Introduction
Cryptococcosis, or cryptococcal disease, is a
potentially fatal fungal disease caused by Cryptococcus
neoformans or Cryptococcus gattii in patients with
acquired immunodeficiency syndrome. Cryptococcal
meningoencephalitis results in >600,000 fatalities every year
(1). As a fungal infection, the
limitation and clearance of cryptococcosis is mostly dependent on
innate immune cells, such as dendritic cells (DC), natural killer
cells (NK) and neutrophils. Briefly, Cryptococcus infection induces
secretion of pro-inflammatory cytokines, including type-I
interferon (IFN-I), tumor necrosis factor-α, interleukin (IL)-1β
and IL-6, from innate immune cells (2). Studies have suggested that myeloid
differentiation primary response gene 88 (MyD88)-mediated Toll-like
receptor (TLR) signaling pathways, such as TLR2, TLR4 and TLR9, are
involved in this secretory process (3,4).
However, in a more recent study, TLR4 was observed to not be
required for host defense against cryptococcal infection (5). In addition, Ley et al
(6)suggested that TLR signals have
a limited role in the clearance of Cryptococcus, as MyD88- and
TLR-deficient mice were able to survive following cryptococcal
infection (6).
By contrast, the roles of adaptive immune cells in
the generation of protective anti-cryptococcal infection immune
responses have been widely accepted. Studies have shown that
increased IL-17A production, a pro-inflammatory cytokine which is
predominantly secreted by CD4+ T cells, is associated
with cryptococcal burden (5,7).
Furthermore, IL-17A secreted by CD4+ T helper (Th)17
cells is involved in multiple roles as a ‘bridge’ that is
associated with innate and adaptive immune responses. Its primary
functions include inducing pro-inflammatory secretion in mucosal
tissues (8) as a potent inducer.
IL-17A can also enhance neutrophil chemotaxis by upregulating the
production of granulocyte-colony stimulating factor and chemokine
(C-X-C motif) ligand 1 (9). An
increased Th17 cell population has also been found to inhibit the
generation of Treg cells (10) and
promote the clearance of fungal infections. Notably, IL-17A is also
secreted by other cells, such as γδ T cells, CD8+ T
cells, NK T cells, NK cells and neutrophils (11–13).
Differentiation of Th17 cells is dependent on IL-6
and transforming growth factor-β (TGF-β) signals; however, MyD88
was previously identified to enhance Th17 polarization by inducing
IL-1β and IL-23 production (14).
Activation of TLR signals in antigen-presenting cells (APCs) skewed
Th subset development in the thymus and the peripheral tissues
(15). Additionally, endotoxin
exposure, which can specifically activate TLR4 signaling pathways
in the Blomia tropicalis allergens infection model, shifted
Th2 cell response towards the Th17 cell-mediated immune response
(16). These results indicate that
TLR signaling may be involved in Th17 cell-mediated host
anti-cryptococcal infection, particularly in Th17 cell
differentiation. In the present study, the effect of C.
neoformans-induced release of IFN-I on immune regulation was
determined. TLR signaling pathways were blocked and IFN-I secretion
was examined with neutralizing antibodies.
Materials and methods
Cell culture and Pam3CSK4 treatment
Human peripheral blood mononuclear cells (PBMCs)
were cultured in Dulbecco’s modified Eagle’s medium (Gibco-BRL,
Carlsbad, CA, USA) supplemented with 10% heat-inactivated fetal
calf serum, 100 U/ml penicillin and 100 μg/ml streptomycin, then
incubated at 37°C in 5% CO2. Cultured cells were treated
with 0.1 μg/ml tripalmitoyl-S-glyceryl-cystein (Pam3CSK4;
910.5 Da; EMC microcollections; Tübingen, Germany) in the medium to
activate the TLR2 signaling pathway.
Cryptococcus strains and culture
conditions
The encapsulated C. neoformans strain 11959
(ATCC 90112) was cultured in medium containing 1% yeast extract, 1%
peptone and 2% glucose at 30°C for 2 days. The cell suspension was
mixed with glycerine and stored at −80°C. C. neoformans was
heat-killed at 65°C for 30 min and 109 fungi/ml medium
was added to the co-culture.
Quantitative polymerase chain reaction
(qPCR)
Total RNA was harvested from cells, treated with
DNase and reverse transcribed as previously described (17). Universal human (h)IFN-I primers
were as follows: Forward, 5′-ATG GCT AGR CTC GTG CTT TCC T-3′ (R is
a wobble of A or G) and reverse, 5′-AGG GCT CTC CAG AYT TCT GCT
CTG-3′ (Y is a wobble of C or T); hIFN-γ forward, 5′-TCA AGT GGC
ATA GAT GTG GAA-3′ and reverse, 5′-CAC TCG GAT GAG CTC ATT GA-3′;
hIL-1β forward, 5′-AAA CCT CTT CGA GGC ACA AG-3′ and reverse,
5′-CTG TTT AGG GCC ATC AGC TT-3′; hIL-6 forward, 5′-GAC AAC TTT GGC
ATT GTG G-3′ and reverse, 5′-ATG CAG GGA TGA TGT TCT G-3′; hIL-17A
forward, 5′-CTG TGT CTC TGA TGC TGT TG-3′ and reverse, 5′-ATG TGG
TGG TCC AGC TTTC-3′; and hGAPDH, forward 5′-ACC ACA GTC CAT
GCC ATC AC-3′ and reverse, 5′-CAC CAC CCT GTT GCT GTA GCC-3′.
Relative abundance of each cDNA was normalized to corresponding
GAPDH levels and quantified using the ΔCT method.
Cytokine assays
T-cell derived cytokine levels, IL-17A, IL-22 and
IL-23, were determined in the cultured supernatant after 7 days of
incubation using commercially available ELISA kits (R&D
Systems, Minneapolis, MN, USA) according to the manufacturer’s
instructions. Lower detection limits were 40 and 78 pg/ml for IL-17
and IL-22/23, respectively.
Blockade of IFN-I signal with antibody in
mice splenocytes
Splenocytes were isolated from the spleen of female
C56Bl/6 mice and treated with isotype antibody or anti-IFN-IR1
antibody 0.1 mg/ml (Invitrogen, Carlsbad, CA, USA).
Statistical analysis
Results from at least three repeat experiments were
pooled and analyzed using GraphPad Prism 5 software (GraphPad
Software, Inc., San Diego, CA, USA). Data are expressed as the mean
± standard error. P<0.05 was considered to indicate a
statistically significant difference.
Results
Cryptococcal infection induces early
IFN-I expression prior to Th17 cell activation in vitro
C. neoformans infection was established in
vivo with co-cultured human PBMCs and heat-killed C.
neoformans in vitro. As previously reported, Cryptococcus
infection induces pro-inflammatory cytokine secretion in innate
cells and is mostly independent on peroxisome
proliferator-activated receptor (PPAR), and other factors including
TLR family, MyD88 and NF-κB were reported activated in the
clearance of C. neoformans. In the present study, the
production of TLR signaling molecules and the levels of IFN-I and
IFN-γ were examined following C. neoformans infection, as
well as various cytokines that are critical for Th17 cell
differentiation, such as IL-1β, IL-6 and IL-17A, at various time
points following qPCR. The results suggested there were no
significant difference in IL-17A expression levels in the early
phase (<4 h) following infection when compared mRNA expression
in 1 h and 2 h with that in 0.5 h (P0.5 vs. 1=0.1182 and
P0.5 vs. 2=0.2431). However, C. neoformans
treatment induced strong expressions of pro-inflammatory cytokines
IFN-I, IFN-γ and IL-1β, which were quite different from the media
mock group.
Blockade of IFN-I signals decreases
IL-17A expression levels in T cells
Previous studies on IFN-I have suggested that
members of the IFN-I family induce transcription of numerous target
genes involved in host anti-virus and -bacterial infection
(13,14). However, fungal infection is
commonly recognized via the TLR2, TLR4 and TLR6 signaling pathways
and does not result from the secretion of IFN-I. In the present
study, a peak expression of IFN-I was noted in the early phase
(<0.5 h) of C. neoformans infection. This transient
expression of IFN-I occurred prior to the release of additional
cytokines and its function was clear such as inducing synthesis of
NO and activation of macrophages. To examine whether heightened
IFN-I secretion was necessary for Th17 cell development and IL-17A
production, the IFN-I-receptor (R) was blocked with anti-IFN-I
receptor neutralizing antibodies in the co-culture model and the
TLR2 agonist, Pam3CSK4, was used to activate TLR signaling pathways
as the control. Notably, no significant differences in IL-17A
production were observed via ELISA (data not shown). Considering
that neutrophils in human PBMCs are a major source of IL-17A
(13), this treatment was repeated
in mice splenocytes, which have a lower number of neutrophils than
PBMCs, and found that blockade of IFN-I reduced IL-17A secretion
levels after 7 days of co-culture. In addition, Pam3CSK4-treated
mice splenocytes did not express the high levels of IFN-I (data not
shown) and IL-17A observed in the control group (Fig. 2).
Blockade of IFN-I signals inhibits Th17
cell development in vitro
To understand IFN-I and its roles in Th17 cell
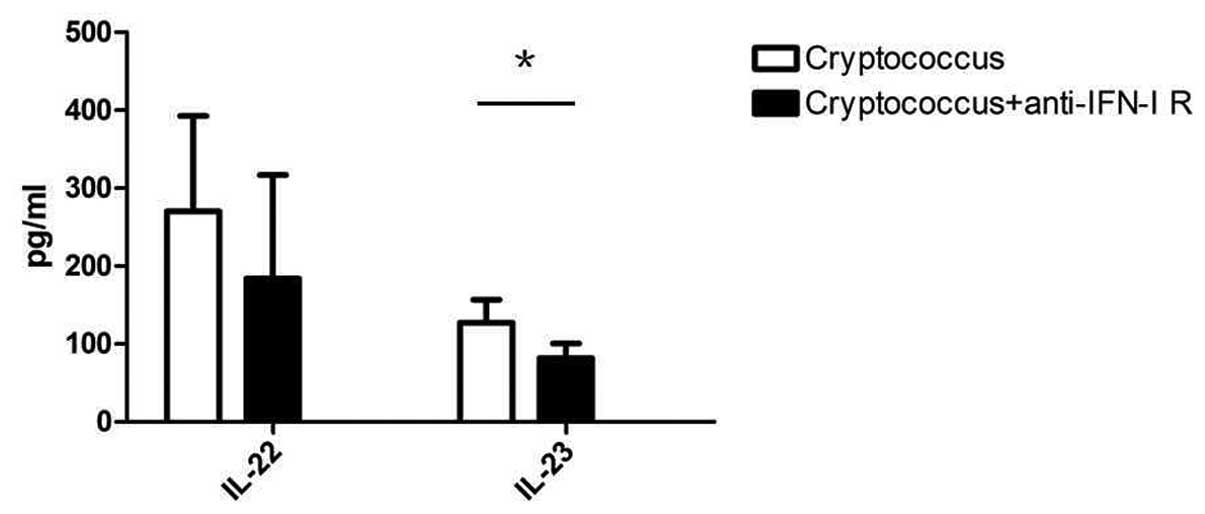
development, the expression of IL-22 and IL-23 was determined in
co-cultured mice splenocytes. IL-22 is the key factor in the
mucosal immune response and clearance of pathogenic microbes
(15). IL-23 promotes naive
CD4+ T cell differentiation towards Th17 cells (18,19),
and IL-22 and IL-23 are produced by Th17 cells. In the present
model, blockade of IFN-I signals in C. neoformans
co-cultured mice splenocytes with anti-IFN-I receptor neutralizing
antibodies resulted in decreased expression levels of IL-22 and
IL-23 compared with that of the control group after 7 days of
culture (P=0.0453) (Fig. 3). These
results suggest that the expression levels of IFN-I in the early
phase of C. neoformans infection may have a guiding role in
Th17 cell differentiation and host anti-fungal immune
responses.
Discussion
Cryptococcosis is a disease resulting from fungal
infection. Although a number of cell subsets, such as neutrophils
and DCs, are involved in the clearance of Cryptococcus spp.,
Th17 cells and innate cells are accepted as the major regulatory
factors (16). The recognition of
pathogenic microbes via PPAR family members is important to
activate local inflammations in the early phase of infection.
Recent studies (20–22) have shown that MyD88-mediated TLR
signals are involved in the clearance of Cryptococcus via cytokine
secretion; however, the mechanisms of these cytokines in immune
regulation remain unclear.
In the present study, human PBMCs and heat-killed
C. neoformans were co-cultured to mimic cryptococcal
infection in human PBMCs to examine the cytokine profile at various
time points. Results suggest that heat-killed C. neoformans
increased the expression levels of IFN-I, IL-1β and IL-6 in the
acute phase of infection and enhanced IL-17A production after 2 h
(data not shown). Notably, the recognition of C. neoformans
was mediated by TLR2, TLR4 and/or TLR6, which did not result in
high levels of IFN-I secretion. To understand whether IFN-I is
necessary in the host anti-fungal immune response, the IFN-I-R
signal was blocked with anti-IFN-I-R neutralizing antibodies. This
blockade of IFN-I was found to specifically reduce IL-17A
expression in mice splenocytes, but had almost no effect on human
PBMCs. Considering that there were numerous IL-17A-producing innate
cells (such as neutrophils and γδ T cells) in human PBMCs, we
speculated that IFN-I may be important for Th17 cell polarization,
however, the mechanisms remain unclear. Further examination of
IL-22 and IL-23 expression levels were also consistent with this
hypothesis.
The common opinion regarding Th17 cell development
both in vitro and in vivo suggests that the
polarization of Th17 cells is regulated by IL-6 and TGF-β, which is
secreted by adipocytes (23,24).
However, the mechanisms may be more complex. A previous
bioinformatic study of Th17 cell differentiation (25) suggested that a number of
transcription factors, such as signal transducer and activator of
transcription 3 (STAT3), interferon regulatory factor-4 (IRF4) and
basic leucine zipper transcription factor, ATF-like, are activated
much earlier than IL-17A gene transcription. In addition, a study
of DC subsets identified that IRF4 transcription-factor-dependent
CD103+ DCs specifically directed Th17 cell polarization
(26). In the present study, we
reported that IFN-I expression was detected in the early phase in
C. neoformans infection but not IL-6 or IL-17 expression. In
addition, the activation of IFN-I expression was required in the
followed immune responses as blockade of IFN-I signal downregulated
cytokines IL-17, IL-22 and IL-23 secretions in vitro. These
finding suggest the critical roles of IFN-I in the clearance of
fungi infection and provided direction for the future treatment of
cryptococcosis.
References
|
1
|
Biondo C, Midiri A, Messina L, Tomasello
F, Garufi G, Catania MR, et al: MyD88 and TLR2, but not TLR4, are
required for host defense against Cryptococcus neoformans.
Eur J Immunol. 35:870–878. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nakamura K, Miyagi K, Koguchi Y, Kinjo Y,
Uezu K, Kinjo T, et al: Limited contribution of Toll-like receptor
2 and 4 to the host response to a fungal infectious pathogen,
Cryptococcus neoformans. FEMS Immunol Med Microbiol.
47:148–154. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wormley FL Jr, Perfect JR, Steele C and
Cox GM: Protection against cryptococcosis using a murine gamma
interferon-producing Cryptococcus neoformans strain. Infect
Immun. 75:1453–1462. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wozniak KL, Ravi S, Macias S, Young ML,
Olszewski MA, Steele C and Wormley FL: Insights into the mechanisms
of protective immunity against Cryptococcus neoformans
infection using a mouse model of pulmonary cryptococcosis. PLoS
One. 4:e68542009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Peck A and Mellins ED: Precarious balance:
Th17 cells in host defense. Infect Immun. 78:32–38. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ley K, Smith E and Stark MA:
IL-17A-producing neutrophil-regulatory Tn lymphocytes. Immunol Res.
34:229–242. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bettelli E, Carrier Y, Gao W, Korn T,
Strom TB, Oukka M, Weiner HL and Kuchroo VK: Reciprocal
developmental pathways for the generation of pathogenic effector
TH17 and regulatory T cells. Nature. 441:235–238. 2006. View Article : Google Scholar
|
|
8
|
Korn T, Bettelli E, Oukka M and Kuchroo
VK: IL-17 and Th17 cells. Annu Rev Immunol. 27:485–517. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ferretti S, Bonneau O, Dubois GR, Jones CE
and Trifilieff A: IL-17, produced by lymphocytes and neutrophils,
is necessary for lipopolysaccharide-induced airway neutrophilia:
IL-15 as a possible trigger. J Immunol. 170:2106–2112. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang J, Burkett PR, Borges CM, Kuchroo
VK, Turka LA and Chang CH: MyD88 is essential to sustain mTOR
activation necessary to promote T helper 17 cell proliferation by
linking IL-1 and IL-23 signaling. Proc Natl Acad Sci USA.
110:2270–2275. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jin B, Sun T, Yu XH, Yang YX and Yeo AE:
The effects of TLR activation on T-cell development and
differentiation. Clin Dev Immunol. 2012:8364852012.PubMed/NCBI
|
|
12
|
Barboza R, Câmara NO, Gomes E, Sá-Nunes A,
Florsheim E, Mirotti L, et al: Endotoxin exposure during
sensitization to Blomia tropicalis allergens shifts TH2
immunity towards a TH17-mediated airway neutrophilic inflammation:
role of TLR4 and TLR2. PLoS One. 8:e671152013.PubMed/NCBI
|
|
13
|
Perry AK, Chen G, Zheng D, Tang H and
Cheng G: The host type I interferon response to viral and bacterial
infections. Cell Res. 15:407–422. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rifkin IR, Leadbetter EA, Busconi L,
Viglianti G and Marshak-Rothstein A: Toll-like receptors,
endogenous ligands, and systemic autoimmune disease. Immunol Rev.
204:27–42. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lilly LM, Gessner MA, Dunaway CW, Metz AE,
Schwiebert L, Weaver CT, Brown GD and Steele C: The β-glucan
receptor dectin-1 promotes lung immunopathology during fungal
allergy via IL-22. J Immunol. 189:3653–3660. 2012.
|
|
16
|
Wozniak KL, Hardison SE, Kolls JK and
Wormley FL: Role of IL-17A on resolution of pulmonary C.
neoformans infection. PLoS One. 6:e172042011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Clifford JL, Walch E, Yang X, Xu X,
Alberts DS, Clayman GL, et al: Suppression of type I interferon
signaling proteins is an early event in squamous skin
carcinogenesis. Clin Cancer Res. 8:2067–2072. 2002.PubMed/NCBI
|
|
18
|
Frazer LC, Scurlock AM, Zurenski MA, et
al: IL-23 induces IL-22 and IL-17 production in response to
Chlamydia muridarum genital tract infection, but the absence
of these cytokines does not influence disease pathogenesis. Am J
Reprod Immunol. 70:472–284. 2013.PubMed/NCBI
|
|
19
|
Yang CY, Ma X, Tsuneyama K, et al:
IL-12/Th1 and IL-23/Th17 biliary microenvironment in primary
biliary cirrhosis: Implications for therapy. Hepatology. Dec
21–2013.(Epub ahead of print).
|
|
20
|
Dan JM, Wang JP, Lee CK and Levitz SM:
Cooperative stimulation of dendritic cells by Cryptococcus
neoformans mannoproteins and CpG oligodeoxynucleotides. PLoS
One. 3:e20462008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang JP, Lee CK, Akalin A, Finberg RW and
Levitz SM: Contributions of the MyD88-dependent receptors IL-18R,
IL-1R, and TLR9 to host defenses following pulmonary challenge with
Cryptococcus neoformans. PLoS One. 6:e262322011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Redlich S, Ribes S, Schütze S, Eiffert H
and Nau R: Toll-like receptor stimulation increases phagocytosis of
Cryptococcus neoformans by microglial cells. J
Neuroinflammation. 10:712013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
O’Shea JJ, Steward-Tharp SM, Laurence A,
Watford WT, Wei L, Adamson AS and Fan S: Signal transduction and
Th17 cell differentiation. Microbes Infect. 11:599–611.
2009.PubMed/NCBI
|
|
24
|
Zhou L and Littman D: Transcriptional
regulatory networks in Th17 cell differentiation. Curr Opin
Immunol. 21:146–152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yosef N, Shalek AK, Gaublomme JT, et al:
Dynamic regulatory network controlling TH17 cell differentiation.
Nature. 496:461–468. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Persson EK, Uronen-Hansson H, Semmrich M,
et al: IRF4 transcription-factor-dependent CD103(+)CD11b(+)
dendritic cells drive mucosal T helper 17 cell differentiation.
Immunity. 38:958–969. 2013.PubMed/NCBI
|