for the
cholinesterase inhibitors. Alzheimer Dis Assoc Disord. 20(2 Suppl
1): S19–S26. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Riepe MW: Cholinergic treatment: what are
the early neuropathological targets. Eur J Neurol. 12:3–9. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Alcántara-González F, Mendoza-Perez CR,
Zaragoza N, et al: Combined administration of cerebrolysin and
donepezil induces plastic changes in prefrontal cortex in aged
mice. Synapse. 66:938–949. 2012.PubMed/NCBI
|
|
40
|
Yoshiyama Y, Kojima A, Ishikawa C and Arai
K: Anti-inflammatory action of donepezil ameliorates tau pathology,
synaptic loss, and neurodegeneration in a tauopathy mouse model. J
Alzheimers Dis. 22:295–306. 2010.PubMed/NCBI
|
|
41
|
Autio H, Mätlik K, Rantamäki T, et al:
Acetylcholinesterase inhibitors rapidly activate Trk neurotrophin
receptors in the mouse hippocampus. Neuropharmacology.
61:1291–1296. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shen H, Kihara T, Hongo H, et al:
Neuroprotection by donepezil against glutamate excitotoxicity
involves stimulation of alpha7 nicotinic receptors and
internalization of NMDA receptors. Br J Pharmacol. 161:127–139.
2010. View Article : Google Scholar
|

















